Introduction
Gastroesophageal reflux (GER) is common in infants, children, and adolescents.[1,2] Based on symptom surveys, recurrent vomiting is reported in two thirds of 4-month-old infants, but is present in only 5% to 10% of infants by 1 year of age.[2] Beyond infancy, up to one fourth of children and adolescents have recurrent abdominal pain, whereas only 5% report heartburn or epigastric pain.[2,3] Symptoms of gastroesophageal reflux disease (GERD) are more common in adults, with heartburn occurring in 40% of survey respondents.[4] In one study,[5] adults with GERD were more likely to recall experiencing acid reflux symptoms in childhood, including abdominal pain, heartburn, recurrent vomiting, dysphagia, chronic cough, or hoarseness.[5] Symptoms of GERD vary with age. Common GERD symptoms in infants are regurgitation, choking, gagging, irritability, opisthotonic posturing, and excessive hiccups. GERD symptoms in young children are similar to those experienced by adults, such as abdominal pain, vomiting, excessive belching, and dysphagia. These results suggest that in some individuals, GERD is a lifelong disease that may require aggressive therapy early in life to reduce the risk of long-term sequelae, such as erosive esophagitis or Barrett's esophagus. Therefore, because GER is common, it is important to distinguish pediatric patients with pathologic reflux that may lead to complications of GERD from those with physiologic GER who have a better prognosis. The presence of associated symptoms such as poor weight gain, excessive crying, disturbed sleep, and feeding or respiratory problems distinguishes infants with GERD from those with physiologic gastroesophageal reflux.
Diagnostic Evaluation of GERD in Children
Management of GERD in both adults and children is based on disease severity, the degree of symptoms, and presence or absence of complications of GERD determined by the diagnostic evaluation. Many diagnostic approaches are used to evaluate GERD in pediatric patients. GERD can be diagnosed by typical history and physical examination findings (Table 1) as a basis for a trial of therapy.[6] However, typical symptoms do not always predict which patients will respond to treatment. In one study,[7] there was poor correlation between irritability and positive esophageal biopsy or esophageal pH study findings of GERD in infants. In the diagnostic evaluation, the upper gastrointestinal series is neither sensitive nor specific for the diagnosis of GERD, but can demonstrate anatomic causes of vomiting, particularly congenital abnormalities in infants. Esophageal pH monitoring can quantitate esophageal acid exposure by measuring the frequency and duration of acid reflux events in children with suspected GERD.[8] In the evaluation of GERD in pediatric patients, esophageal pH monitoring determines the degree of esophageal acid exposure, whether a temporal association exists between atypical symptoms and acid reflux, as well as the adequacy of therapy in patients who respond poorly to treatment.[8] Esophageal pH monitoring is particularly valuable in correlating acid reflux and atypical symptoms of GERD in infants and children, such as chronic cough, stridor, wheezing, apnea, irritability, or opisthotonic posturing.
Table 1.
Signs and Symptoms of Pediatric GERD
| Infants | Older Children and Adolescents |
| Feeding resistance | Abdominal pain |
| Recurrent vomiting | Heartburn |
| Failure to thrive | Recurrent vomiting |
| Fussiness/irritability | Dysphagia |
| Apnea/choking episodes | Chronic cough/wheezing |
| Opisthotonic posturing | Hoarseness |
Esophagoscopy with biopsy can confirm the diagnosis of reflux esophagitis and screen for other upper gastrointestinal disorders whose symptoms may mimic those of GERD in children. Esophageal biopsy can provide additional information beyond visual appearance, and thus is recommended in pediatric endoscopy for evaluation of GERD because there is poor correlation between endoscopic and histologic findings in infants and children.[9] Many of these patients may have histologic evidence of reflux esophagitis despite the appearance of grossly normal mucosa endoscopically, while other children with esophagitis may have conditions that mimic GERD, such as eosinophilic esophagitis. One endoscopic finding associated with GERD in children, as opposed to eosinophilic esophagitis, is the presence of vertical red lines in the distal esophagus (Figure 1).[10]
Figure 1.
