Abstract
We report here the molecular cloning and expression of a hemolytic sphingomyelinase from an aquatic bacterium, Pseudomonas sp. strain TK4. The sphingomyelinase gene was found to consist of 1,548 nucleotides encoding 516 amino acid residues. The recombinant 57.7-kDa enzyme hydrolyzed sphingomyelin but not phosphatidylcholine, phosphatidylserine, phosphatidylglycerol, phosphatidic acid, or phosphatidylethanolamine, indicating that the enzyme is a sphingomyelin-specific sphingomyelinase C. The hydrolysis of sphingomyelin by the enzyme was found to be most efficient at pH 8.0 and activated by Mn2+. The enzyme shows quite a broad specificity, i.e., it hydrolyzed 4-nitrobenz-2-oxa-1,3-diazole (NBD)-sphingomyelin with short-chain fatty acids and NBD-sphingosylphosphorylcholine, the latter being completely resistant to hydrolysis by any sphingomyelinase reported so far. Significant sequence similarities were found in sphingomyelinases from Bacillus cereus, Staphylococcus aureus, Listeria ivanovii, and Leptospira interrogans, as well as a hypothetical protein encoded in Chromobacterium violaceum, although the first three lacked one-third of the sequence corresponding to that from the C terminus of the TK4 enzyme. Interestingly, the deletion mutant of strain TK4 lacking 186 amino acids at the C-terminal end hydrolyzed sphingomyelin, whereas it lost all hemolytic activity, indicating that the C-terminal region of the TK4 enzyme is indispensable for the hemolytic activity.
Sphingomyelin (SM) is a ubiquitous component of the plasma membrane of vertebrates (24). Sphingomyelinase (SMase; EC 3.1.4.12; also known as SMase C) is a phosphodiesterase that is specific to SM but not phosphatidylcholine (PC). Four bacterial SMase genes have been cloned to date, those from Bacillus cereus (30), Staphylococcus aureus (4), Listeria ivanovii (7), and Leptospira interrogans (21); however, no genetic analysis of an SMase from gram-negative bacteria has been reported so far.
All bacterial SMases described above cause hemolysis and thus are suspected to be potential virulence factors. Disruption of the SMase genes of S. aureus and L. ivanovii resulted in decreased infectivity (2, 8), although the relationship between SMase activity and hemolytic activity is still unknown at the molecular level.
In this study, we report the molecular cloning and expression of a Mn2+-dependent SMase/hemolysin of an aquatic gram-negative bacterium, Pseudomonas sp. strain TK4. This report also shows that the C-terminal region of TK4 SMase is indispensable for the hemolytic activity but not for the SMase activity.
MATERIALS AND METHODS
Materials.
Escherichia coli strains DH5α and BL21(DE3)pLysS, plasmid pUC118, and Pyrobest DNA polymerase were obtained from Takara Shuzo (Shiga, Japan). Sheep blood agar medium was from Japan Biotest (Tokyo, Japan). Plasmid pET23b was purchased from Novagen (Madison, Wis.), and plasmid pBluescript II SK was purchased from Stratagene (La Jolla, Calif.). Restriction enzymes, T4 DNA ligase, and bacterial alkaline phosphatase were obtained from Wako (Osaka, Japan). SM (from egg yolk), L-α-PC, l-α-phosphatidylethanolamine, l-α-phosphatidylglycerol, l-α-phosphatidic acid, l-α-phosphatidylserine, and SMase from S. aureus were purchased from Sigma (St. Louis, Mo.). Recombinant SMase from B. cereus was obtained from Higeta Shoyu Co., Ltd. (Tokyo, Japan). Sphingosylphosphorylcholine (SPC) was prepared as described previously (26). 4-Nitrobenz-2-oxa-1,3-diazole (NBD)-labeled substrates were enzymatically synthesized by using sphingolipid ceramide N-deacylase as described previously (19). l-α-Lysophosphatidylcholine was prepared by the hydrolysis of PC with phospholipase A2 from bee venom (Sigma) as described in reference 18. All other reagents were of the highest purity available.
Bacterial strains and culture conditions.
Pseudomonas sp. strain TK4 was isolated from a pond where eel culture has been conducted (11). TK4 was grown at 25°C in a medium containing 0.5% tryptone, 0.1% yeast extract, and 0.2% NaCl, pH 7.4, with shaking. E. coli DH5α and BL21(DE3)pLysS cells were used as host cells for the expression of SMase. DH5α was grown in Luria-Bertani medium or on sheep blood agar plates containing 100 μg of ampicillin/ml at 37°C. BL21(DE3)pLysS cells transformed with pET/SM-T or pET/SM-Δ186 were grown at 37°C in Luria-Bertani medium containing 100 μg of ampicillin/ml until the optical density at 600 nm reached 0.9. Then, the cultivation was continued for an additional 2 h at 37°C with or without 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG).
Physiological and biochemical tests of bacteria.
