Abstract
Epr is a minor extracellular protease secreted by Bacillus subtilis 168. In this study, we show that epr is transcribed by EςD, the RNA polymerase associated with transcription of genes involved in chemotaxis and motility. Disruption of epr abolished swarming of Bacillus subtilis, suggesting its involvement in motility.
At the onset of stationary phase, Bacillus subtilis secretes at least seven extracellular proteases of which subtilisin (aprE) and a neutral metalloprotease E (nprE) are the most abundant (6). Of the remaining five minor extracellular proteases, Epr (epr) contributes 2 to 4% to this pool (18). The expression of these enzymes is stringently regulated, as best exemplified by the aprE gene (23). However, these proteases appear to be dispensable for growth (11, 19), and their relevance in the physiology of the cell remains to be determined.
The transition state is also characterized by the acquisition of specific functions such as competence and motility (23). The expression of genes related to motility, chemotaxis, and flagellar assembly is governed by the alternate sigma factor ςD (14). ςD complexed to the core RNA polymerase (EςD) recognizes and binds the bipartite promoter sequences 5′-CTAAA-3′ (−35) and 5′-CCGATAT-3′ (−10) (14). We present here evidence that epr is transcribed from a ςD-dependent promoter and that this gene is involved in the swarming of B. subtilis.
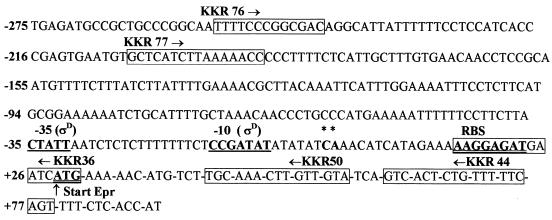
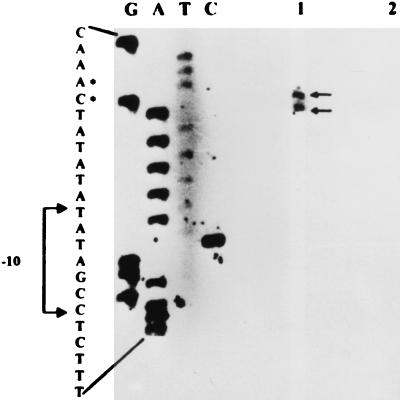
Although epr has been cloned and sequenced (4, 18), the identity of its promoter and the possible physiological role(s) of this gene have so far not been elucidated. To identify the promoter for epr, we examined the DNA sequence upstream of the ribosome-binding site (RBS) for a putative bipartite promoter sequence that may be related to known promoter sequences for the different sigma factors in B. subtilis. A ςD-type promoter with a −35 (CTATT) and a −10 (CCGATAT) element was identified (Fig. 1) that showed matches of 3 of 5 and 7 of 7, respectively, with the consensus ςD promoter (14) and an optimal spacing of 17 bp between the two elements. To determine that indeed this promoter was utilized, primer extension analysis was employed to identify the epr transcription start site. Total RNA was isolated from B. subtilis 168/pIC56-4 and 1A716/pIC56-4 carrying the epr gene in a multicopy plasmid, pIC56-4 (Table 1). Since the levels of Epr are very low (4, 18), we were unable to detect epr mRNA from a single-copy gene and hence we have used a multicopy vector. A polyacrylamide gel electrophoresis-purified primer, KKR50 (5′-CGAGGATCCTGTACAACAAGTTTGCA-3′) (Fig. 1), end labeled with [γ-32P]ATP (5,000 Ci/mM) and T4 polynucleotide kinase, was annealed with 10 μg of RNA, and the primer was extended with Moloney murine leukemia virus reverse transcriptase (1, 17). The extended product(s) was analyzed on a 7 M urea-8% polyacrylamide denaturing gel. To obtain the template for the sequencing reactions, a DNA fragment of 360 bp that overlapped the putative start site was obtained from pIC56-4 by PCR amplification with the primers KKR76 (5′-CGAGATCTCTGCAGTTTTCCCGGCGAC-3′) and KKR44 (5′CTGGATCCAGGGGGCTGAAAAACAGAGTGAC-3′) (Fig. 1) that carry PstI and BamHI restriction sites, respectively. The amplified product was cloned in M13mp19, and single-stranded circular DNA was isolated (17) and used as a template in the sequencing reactions with end-labeled KKR50 and Sequenase version 2.0 (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. Figure 2 (lane 1) shows two extended products for RNA derived from 168/pIC56-4. The extended products correspond to a C (plus strand) and an A (plus strand) located six and seven bases downstream of the −10 position of the putative ςD promoter. This result demonstrates that epr transcription is governed by the ςD promoter. No extended products were obtained for RNA derived from 1A716/pIC56-4 (Fig. 2, lane 2), in which sigD is mutated, indicating that transcription of epr occurs only when ςD is present. The relative intensities of the two extended products were similar, suggesting that the two RNA transcripts are made in equal proportion. Alternatively, one of the products which terminates at A may have arisen as a result of premature termination during reverse transcription.