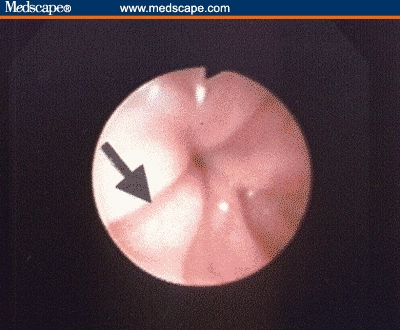
In addition to confirming the histologic features of reflux esophagitis, esophageal biopsy can help diagnose atypical causes of vomiting, such as eosinophilic esophagitis, which occurs in an estimated 10% to 15% of children undergoing upper endoscopy for GERD symptoms.[6,11] The diagnostic tests that are useful in the evaluation of suspected GERD in pediatric patients are listed in Table 2.
Table 2.
Diagnostic Evaluation of Pediatric GERD
| History and physical examination |
| Upper gastrointestinal series |
| Esophageal pH monitoring |
| Esophagoscopy with biopsy |
Eosinophilic esophagitis is characterized by an isolated, severe eosinophilic infiltration of the esophagus. The presenting symptoms of eosinophilic esophagitis are indistinguishable from GERD, and include vomiting/regurgitation, abdominal pain, chest pain, heartburn, and dysphagia. Choking, food impaction, and dysphagia may be more prominent symptoms in these patients. However, these symptoms in children with eosinophilic esophagitis do not respond to acid-suppression therapy. Many infants with this condition respond to strict elimination diets and amino acid-based formulas. Systemic or swallowed doses of inhaled corticosteroids have been reported as treatments in older children or adolescents with eosinophilic esophagitis.
Management — Lifestyle Changes for GERD in Children
The goals for treatment of GERD are to relieve symptoms, heal esophagitis if present, maintain remission of symptoms, and manage or prevent complications. Treatment options to achieve these goals include dietary or behavioral modifications, pharmacologic intervention, and surgical therapy. Recommendations regarding dietary and behavioral changes in the management of pediatric GERD are age-dependent. For infants, dietary changes include modifying infant feeding techniques and changing formula composition by the addition of thickening agents or by changing the milk protein source. The addition of rice cereal (1 tablespoon per ounce of formula) decreases the volume and frequency of regurgitation, but does not reduce esophageal acid exposure.[12] Alternatively, commercially available formula with osmotic agents has also been reported to have similar effects as formula thickened with rice cereal.[13] Because symptoms of milk protein allergy can overlap those of GERD in infants, a 2-week trial of hypoallergenic formula can be recommended in this age group.[6,11] Nonpharmacologic conservative therapy improved symptoms of vomiting and irritability in approximately one fourth of infants with GERD symptoms in one study.[14]
Lifestyle changes for management of GERD in adolescents are similar to adult recommendations, and include dietary modification, weight reduction, avoidance of alcohol, and smoking cessation (Table 3). Passive tobacco smoke exposure is a risk factor for esophagitis in children; therefore, avoidance of tobacco smoke is recommended for a child diagnosed with GERD.[15]
Table 3.
Lifestyle Changes in the Management of Pediatric GERD
| Infants | Older Children and Adolescents |
| Thickened feedings | Weight reduction if overweight |
| Smaller, more frequent feedings | Dietary modification |
| Antireflux positioning after feedings | Smoking cessation |
| Avoid passive tobacco smoke exposure |
Management — Pharmacologic Therapy for GERD in Children
Therapeutic agents that decrease gastric acid secretion are the most effective treatment for management of GERD in children and adults. Histamine-2 receptor antagonists (H2RAs) and proton-pump inhibitors (PPIs) are recommended for antisecretory therapy of GERD.[6,16] Cimetidine and nizatidine were found to be more effective than placebo in healing erosive esophagitis in randomized controlled trials.[17,18] PPIs are more cost-effective when compared with H2RAs because of their better treatment outcome; PPIs are also less costly than surgical therapy in adults.[19] Omeprazole and lansoprazole are approved by the US Food and Drug Administration for pediatric use. Studies are currently being conducted or are being planned to evaluate the safety and efficacy of the use of other PPIs in children.