The identification of strain TK4 was conducted according to the procedure outlined in the ninth edition of Bergey’s Manual of Systematic Bacteriology (14). Motility, morphology, and Gram-staining characteristics were determined by light microscopy. A transmission electron microscope was employed for the observation of flagella with 2% p-tungstic acid. Utilization of each carbohydrate was determined with Hugh-Leifson medium (10). King’s medium was used to test for the production of diffusible pigment (12). Kovacs’ oxidase test was employed (13). Sensitivity to the vibriostatic agent 0/129 was tested according to the method of Shewan et al. (22). Bacterial DNAs were purified by the method of Marmur (17), and guanine-plus-cytosine (G+C) contents were determined by high-performance liquid chromatography (HPLC) as reported by Tamaoka and Komagata (27). A standard mixture of the four deoxyribonucleotides was purchased from Yamasa Shoyu (Chiba, Japan).
Phylogenetic analysis.
The 16S ribosomal DNA (rDNA) was amplified using universal primers p27f (5prime;-AGA GTT TGA TCM TGG CTC AG-3prime;; positions 8 to 27; E. coli numbering [3]) and p1492r (5prime;-GGC TAC CTT GTT ACG ACT T-3prime;). PCR products were purified from a 1.0% agarose gel and were sequenced directly using nine different sequencing primers (9). The nucleotide sequences were aligned, and phylogenetic relationships were analyzed using CLUSTAL W (28), with other 16S rDNA sequences being obtained from the Ribosomal Database Project II (16).
Construction of a genomic DNA library of Pseudomonas sp. strain TK4.
Genomic DNA was isolated from Pseudomonas sp. strain TK4 and partially digested with Sau3AI. The Sau3AI fragments (2 to 10 kbp) were gel purified and ligated to BamHI-digested pUC118 DNA. The plasmids were used for the transformation of E. coli DH5α.
Expression screening and cloning of the gene encoding SMase.
Transformants of E. coli DH5α containing the plasmids were seeded (approximately 400 colonies/9.2-cm-diameter plate) on the sheep blood agar plates supplemented with 100 μg of ampicillin/ml and incubated at 37°C for 16 h. Positive colonies were surrounded by a transparent hemolytic zone on the agar. Among approximately 4,000 colonies of E. coli DH5α transformed with plasmids containing a Sau3AI-digested genomic DNA of strain TK4, one positive clone, designated H2, was isolated. The plasmid pH2 contains a 6.3-kbp insert. To obtain a shortened insert having full activity, the insert was further digested with NotI, SmaI, and SacII, and the resulting fragments were ligated into the multiple cloning site of the plasmid pBluescript II SK. Among three clones having the deleted inserts, only clone pNS2, carrying a 2.4-kbp NotI-SmaI fragment, was found to possess distinct hemolytic and SMase activity. The nucleotide sequence of pNS2 was determined as described below.
DNA sequencing and sequence analysis.
The nucleotide sequences were determined by the dideoxynucleotide chain termination method with a BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems Japan, Tokyo, Japan) and a DNA sequencer (Applied Biosystems Japan; model 377). DNA alignment and analysis were performed using DNASIS (Hitachi Software Engineering, Tokyo, Japan).
Assay of hemolysis.
The hemolytic activity of the strain TK4 SMase was examined by adding enzyme to a 10% sheep erythrocyte suspension in 10 mM Tris-buffered saline (TBS), pH 7.5, containing 5 mM MnCl2, followed by incubation at 37°C for 30 min. To measure hot-cold hemolysis, aliquots from the incubation mixture were immediately cooled in an ice bath and centrifuged. The supernatants were then diluted to 4 volumes with distilled water and measured spectrophotometrically at 540 nm. A complete lysis of erythrocytes was obtained by adding distilled water instead of TBS.
Assay of SMase.
The activity of SMase was measured using C12-NBD-SM as a substrate as described below. The reaction mixture contained 500 pmol of C12-NBD-SM and an appropriate amount of the enzyme in 20 μl of 25 mM Tris-HCl buffer, pH 7.5, containing 0.1% (wt/vol) Triton X-100 and 5 mM MnCl2. Following incubation at 37°C for 30 min, the reaction was terminated by heating the mixture in a boiling water bath for 5 min. The sample was dried with a Speed Vac concentrator, dissolved in 20 μl of chloroform-methanol (2/1 [vol/vol]), and applied to a thin-layer chromatography (TLC) plate, which was developed with chloroform–methanol–0.02% CaCl2 (5/4/1 [vol/vol]) as a developing solvent. C12-NBD-Cer (NBD-dodecanoylsphingosine) released by the action of the enzyme and the remaining C12-NBD-SM were separated by TLC and then analyzed and quantified with a Shimadzu CS-9300 chromatoscanner (Shimadzu, Kyoto, Japan) (excitation, 475 nm; emission, 525 nm). One enzyme unit was defined as the amount capable of catalyzing the release of 1 μmol of C12-NBD-Cer/min from C12-NBD-SM under the conditions described above.