FIG. 1.
Sequence of epr (4, 18) (accession no. X73124) with positions of the primers used in the study. The transcription start sites are marked with asterix. C* has been denoted as +1.
TABLE 1.
Plasmids and strains used in this study
| Plasmid or strain | Genotypea | Source or reference |
|---|---|---|
| Plasmids | ||
| pIC56-4 | Plasmid pIC56 (Emr) (20) bearing epr gene at the BamHI site | Laboratory Stock (16) |
| pIC216-epr | Plasmid pIC216 (Spr) (21) bearing epr gene at the SalI site; the epr gene was excised as a SalI fragment from pIC56-4 | This study |
| pRB381 | E. coli-B. subtilis shuttle vector for translational fusion with β-galactosidase gene | Bacillus Genetic Stock Center (3) |
| pRBU7 | pRB381 bearing a 250-bp (−205 to +31 from transcription start site of epr) insert containing the epr promoter region, RBS, and ATG in translational frame with the lacZ gene | This study |
| Strains | ||
| 168 | trpC2 | Bacillus Genetic Stock Center |
| 1A716 | sigD::pLM5 CmrtrpC2 | Bacillus Genetic Stock Center |
| 168Δepr | trpC2 epr::Emr | Laboratory stock (16) |
| 168/pIC56-4 | B. subtilis 168 bearing pIC56-4 | This study |
| 1A716/pIC56-4 | B. subtilis 1A716 bearing pIC56-4 | This study |
| 168/pRB381 | B. subtilis 168 bearing pRB381 | This study |
| 168/pRBU7 | B. subtilis 168 bearing pRBU7 | This study |
| 1A716/pRBU7 | B. subtilis 1A716 bearing pRBU7 | This study |
| 168Δepr/pIC216-epr | B. subtilis 168Δepr bearing pIC216-epr | This study |
Emr, erythromycin resistance; Cmr, chloramphenicol resistance. Numbers in parentheses are references.
FIG. 2.
Primer extension analysis of RNA from 168/pIC56-4 (lane 1) and 1A716/pIC56-4 (lane 2). Autoradiography exposure was for 72 h. Start sites are marked with dots.
To further confirm that the epr gene is transcribed by EςD, the promoter activity of epr was determined in both B. subtilis 168 and 1A716 containing the ςD promoter, fused to the β-galactosidase gene of E. coli, in plasmid pRB381 (3). A 250-bp DNA segment (U7) containing the ςD promoter, the RBS, and the ATG was PCR amplified from pIC56-4 with the primers KKR77 (5′-CGAGATCTCTGCAGGCTCATCTTAAAAACC-3′) and KKR36 (5′-CTTAGGATCCATGATTCATCTCC-3′) (Fig. 1) that contain restriction enzyme sites for PstI and BamHI, respectively. The amplified product was digested with PstI-BamHI, cloned in pRB381 to give pRBU7, and transformed in B. subtilis 168 and 1A716 to give 168/pRBU7 and 1A716/pRBU7, respectively. The cells were grown at 37°C in Penassay broth to stationary phase (i.e., to an optical density at 600 nm [OD600] of ∼2.0), and the β-galactosidase activities were determined (15). The enzyme activity in 168/pRBU7 was observed to be 4,860 Miller units, whereas no activity was seen in 168/pRB381, indicating that the 250-bp region in pRBU7 carries the epr promoter. However, the β-galactosidase activity in 1A716/pRBU7 that lacks ςD was completely abolished. Thus, the results of the primer extension analysis and the reporter gene assays clearly demonstrate that transcription of epr is ςD dependent.