Two randomized, placebo-controlled trials of H2RA treatment for esophagitis in children have been reported.[17,18] These trials involved use of cimetidine administered at 30–40 mg/kg/day for 6 weeks[17] and nizatidine given at 10 mg/kg/day[18] for 6 weeks; a 70% healing rate was achieved with both cimetidine and nizatidine, compared with 15% for placebo. The safety and efficacy of other H2RAs, including ranitidine and famotidine, have been demonstrated in children in open-label studies.[6] The recommended dosages of H2RAs and PPIs with FDA-approved pediatric indications are shown in Table 4.
Table 4.
Pharmacologic Agents With Pediatric Indications for Treatment of GERD
| Medication | Dose | Frequency |
| H2RAs | ||
| Cimetidine | 40 mg/kg/day | Thrice daily or 4 times daily |
| Famotidine | 1 mg/kg/day | Twice daily |
| Ranitidine | 5–10 mg/kg/day | Twice daily or thrice daily |
| PPIs | ||
| Lansoprazole | 0.4–2.8 mg/kg/day | Once daily |
| Omeprazole | 0.7–3.3 mg/kg/day | Once daily |
A previous study reported a similar outcome for high-dose antacid therapy and cimetidine in children treated for esophagitis.[20] However, serum aluminum concentrations approaching levels reported to cause osteopenia and neurotoxicity were found in children taking aluminum-containing antacids.[21] Thus, chronic antacid therapy is generally not recommended as the primary therapy for the pediatric patient with GERD because safer and more convenient alternatives exist.[6]
Although prokinetic or promotility agents are prescribed for the management of pediatric GERD either in combination with an antisecretory agent or alone, their efficacy is equivocal in controlled trials.[6] Metoclopramide affects esophageal and gastric motility through an antidopaminergic mechanism. However, its clinical efficacy for treatment of pediatric GERD was equivocal in controlled trials, so little evidence exists to support metoclopramide therapy for GERD in infants and children.[6] In patients with a dysmotility disorder associated with GERD, such as significant gastroparesis, erythromycin given in doses of 3–5 mg/kg/day may be considered.[22] Cisapride was effective in decreasing symptoms and improving results of esophageal pH monitoring in 6 controlled pediatric trials, but is no longer available commercially in the United States. Based on available evidence regarding the limited efficacy of prokinetics in the management of GERD, more research is needed in the development of promotility agents that are both safe and effective for use in the pediatric population.
As stated above, H2RAs are frequently used as first-line therapy for treatment of reflux esophagitis in pediatric patients,[23] but PPIs are superior to H2RAs in healing of erosive esophagitis in adults[24] and children.[25–27] PPIs are more potent inhibitors of acid secretion than H2RAs in that they irreversibly bind to H+, K+ -ATPase in the gastric parietal cell canaliculus and inhibit acid secretion.[16,23]
PPI Therapy
Pharmacokinetics studies have shown that the half-life of omeprazole and lansoprazole is shorter in children than in adults.[25,28,29] Therefore, higher weight-adjusted doses of PPIs per kilogram of body weight are needed in children to achieve similar serum concentrations to those found to be effective in adults.[16] In appropriate doses, the pharmacodynamics of lansoprazole were found to be similar to those found in adults, based on control of gastric acidity as measured by intragastric pH monitoring.[29]
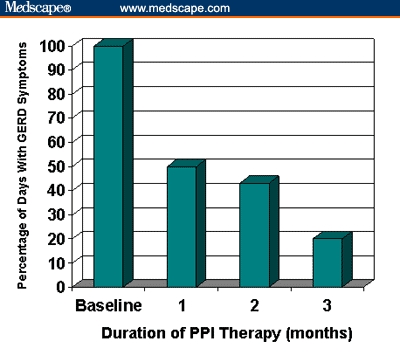
Omeprazole given for 12 weeks resulted in resolution of moderate-to-severe GERD symptoms, such as heartburn, epigastric pain, irritability, dysphagia, odynophagia, coughing, wheezing, or vomiting, in all but 7% of pediatric patients aged 1–16 years.[25] Erosive esophagitis was healed in 82% of subjects treated with omeprazole. In another study[26] that used both PPI and H2RA therapy in 153 patients aged 6–18 years, 70% of the patients with peptic esophagitis responded to an 8-week course of ranitidine (4 mg/kg dose, given twice daily or thrice daily). Of the 30% of patients who did not improve, 87% responded to an 8-week course of omeprazole (20 mg/day), again demonstrating improved response of children to PPI therapy compared with H2RA therapy.[26] Similarly, lansoprazole given at a dose of 1.4 mg/kg/day resulted in endoscopic healing of esophagitis in 80% of children (with a median age of 3.5 years) after 4 weeks of therapy.[28] There was a 100% healing rate for erosive esophagitis and abatement of symptoms of GERD in 80% of subjects treated with lansoprazole in a US multicenter study involving 66 children aged 1–11 years.[27] Figure 2 demonstrates the improvement in the median percentage of days with GERD symptoms over the duration of treatment with lansoprazole in this latter study. As shown in the figure, there was a 50% reduction in symptoms after 1 month of PPI therapy in this age group, but an 80% reduction in symptoms was not achieved until 12 weeks of therapy.[27] A short-term study in 63 adolescents aged 11-17 years receiving lansoprazole therapy reported improvement in GERD symptoms in approximately 70% of patients after only 5 days of treatment.[30]
Figure 2.