Phospholipase assay.
Ten nanomoles of various phospholipids was incubated with an appropriate amount of enzyme in 25 mM Tris-HCl buffer, pH 7.5, containing 0.1% Triton X-100 and 5 mM MnCl2 at 37°C for 2 h. After the reaction, the reaction mixture was dried with a Speed Vac concentrator, dissolved in 20 μl of chloroform-methanol (2/1 [vol/vol]), and applied to a TLC plate, which was developed with chloroform–methanol–0.02% CaCl2 (5/4/1 [vol/vol]). Primulin spray was used to detect phospholipids and their digests (23). The degradation of the substrates was quantified with an ATTO Lane Analyzer 2.2 (ATTO, Tokyo, Japan).
Purification of the recombinant SMase from E. coli.
E. coli DH5α cells transformed with pNS2 were grown at 37°C in Luria-Bertani medium (1 liter) containing 100 μg of ampicillin/ml for 16 h. The cells were then harvested by centrifugation and suspended in 50 ml of ice-cold extraction buffer (10 mM Tris-HCl buffer [pH 8.0], 20% sucrose, 10 mg of lysozyme/ml). After shaking for 10 min on ice, the solution was centrifuged at 5,000 × g for 10 min, and the supernatant obtained (periplasmic fraction) was used for the purification of SMase. The periplasmic fraction (50 ml) was dialyzed against buffer A (20 mM Tris-HCl buffer [pH 7.5] containing 2 M NaCl) at 4°C for 16 h and applied to a HiTrap Phenyl Sepharose FF column (1 ml; Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom) preequilibrated with buffer A. The column was washed with 10 ml of buffer A, and then SMase was eluted with buffer B (20 mM Tris-HCl buffer, pH 7.5) and buffer C (20 mM Tris-HCl buffer [pH 7.5] containing 0.1% Triton X-100). The active fractions were pooled (28 ml) and applied to a HiTrap Q column (1 ml; Amersham Pharmacia Biotech) preequilibrated with buffer C. After the column had been washed with 10 ml of buffer C, the enzyme was eluted with buffer D (20 mM Tris-HCl buffer [pH 7.5], containing 0.1% Triton X-100 and 1 M NaCl) and buffer E (50 mM acetate buffer [pH 5.0], containing 0.1% Triton X-100 and 2 M NaCl).
Protein assay and PAGE.
Protein content was determined by the bicinchoninic acid method (Pierce, Rockford, Ill.) or sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using bovine serum albumin as the standard. SDS-PAGE was carried out according to the method of Laemmli (15). The proteins on the SDS-polyacrylamide gel were visualized by staining with silver staining solution (20) and determined with a Shimadzu CS-9300 chromatoscanner with the reflectance mode set at 540 nm.
Western blotting.
The recombinant proteins, separated by SDS–10% PAGE, were transferred onto a polyvinylidene difluoride membrane by using a semidry blotter (Bio-Rad Laboratories, Hercules, Calif.). The membrane was then incubated with antipolyhistidine-tagged mouse monoclonal antibody (Invitrogen Japan, Tokyo, Japan) or anti-T7-tagged mouse monoclonal antibody (Novagen, Darmstadt, Germany) for 6 h at room temperature. The bands were visualized with anti-mouse immunoglobulin G-horseradish peroxidase antibody and a peroxidase staining kit (Nacalai Tesque, Kyoto, Japan).
Construction of expression vector and deletion mutants.
The following primers were used for PCR: USM52 (5prime;-GCA AGC TTA TGA CCC AGG CCC AGG CCG CC-3prime;), LSM1531X (5prime;-TTT CTC GAG GAA ACG CAG CTT GCT GCG-3prime;), and LSM973X (5prime;-TTT CTC GAG GTC GGC GTA GGT GAA GGC-3prime;). USM52 contained a HindIII restriction site (underlined), and LSM1531X and LSM973X contained a XhoI restriction site (double underlined). PCRs were performed in a Gene Amp PCR System 2400 (Applied Biosystems Japan) for 30 cycles (each consisting of denaturation at 98°C for 10 s and extension at 68°C for 2 min) using Pyrobest DNA polymerase, pNS2 as a template, and the following sets of primers: for the construction of pET/SM-T, USM52-LSM1531X, and for that of pET/SM-Δ186, USM52-LSM973X. After gel purification, the amplified products were digested with HindIII and XhoI. The HindIII-XhoI fragments were cloned into the HindIII/XhoI-digested pET23b.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the GenBank/EBI data bank with accession no. AB066097.
RESULTS
Identification of TK4.
Strain TK4, isolated from pond water in Miyazaki prefecture (Japan), is a short, rod-shaped, gram-negative bacterium with a long polar flagellum. TK4 was assigned to the genus Pseudomonas on the basis of morphological and biochemical characteristics. TK4 is very similar to Pseudomonas anguilliseptica, which is known to be a pathogen of eels (29). The 16S rDNA analysis showed that TK4 and P. anguilliseptica should be classified into group I of the genus Pseudomonas, although the GC contents of the two species were slightly different (TK4, 66.4%; P. anguilliseptica, 62.4%).