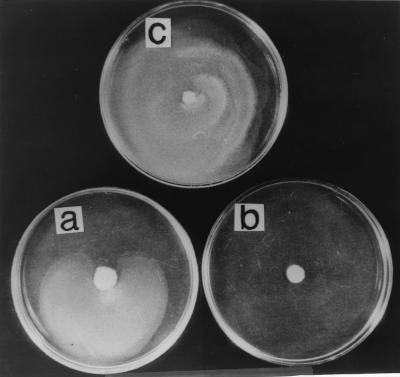
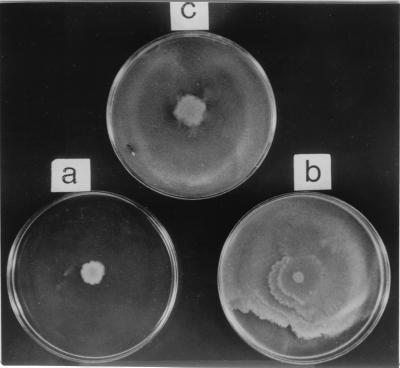
Among the many functions governed by ςD in B. subtilis, several of these genes relate to chemotaxis and to motility that includes flagellin biosynthesis (5, 14). We thus decided to investigate whether Epr may be involved in either chemotaxis or motility. Swarming of bacterial cells is one of the several motility related functions involving surface translocation on soft agar that is accompanied by changes in cell shape and hyperflagellation (9). In nature, swarming provides a means for colonization of bacteria to achieve an ecological niche (8). Proteolysis appears to be one of the mechanisms that regulate the differentiation of the swarmer cells. For example, in the swarming of Vibrio paraheamolyticus, the LonS protease, which is functionally homologous to E. coli Lon, regulates cell elongation and lateral flagellum expression (7, 22). Mutants that lack LonS have a constitutive elongated phenotype. Attempts to demonstrate a “swarm+” phenotype in lonS mutants were unsuccessful (22). We were thus interested in determining whether there was a relationship between the swarming of B. subtilis and epr. Swarm assays were carried out with B. subtilis 168, 168Δepr, and 168Δepr/pIC216-epr (Table 1). The cells were grown in Luria-Bertani (LB) broth at 37°C to an OD600 of ∼4.0, and 1-μl portions of the cultures were spotted onto LB agar plates containing 0.8% agar. The plates were incubated at 37°C for 24 h. The results (Fig. 3) clearly show that the wild-type B. subtilis 168 (Fig. 3a) exhibited swarming on soft agar plates, whereas 168Δepr (Fig. 3b) did not. The ability of 168Δepr to swarm was restored (Fig. 3c) when a functional epr gene was introduced (168Δepr/pIC216-epr). It is important to distinguish here between swimming and swarming motility. Swimming motility is exhibited by individual cells, does not involve communication with other cells, and is observed in liquid medium as well as on plates containing low percentages of agar (0.2 to 0.4%) (8). Swarming, on the other hand, is exhibited on plates containing higher percentages of agar (>0.5%) and requires close cell-to-cell contact, and signaling of information between individual cells is an integral part of this phenomenon (8). Microscopic observation of 168Δepr reveals that it is motile and that the vigor of its motility is comparable to that of the wild type. Motility assays performed with B. subtilis 168 and 168Δepr on LB agar plates containing increasing concentrations of agar (0.3 to 0.8%) revealed that disruption of epr did not affect the spreading of the cells at up to 0.5% agar (swimming) but that the cells were incapable of spreading on plates containing 0.8% agar (swarming) (data not shown). We thus believe that this phenomenon is swarming motility and that Epr is involved in the swarming of B. subtilis. It is possible that Epr may be involved in providing signals to mediate cell-cell communication, a property of swarmer cells. Communication between cells in processes such as quorum sensing and competence appears to involve peptides that are secreted and reimported (2, 13). We propose that the role of Epr in swarming motility is to provide the signal(s) utilizing its proteolytic activity, or Epr itself may be proteolysed by other proteases to generate the signal(s). In support of the above hypothesis, swarm assays were performed with 168Δepr on 0.8% LB agar plates as well as on 0.8% conditioned LB agar plates (i.e., containing a 1:1 mixture of fresh LB and spent medium supernatant derived from B. subtilis 168, grown to an OD600 of ∼4.0 and filter sterilized). The results in Fig. 4 demonstrate that the 0.8% conditioned LB agar plate supported the swarming of 168Δepr (Fig. 4b), whereas it was unable to swarm on a 0.8% LB agar plate (Fig. 4a). This clearly indicates that a signal released from wild-type cells into the medium supported the swarming of 168Δepr that was otherwise missing in the mutant, thus implicating Epr in the signaling process for swarming. Work is in progress to determine the identity of the signal and the signaling mechanism. Epr is also known to exist in multiple forms due to posttranslational processing. One such product of Epr is a C-terminal domain of 240 amino acids that is lysine rich and whose function is unknown (4, 18). Intriguingly, a homology search of this domain with BLASTP in GenBank (http://www.ncbi.nlm.nih.gov) showed considerable homology with various isoforms of tropomyosin across eukaryotes. Tropomyosins are known to regulate actin-myosin interactions in eukaryotes (12). Recently, actin homologs have been identified in B. subtilis that are responsible for maintaining cell shape (10). It may well be that an additional role of Epr is to regulate morphologic changes such as the cell elongation that accompanies swarming.