Multiple pediatric clinical trials have demonstrated that PPIs are safe and well tolerated in children of all ages.[25,28,30–32] The most commonly reported adverse events (headache, constipation, diarrhea, and abdominal pain) occurred in less than 5% of patients, which is similar to the incidence of adverse events experienced with placebo in studies in adults.[16] As in adults, some pediatric patients may require maintenance therapy for treatment of GERD. However, there are limited data from randomized, controlled trials of maintenance therapy for GERD in children.
Many dosing options are available for PPIs, including capsules, tablets, suspensions, and an orally disintegrating tablet. For children who can swallow pills, omeprazole and lansoprazole are available in capsules and tablets. Omeprazole in oral suspension was safe and effective in pediatric clinical trials conducted in infants aged 1–24 months, but is only available after compounding by a local pharmacist. Lansoprazole is available in an orally disintegrating tablet that is bioequivalent to capsules and can be placed directly on the tongue or suspended in 5 mL of water and given via oral syringe.[33]
Management — Surgery for GERD in Children
Indications for surgical therapy of GERD are based on failed response to medical therapy or recurrence of symptoms after weaning from medical therapy.[6] Many adults with GERD symptoms who fail to respond to acid-suppression therapy may have functional dyspepsia, in which case their symptoms are unlikely to improve after surgery. In children, recurrent vomiting is frequently a predominant symptom, and fundoplication has been reported to be effective as treatment for the symptom of recurrent vomiting in children with GERD. However, fundoplication is not required for management of vomiting in the absence of other complications of GERD. In contrast, potentially life-threatening complications of GERD, such as respiratory symptoms associated with GER, are more likely in infants than older children and may be an indication for surgery. Reports of complete relief of GERD symptoms in children have ranged from 57% to 92% after fundoplication.[6] Mortality rates for pediatric fundoplication have ranged from 0% to 4.7%.[6] A review of 15 published reports involving over 11,000 children who had undergone fundoplication for GERD revealed that a weighted average of the most common postoperative complications were GERD and dysphagia, which occurred in approximately 6% of children.[34] However, because the prognosis for resolution of GERD is better in infants than in older patients, the need for surgery for GERD should be weighed against the fact that only a small percentage of infants with GERD will have lifelong disease.
Summary
As should be evident from the previous discussion, GERD is common in infants and children. Identifying pediatric patients with complications of GERD, such as erosive esophagitis, is important because effective treatment is available in this age group, as it is in adults. Antisecretory therapy is the most effective pharmacologic treatment for GERD, and PPIs in general provide faster symptom relief and are effective in healing erosive esophagitis in patients unresponsive to H2RAs. Surgical management of GERD is an option for children in whom pharmacologic therapy is unsuccessful, but the risks associated with surgical intervention must be considered against the relatively better prognosis in infants without life-threatening complications of GERD.