Cloning, sequencing, and alignment of SMase/hemolysin of Pseudomonas sp. strain TK4.
The SMase/hemolysin gene of TK4 was cloned as described in Materials and Methods. A colony of E. coli cells surrounded by a clear hemolytic zone was found on a sheep blood agar plate when the host cell was transformed with pNS2 carrying a 2.4-kbp NotI-SmaI fragment. SMase activity was also detected in the lysates of E. coli carrying pNS2, indicating that pNS2 contains the SMase/hemolysin gene. Two putative open reading frames (ORFs) were identified in pNS2. The shorter ORF (ORF1), possibly lacking the N-terminal sequence, shows 63% identity at the amino acid level with the C-terminal portion of acyl-coenzyme A dehydrogenase from Pseudomonas aeruginosa PAO1 (25). The longer ORF (ORF2), consisting of 1,548 nucleotides, encoded a polypeptide of 516 amino acids that exhibited significant identity to the sequences of SMases reported previously.
The amino acid sequence of TK4 SMase showed 32, 30, 31, and 38% identity to that of the enzymes from B. cereus, S. aureus, L. ivanovii, and L. interrogans, respectively (Fig. 1). It should be noted, however, that the first three lacked one-third of the sequence of strain TK4 at the C-terminal end. The putative binding sites for Mg2+ and substrates glutamic acid (E, ⋆) and asparagine (N, ★), respectively, were well conserved in these bacterial SMases (Fig. 1). Interestingly, the amino acid sequence of a hypothetical protein of unknown function from Chromobacterium violaceum (accession no. AF172851-1) showed 23% identity to that of TK4 SMase.
FIG. 1.
Alignment of TK4 SMase and other bacterial homologs. Four bacterial SMases and one unknown protein from C. violaceum are aligned with TK4 SMase. Residues conserved in more than 30% of strains having an SM-specific SMase are shown on a black background, while those found in all the strains are indicated by asterisks. Gaps inserted into the sequences are indicated by dots. Alignment was performed using CLUSTAL W (28). The Mg2+-complexing glutamic acid (⋆) and asparagine involved in substrate binding (★) are indicated below the sequence.
The molecular weight and pI of the mature enzyme of TK4 were estimated to be 58,110 and 6.33, respectively, from the deduced amino acid sequence. A hydrophobic region was found at the N-terminal end of the deduced amino acid sequence of TK4 SMase. This sequence seems to be a putative signal peptide, since it had a positively charged N terminus followed by a hydrophobic core and a string of polar residues (1). This is consistent with the observation that TK4 SMase was released into the culture medium. The presence of the hydrophobic motif was also clearly indicated by hydrophobicity plot analysis (data not shown).
Purification of recombinant SMase from E. coli carrying pNS2.
E. coli DH5α carrying pNS2 produced an SMase at 11 mU/mg of protein regardless of the presence or absence of IPTG, indicating that the SMase was probably transcribed from an original TK4 promoter. TK4 SMase was extracted from the periplasmic space of the recombinant E. coli and purified by sequential chromatography on HiTrap Phenyl Sepharose FF and HiTrap Q as described in Materials and Methods. The recombinant SMase was finally purified more than 3,000-fold with an overall yield of 5.4% (Table 1). The most effective step for purification of the enzyme was chromatography using HiTrap Q, by which most of the contaminating proteins were eluted with buffer D (20 mM Tris-HCl buffer [pH 7.5] containing 1 M NaCl and 0.1% Triton X-100), whereas the SMase was eluted with buffer E (50 mM acetate buffer [pH 5.0] containing 2 M NaCl and 0.1% Triton X-100), indicating that the enzyme was strongly adsorbed on an anion-exchange column. Since the pI of the TK4 SMase is not that high, the binding of the enzyme to the column seems to be not entirely due to ionic binding. By this chromatography, the specific activity of the enzyme was increased by at least 10-fold with 10% recovery. The final preparation of the enzyme gave a single protein band corresponding to a molecular mass of 57.7 kDa on SDS-PAGE after staining with silver staining solution under reducing conditions (Fig. 2). This value agreed well with the molecular weight deduced from the nucleotide sequence.
TABLE 1.
Purification of SMase from E. coli carrying pNS2
| Step | Total activity (mU) | Total protein (mg) | Sp act (mU/mg) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| Total lysate | 25,000 | 2,300 | 11 | 100 | 1 |
| Periplasm | 9,450 | 12.7 | 740 | 37.8 | 67.3 |
| HiTrap Phenyl Sepharose FF | 12,636 | 3.93 | 3,200 | 50.5 | 290.9 |
| HiTrap Q | 1,357 | 0.041a | 33,000 | 5.4 | 3,000 |
Estimated by SDS-PAGE as described in the text.