FIG. 3.
Swarm assay for B. subtilis 168 (a), 168Δepr (b), and 168Δepr/pIC216-epr (c) on 0.8% LB agar plates.
FIG. 4.
Swarm assay for B. subtilis 168 (c) and 168Δepr (a) on a 0.8% LB agar plates and 168Δepr on a 0.8% conditioned LB agar plate (b).
Acknowledgments
This work was supported by the Department of Science and Technology (SP/SO/D-58/96) and the Council of Scientific and Industrial Research [37(950) 97-EMR-II] to K.K.R.
REFERENCES
- 1.Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1995. Short protocols in molecular biology, 3rd ed., p.4-20–4-22. John Wiley & Sons, Inc., New York, N.Y.
- 2.Bassler, B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582–587. [DOI] [PubMed] [Google Scholar]
- 3.Bruckner, R. 1992. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene 122:187–192. [DOI] [PubMed] [Google Scholar]
- 4.Bruckner, R., O. Shosheyov, and R. H. Doi. 1990. Multiple active forms of a novel serine protease from Bacillus subtilis. Mol. Gen. Genet. 221:486–490. [DOI] [PubMed] [Google Scholar]
- 5.Chen, L., and J. D. Helmann. 1994. The Bacillus subtilis ςD-dependent operon encoding the flagellar proteins FliD, FliS, and FliT. J. Bacteriol. 176:3093–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari, E., A. S. Jarnagin, and B. F. Schmidt. 1993. Commercial production of extracellular enzymes, p.917–937. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Amercian Society for Microbiology, Washington, D.C.
- 7.Fraser, G. M., and C. Hughes. 1991. Swarming motility. Curr. Opin. Microbiol. 2:630–635. [DOI] [PubMed] [Google Scholar]
- 8.Harshey, R. M. 1994. Bees aren’t the only ones: swarming in gram-negative bacteria. Mol. Microbiol. 13:389–394. [DOI] [PubMed] [Google Scholar]
- 9.Harshey, R. M., and T. Matsuyama. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. USA 91:8631–8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, L. J. F., R. Carballido-Lopez, and J. Errington. 2001. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104:913–922. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura, F., and R. H. Doi. 1984. Construction of Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J. Bacteriol. 160:442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, H., and A. Bretcher. 1989. Disruption of single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell 57:233–242. [DOI] [PubMed] [Google Scholar]
- 13.Magnuson, R., J. Solomon, and A. D. Grossman. 1994. Biochemical and genetic characterization of a competence pheromone from B. sutbilis. Cell 77:207–216. [DOI] [PubMed] [Google Scholar]
- 14.Moran, C. P., Jr. 1993. RNA polymerase and transcription factors, p.653–667. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 15.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p.442–443. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Inc., New York, N.Y.
- 16.Samant, R. S. 1998. Ph.D. thesis. Indian Institute of Technology, Mumbai, India.
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p.7.79–7.83. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Sloma, A., A. Ally, D. Ally, and J. Pero. 1988. Gene encoding a minor extracellular protease in Bacillus subtilis. J. Bacteriol. 170:5557–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahl, M. L., and E. Ferrari. 1984. Replacement of the Bacillus subtilis subtilisin structural gene with an in vitro derived deletion mutation. J. Bacteriol. 158:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinmetz, M., and R. Richter. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79–83. [DOI] [PubMed] [Google Scholar]
- 21.Steinmetz, M., and R. Richter. 1994. Easy cloning of mini-Tn10 insertions from the Bacillus subtilis chromosome. J. Bacteriol. 176:1761–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart, B., J. L. Enos-Berlage, and L. L. McCharter. 1997. The lonS gene regulates swarmer cell differentiation of Vibrio parahaemolyticus. J. Bacteriol. 179:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauch, M. A., and J. A. Hoch. 1993. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol. Microbiol. 7:337–342. [DOI] [PubMed] [Google Scholar]