References
- 1. Nelson SP, Chen EH, Syniar GM, Christoffel KK. Prevalence of symptoms of gastroesophageal reflux during infancy: a pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediatr Adolesc Med. 1997; 151: 569-572. [DOI] [PubMed] [Google Scholar]
- 2. Nelson SP, Chen EH, Syniar GM, Christoffel KK. Pediatric Practice Research Group. Prevalence of symptoms of gastroesophageal reflux during childhood. Arch Pediatr Adolesc Med. 2000; 154: 150-154. [DOI] [PubMed] [Google Scholar]
- 3. Treem WR, Davis PM, Hyams JS. Gastroesophageal reflux in the older child: Presentation, response to treatment and long-term follow-up. Clin Pediatr. 1991; 30: 435-440. [DOI] [PubMed] [Google Scholar]
- 4. Locke GR, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997; 112: 1448-1456. [DOI] [PubMed] [Google Scholar]
- 5. Waring JP, Feiler MJ, Hunter JG, Smith CD, Gold BD. Childhood gastroesophageal reflux symptoms in adult patients. J Pediatr Gastroenterol Nutr. 2002; 35: 334-338. [DOI] [PubMed] [Google Scholar]
- 6. Rudolph CD, Mazur LJ, Liptak GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001; 32 (suppl): S1-S31. [DOI] [PubMed] [Google Scholar]
- 7. Heine RG, Cameron DJS, Chow CW, Hill DJ, Catto-Smith AG. Esophagitis in distressed infants: poor diagnostic agreement between esophageal pH monitoring and histopathologic findings. J Pediatr. 2002; 140: 14-19. [DOI] [PubMed] [Google Scholar]
- 8. Colletti RD, Christie DL, Orenstein SR. Statement of the North American Society for Pediatric Gastroenterology and Nutrition (NASPGN). Indications for pediatric esophageal pH monitoring. J Pediatr Gastroenterol Nutr. 1995; 21: 253-262. [DOI] [PubMed] [Google Scholar]
- 9. Gilger MA, Dietrich C, Gold B, et al. Does the endoscopic impression accurately predict histology in pediatric EGD. Gastroenterology. 2002; 122: 42. [Google Scholar]
- 10. Gupta SK, Fitzgerald JF, Chong SK, Croffie JM, Collins MH. Vertical lines in the distal esophageal mucosa (VLEM): a true endoscopic manifestation of esophagitis in children? Gastrointest Endosc. 1997; 45: 485-489. [DOI] [PubMed] [Google Scholar]
- 11. Orenstein SR, Shalaby TM, Di Lorenzo C, et al. The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol. 2000; 95: 1422-1430. [DOI] [PubMed] [Google Scholar]
- 12. Orenstein SR, Magill HL, Brooks P. Thickening of infant feedings for therapy of gastroesophageal reflux. J Pediatr. 1987; 110: 181-186. [DOI] [PubMed] [Google Scholar]
- 13. Vanderhoof JA, Moran JR, Harris CL, Merkel KL, Orenstein SR. Efficacy of a pre-thickened infant formula: a multicenter, double-blind, randomized, placebo-controlled parallel group trial in 104 infants with symptomatic gastroesophageal reflux. Clin Pediatr. 2003; 42: 483-495. [DOI] [PubMed] [Google Scholar]
- 14. Shalaby TM, Orenstein SR. Efficacy of telephone teaching of conservative therapy for infants with symptomatic gastroesophageal reflux referred by pediatricians to pediatric gastroenterologists. J Pediatr. 2003; 142: 57-61. [DOI] [PubMed] [Google Scholar]
- 15. Shabib SM, Cutz E, Sherman PM. Passive smoking is a risk factor for esophagitis in children. J Pediatr. 1995; 127: 435-437. [DOI] [PubMed] [Google Scholar]
- 16. Gremse DA. Lansoprazole: pharmacokinetics, pharmacodynamics, and clinical uses. Exp Opin Pharmacother. 2001; 2: 1663-1670. [DOI] [PubMed] [Google Scholar]
- 17. Cucchiara S, Gobio-Casali L, Balli F, et al. Cimetidine treatment of reflux esophagitis in children: an Italian multicentric study. J Pediatr Gastroenterol Nutr. 1989; 8: 150-156. [DOI] [PubMed] [Google Scholar]