FIG. 2.
SDS-PAGE of the purified recombinant TK4 SMase. Lanes 1 and 5, standard proteins; lane 2, periplasmic fraction showing SMase activity; lane 3, fractions containing SMase from HiTrap Phenyl Sepharose FF; lane 4, final preparation from HiTrap Q. Proteins were stained with a silver staining solution. Standard proteins (molecular weights in parentheses) are phosphorylase b (94,000), bovine serum albumin (67,000), ovalbumin (43,000), carbonic anhydrase (30,000), soybean trypsin inhibitor (20,100), and lysozyme (14,400).
General properties of TK4 SMase.
The optimal activity of the SMase was found at around pH 8.0 using 150 mM GTA buffer (50 mM 3,3-dimethyl-glutaric acid, 50 mM Tris, and 50 mM 2-amino-2-methyl-1,3-propanediol) when C12-NBD-SM was used as a substrate, indicating that this enzyme should be classified as a neutral-alkaline SMase (data not shown). The enzymatic activity was completely inhibited by Zn2+, Cu2+, Hg2+, and Ni2+ at 5 mM, while Mn2+ and Mg2+ activated the enzyme at the same concentration. Ca2+ and Co2+ had almost no effect on the enzyme. Since EDTA completely abolished the activity at 5 mM, restoration of the activity was examined after addition of Mn2+, Mg2+, or Ca2+ using SM (Fig. 3A) and sheep erythrocytes (Fig. 3B) as a substrate. After EDTA treatment, Mn2+ at 2 to 10 mM reestablished the enzyme activity at 300% of that before EDTA treatment. The addition of Mg2+ or Ca2+ is about half as effective as Mn2+ in reestablishing the activity (Fig. 3A). It was found that Mn2+ at 1 to 10 mM and Mg2+ at 2 to 10 mM caused a stimulation of hemolysis by the enzyme beyond the basal level before EDTA treatment. Ca2+ enhanced the hemolysis by the enzyme at 2 mM but strongly inhibited it at 10 mM (Fig. 3B).
FIG. 3.
General properties of the recombinant TK4 SMase. The figure shows restoration of SMase activity after EDTA treatment. SMase was inactivated with 1 mM EDTA, and the activity was restored by addition of cations. □, MgCl2; ○, MnCl2; ▪; CaCl2. Restoration of SMase activity was examined by C12-NBD-SM hydrolysis (A) and hemolysis (B). The dotted line in panel B shows the basal activity before EDTA treatment.
Substrate specificity of TK4 SMase.
The specificity of TK4 SMase was determined using various phospholipids at pH 7.5. The enzyme hydrolyzed SM most efficiently (84.1%) followed by SPC (25.4%) and lysophosphatidylcholine (8.1%) among the substrates tested (the values in parentheses represent the extent of hydrolysis of each substrate). However, the TK4 enzyme did not hydrolyze PC, phosphatidylethanolamine, phosphatidylglycerol, phosphatidic acid, or phosphatidylserine under the present conditions. These results show that the TK4 enzyme should be classified as an SMase C. To examine the effects of the fatty acid moiety of SM on the activity, fluorescence (NBD)-labeled SPC (no fatty acid), C6-SM (hexanoic acid-containing SM), and C12-SM (dodecanoic acid-containing SM) were incubated with SMases of different origins. Interestingly, TK4 SMase hydrolyzed SPC, C6-SM, and C12-SM, and the relative extent of the hydrolysis of these substrates by the enzyme was in the reverse order. In contrast, SPC and C6-SM were and SPC was completely resistant to hydrolysis by SMases from Staphylococcus and Bacillus, respectively. These results indicate that the specificity of TK4 SMase for the fatty acid moiety of SM is different from that of the other two SMases.
SM hydrolysis and hemolytic activity of deletion mutants of TK4 SMase.
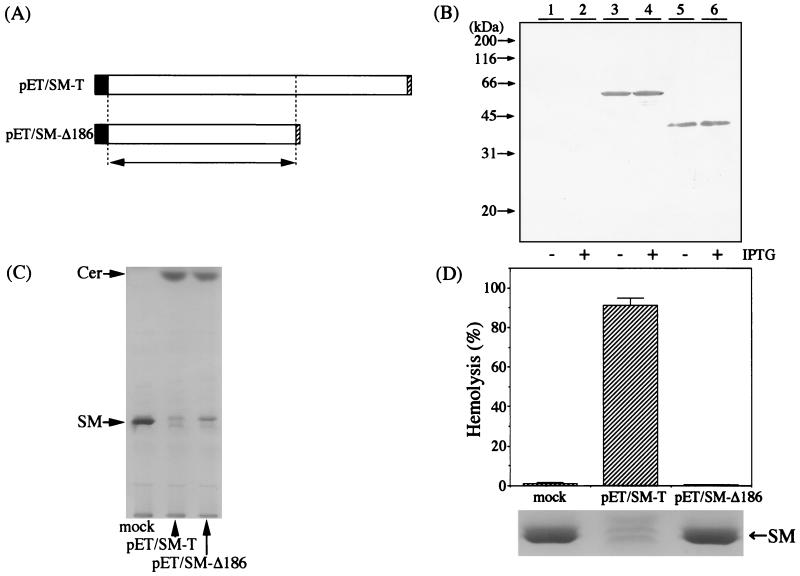
The TK4 SMase is quite large in comparison with the SMases of Bacillus, Staphylococcus, and Listeria, in which the sequence corresponding to the TK4 carboxyl-terminal region is absent, although the deduced amino acid sequences of the four enzymes were similar (Fig. 1). Thus, we attempted to construct a deletion mutant of TK4 SMase which lacks 186 amino acids at the carboxyl terminus and to determine whether the carboxyl-terminal end of the TK4 enzyme is essential for the activities of SM hydrolysis and hemolysis. The recombinant TK4 enzyme (transfectant with pET/SM-T) and its deletion mutant (transfectant with pET/SM-Δ186) were labeled with T7 tag and polyhistidine tag at the amino and carboxyl termini, respectively (Fig. 4A), and subjected to Western blotting using anti-T7 or antipolyhistidine monoclonal antibody. As a result, it was found that recombinant TK4 SMase and its deletion mutant had molecular masses of 59 and 38 kDa on SDS-PAGE, respectively (Fig. 4B). The addition of IPTG did not affect the molecular mass or expression of the enzyme in soluble fractions, although the insoluble enzyme, perhaps that in the inclusion body, increased markedly (data not shown). In the presence of 0.1% Triton X-100, the activity of the deletion mutant was almost the same as that of the wild-type enzyme when egg yolk SM was used as a substrate (Fig. 4C). Interestingly, however, a distinct difference was found in the hemolytic activity, i.e., the deletion mutant lost all the hemolytic activity for sheep erythrocytes even when an excess amount of the enzyme was used for the reaction (Fig. 4D, upper panel). It should be noted that SM hydrolysis was observed for intact erythrocytes after treatment with wild-type enzyme but not with the deletion mutant in TBS without addition of detergents (Fig. 4D, lower panel). These results clearly indicate that the C-terminal region of TK4 SMase is essential for the SM hydrolysis of intact erythrocytes followed by hemolysis, whereas deletion of the region did not affect the SM hydrolysis in detergent micelles.
FIG. 4.
SM hydrolysis and hemolytic activity of deletion mutant. (A) Construction of the SMase expression vector and its deletion mutants. The black and hatched boxes indicate the T7 tag and polyhistidine tag, respectively. The region homologous to the B. cereus SMase is indicated by an arrow. Details are given in Materials and Methods. (B) Western blotting of SMase and its deletion mutant. Lanes 1 and 2, mock transfectants; lanes 3 and 4, wild-type SMase; lanes 5 and 6, deletion mutant. −, no addition of IPTG; +, addition of 1 mM IPTG. Crude soluble proteins, excluding inclusion bodies, were subjected to SDS–10% PAGE, transferred onto a polyvinylidene difluoride membrane, and detected with anti-T7 monoclonal antibody as described in Materials and Methods. (C) SMase activity for egg yolk SM. SM (20 nmol) was incubated at 37°C for 30 min with lysates from transfectants in 25 mM Tris-HCl buffer, pH 7.5, containing 5 mM MnCl2 and 0.1% Triton X-100. (D) Hemolytic activity for sheep erythrocytes (upper panel). Intact sheep erythrocytes were incubated at 37°C for 30 min with lysates (130 mU/100 μl). Hemolysis was determined by the method described in Materials and Methods. After incubation, SM was extracted from erythrocytes and analyzed by TLC (lower panel). TLC was conducted with chloroform–methanol–0.02% CaCl2 (5/4/1 [vol/vol]) as a developing solvent and by staining with Coomassie brilliant blue R-250. mock, no SMase gene vector transfectants; pET/SM-T, TK4 SMase gene transfectants; pET/SM-Δ186, 186-amino-acid-deletion mutant.
DISCUSSION
Three types of SM-degrading enzymes have been reported: (i) SM phosphodiesterase (SMase C, EC 3.1.4.12), which hydrolyzes the ester linkage between Cer and phosphorylcholine; (ii) SM phosphodiesterase D (SMase D, EC 3.1.4.41), which hydrolyzes the ester linkage between Cer phosphate and choline; and (iii) SM-deacylase (sphingolipid Cer N-deacylase), which hydrolyzes the N-acyl linkage between SPC and fatty acid. SMase C and bacterial phospholipase C (EC 3.1.4.3) can hydrolyze SM. However, the two enzymes are completely different, i.e., the former is specific to SM and has little activity for PC, while the latter, known as a zinc protein, hydrolyzes PC much faster than it hydrolyzes SM. This study identified the TK4 SMase as an SM-specific SMase C.