- 18. Simeone D, Caria MC, Miele E, Staiano A. Treatment of childhood peptic esophagitis: a double-blind placebo-controlled trial of nizatidine. J Pediatr Gastroenterol Nutr. 1997; 25: 51-55. [DOI] [PubMed] [Google Scholar]
- 19. O'Connor JB, Provenzale D, Brazer S. Economic considerations in the treatment of gastroesophageal reflux disease: a review. Am J Gastroenterol. 2000; 95: 3356-3364. [DOI] [PubMed] [Google Scholar]
- 20. Cucchiara S, Staiano A, Romaniello G, et al. Antacids and cimetidine treatment for gastro-oesophageal reflux and peptic oesophagitis. Arch Dis Child. 1984; 59: 842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsou VM, Young RM, Hart MH, Vanderhoof JA. Elevated plasma aluminum levels in normal infants receiving antacids containing aluminum. Pediatrics. 1991; 87: 148-151. [PubMed] [Google Scholar]
- 22. Curry JI, Lander TD, Stringer MD. Review article: erythromycin as a prokinetic agent in infants and children. Aliment Pharamacol Therap. 2001; 15: 595-603. [DOI] [PubMed] [Google Scholar]
- 23. Cucchiara S, Franco MT, Terrin G, et al. Role of drug therapy in the treatment of gastro-oesophageal reflux disorder in children. Paediatr Drugs. 2000; 2: 263-272. [DOI] [PubMed] [Google Scholar]
- 24. Chiba N, De Gara CJ, Wilkinson JM, et al. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta analysis. Gastroenterology. 1997; 112: 1798-1810. [DOI] [PubMed] [Google Scholar]
- 25. Hassall E, Israel D, Shepherd R, et al. Omeprazole for treatment of chronic erosive esophagitis in children: a multicenter study of efficacy, safety, tolerability and dose requirements. J Pediatr. 2000; 137: 800-807. [DOI] [PubMed] [Google Scholar]
- 26. Karjoo M, Kane R. Omeprazole treatment of children with peptic esophagitis refractory to ranitidine therapy. Arch Pediatr Adolesc Med. 1995; 149: 267-271. [DOI] [PubMed] [Google Scholar]
- 27. Tolia V, Ferry G, Gunasekaran T, Huang B, Keith R, Book L. Efficacy of lansoprazole in the treatment of gastroesophageal reflux disease in children. J Pediatr Gastroenterol Nutr. 2002; 35: S308-S318. [DOI] [PubMed] [Google Scholar]
- 28. Faure C, Michaud L, Shaghaghi EK, et al. Lansoprazole in children: pharmacokinetics and efficacy in reflux oesophagitis. Aliment Pharmacol Ther. 2001; 15: 1397-1402. [DOI] [PubMed] [Google Scholar]
- 29. Gremse D, Winter H, Tolia V, et al. Pharmacokinetics and pharmacodynamics of lansoprazole in children with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2002; 35: S319-S326. [DOI] [PubMed] [Google Scholar]
- 30. Gunasekaran T, Gupta S, Gremse D, et al. Lansoprazole in adolescents with gastroesophageal reflux disease: pharmacokinetics, pharmacodynamics, symptom relief efficacy, and tolerability. J Pediatr Gastroenterol Nutr. 2002; 35: S327-S335. [DOI] [PubMed] [Google Scholar]
- 31. Franco MT, Salvia G, Terrin G, et al. Lansoprazole in the treatment of gastro-oesophageal reflux disease in childhood. Dig Liver Dis. 2000; 32: 660-666. [DOI] [PubMed] [Google Scholar]
- 32. Tolia V, Fitzgerald J, Hassall E, Huang B, Pilmer B, Kane R. Safety of lansoprazole in the treatment of gastroesophageal reflux disease in children. J Pediatr Gastroenterol Nutr. 2002; 35: S300-S307. [DOI] [PubMed] [Google Scholar]
- 33. Gremse DA, Donnelly JR, Kukulka MJ, Lloyd E, Lee C. A novel option for dosing of proton pump inhibitors: dispersion of lansoprazole orally disintegrating tablet (LODT) in water via oral syringe. Aliment Pharmacol Ther. 2004; (in press) [DOI] [PubMed] [Google Scholar]
- 34. Di Lorenzo C, Orenstein S. Fundoplication: friend or foe? J Pediatr Gastroenterol Nutr. 2002; 34: 117-124. [DOI] [PubMed] [Google Scholar]