Over the past decade, several microbial SMases have been cloned and characterized (4, 7, 21, 30). The amino acid sequence of TK4 SMase shows about 30 to 40% identity with that of other microbial SMases reported to date; however, little identity was found with mammalian SMases. The genome project for P. aeruginosa has been completed and revealed that this gram-negative bacterium possesses a gene encoding phospholipase C but not SMase C (25).
TK4 SMase has properties clearly different from those of other microbial SMases. (i) For TK4 SMase, Mn2+ is the preferred divalent cation for hydrolysis of SM and hemolysis. In contrast, Bacillus and Staphylococcus enzymes are activated by Mg2+ and by Co2+ and Ni2+, respectively. Interestingly, TK4 enzyme was also activated by Mg2+ but not by Co2+ and was completely inhibited by Ni2+. (ii) TK4 SMase showed broad specificity for the fatty acid moiety of SM, i.e., TK4 enzyme hydrolyzed NBD-SPC, which lacks the fatty acid moiety of SM, while Staphylococcus and Bacillus SMases did not hydrolyze NBD-SPC.
In molecular mass, the SMase from TK4 (58.1 kDa) is similar to that from L. interrogans (63.3 kDa) (21) and completely different from those from S. aureus (37.4 kDa) (4), B. cereus (36.9 kDa) (30), and L. ivanovii (38.5 kDa) (7). The alignment of the five enzymes revealed that Staphylococcus, Bacillus, and Listeria SMases lacked the C-terminal region of the former two enzymes. Interestingly, the recombinant leptospiral SMase had a molecular mass of 39.2 kDa and a sequence identical to the N-terminal region of the deduced amino acid sequence of the 63.3-kDa protein, suggesting that the C-terminal region was lost during the processing of the enzyme in the host cells, E. coli minicells (21). At present, it is not clear whether the 63.3-kDa protein of L. interrogans had SMase activity, although the 39.2-kDa protein showed both SMase and hemolytic activities (21). In the present study, the molecular mass of the purified recombinant TK4 SMase (57.7 kDa) is highly consistent with that deduced from the nucleotide sequence (58.1 kDa), and thus, the enzyme is not likely to be processed or modified in E. coli cells. This study clearly indicated that the deletion of 186 amino acids from the C-terminal end of TK4 SMase completely abolished the hemolytic activity, although the activity for SM in Triton X-100 micelles was not affected (Fig. 4C and D).
SMase is considered to be a virulence factor for various infectious diseases, since SMase producers are usually serious or opportunistic pathogens. For example, L. interrogans is a pathogen causing leptospirosis, an infectious disease in both humans and wild and domestic animals (5), and S. aureus is a well-known opportunistic pathogen (6). Although the relationship between microbial SMase and infectious diseases is still unclear at the molecular level, SMase could cause hemolysis when SMase-producing microbes invade the host. The S. aureus SMase is also known as a β-toxin causing hemolysis. It is worth noting that β-toxin-producing S. aureus showed strong infectivity compared with the toxin-negative mutant (2). It is also noted that all cloned SMases, including the one from TK4, caused hemolysis. The possible role of the SMase of TK4, which was isolated from an eel culture, in fish diseases should be clarified.
Acknowledgments
We acknowledge the help of N. Furuya (Kyushu University) with the observation of bacteria by electron microscopy.
This work was supported in part by a Grant-in Aid for Scientific Research on Priority Areas (B) (12140204) and a Grant-in Aid for Scientific Research (B) (13460044) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Abrahamsen, L., T. Moks, B. Nilsson, U. Hellman, and M. Uhlen. 1985. Analysis of signals for secretion in the staphylococcal protein A gene. EMBO J. 4:3901–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramley, A. J., A. H. Patel, M. O’Reilly, R. Foster, and T. J. Foster. 1989. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect. Immun. 57:2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brousius, J., M. L. Palmer, P. J. Kennedy, and H. F. Noller. 1978. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4801–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman, D. C., J. P. Arbuthnott, H. M. Pomeroy, and T. H. Birkbeck. 1986. Cloning and expression in Escherichia coli and Staphylococcus aureus of the beta-lysin determinant from Staphylococcus aureus: evidence that bacteriophage conversion of beta-lysin activity is caused by insertional inactivation of the beta-lysin determinant. Microb. Pathog. 1:549–564. [DOI] [PubMed] [Google Scholar]
- 5.Ellis, W. A., J. J. O’Brien, D. G. Brynson, and D. P. Mackie. 1985. Bovine leptospirosis: some clinical features of serovar hardjo infection. Vet. Rec. 117:101–104. [DOI] [PubMed] [Google Scholar]
- 6.Gemmell, C. G. 1982. The staphylococcus—new features 100 years after its discovery. J. Infect. 4:5–15. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Zorn, B., G. Dominguez-Bernal, M. Suarez, M. T. Ripio, Y. Vega, S. Novella, and J. A. Vazquez-Boland. 1999. The smcL gene of Listeria ivanovii encodes a sphingomyelinase C that mediates bacterial escape from the phagocytic vacuole. Mol. Microbiol. 33:510–523. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Zorn, B., G. Dominguez-Bernal, M. Suarez, M. T. Ripio, Y. Vega, S. Novella, A. Rodriguez, I. Chico, A. Tierrez, and J. A. Vazquez-Boland. 2000. SmcL, a novel membrane-damaging virulence factor in Listeria. Int. J. Med. Microbiol. 290:369–374. [DOI] [PubMed] [Google Scholar]
- 9.Hiraishi, A. 1992. Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett. Appl. Microbiol. 15:210–213. [DOI] [PubMed] [Google Scholar]
- 10.Hugh, R., and E. Leifson. 1953. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram-negative bacteria. J. Bacteriol. 66:24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito, M., T. Kurita, and K. Kita. 1995. A novel enzyme that cleaves the N-acyl linkage of Cers in various glycosphingolipids as well as sphingomyelin to produce their lyso forms. J. Biol. Chem. 270:24370–24374. [DOI] [PubMed] [Google Scholar]
- 12.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301–307. [PubMed] [Google Scholar]
- 13.Kovacs, N. 1956. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature 178:703. [DOI] [PubMed] [Google Scholar]
- 14.Krieg, N. R. (ed.). 1984. Bergey’s manual of systematic bacteriology. Williams & Wilkins, Baltimore, Md.
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. [DOI] [PubMed] [Google Scholar]
- 16.Maidak, B. L., J. R. Cole, C. T. Parker, Jr., G. M. Garrity, N. Larsen, B. Li, T. G. Lilburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmur, J. 1961. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 3:208–218. [Google Scholar]
- 18.Mason, J. T., A. V. Broccoli, and C. Huang. 1981. A method for the synthesis of isomerically pure saturated mixed-chain phosphatidylcholines. Anal. Biochem. 113:96–101. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa, T., M. Tani, K. Kita, and M. Ito. 1999. Preparation of fluorescence-labeled GM1 and sphingomyelin by the reverse hydrolysis reaction of sphingolipid Cer N-deacylase as substrates for assay of sphingolipid-degrading enzymes and for detection of sphingolipid-binding proteins. J. Biochem. (Tokyo) 126:604–611. [DOI] [PubMed] [Google Scholar]
- 20.Ohsawa, K., and N. Ebata. 1983. Silver stain for detecting 10-femtogram quantities of protein after polyacrylamide gel electrophoresis. Anal. Biochem. 135:409–415. [DOI] [PubMed] [Google Scholar]
- 21.Segers, R. P., A. van der Drift, A. de Nijs, P. Corcione, B. A. van der Zeijst, and W. Gaastra. 1990. Molecular analysis of a sphingomyelinase C gene from Leptospira interrogans serovar hardjo. Infect. Immun. 58:2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shewan, J. M., W. Hodgkiss, and J. Liston. 1954. A method for the rapid differentiation of certain non-pathogenic, asporogenous bacilli. Nature 173:208–209. [DOI] [PubMed] [Google Scholar]
- 23.Skipski, V. P. 1975. Thin-layer chromatography of neutral glycosphingolipids. Methods Enzymol. 35:396–425. [DOI] [PubMed] [Google Scholar]
- 24.Slotte, J. P., A. S. Harmala, C. Jansson, and M. I. Porn. 1990. Rapid turn-over of plasma membrane sphingomyelin and cholesterol in baby hamster kidney cells after exposure to sphingomyelinase. Biochim. Biophys. Acta 1030:251–257. [DOI] [PubMed] [Google Scholar]
- 25.Stover, C. K., X.-Q. T. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrook-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. M. Lim, K. A. Smith, D. H. Spencer, G. K.-S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959–964. [DOI] [PubMed] [Google Scholar]
- 26.Sueyoshi, N., H. Izu, and M. Ito. 1997. Preparation of a naturally occurring D-erythro-(2S, 3R)-sphingosylphosphocholine using Shewanella alga NS-589. J. Lipid Res. 38:1923–1927. [PubMed] [Google Scholar]
- 27.Tamaoka, J., and K. Komagata. 1984. Determination of DNA base composition by reverse-phase high-performance liquid chromatography. FEMS Microbiol. Lett. 25:125–128. [Google Scholar]
- 28.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakabayashi, H., and S. Egusa. 1972. Characteristics of a Pseudomonas sp. from an epizootic of pond-cultured eels (Anguilla japonica). Bull. Jpn. Soc. Sci. Fish. 38:577–587. [Google Scholar]
- 30.Yamada, A., N. Tsukagoshi, S. Udaka, T. Sasaki, S. Makino, S. Nakamura, C. Little, M. Tomita, and H. Ikezawa. 1988. Nucleotide sequence and expression in Escherichia coli of the gene coding for sphingomyelinase of Bacillus cereus. Eur. J. Biochem. 175:213–220. [DOI] [PubMed] [Google Scholar]