Abstract
1. Methods are described for using the changes in respiration of intact Libinia nerve to follow the rate of energy utilization by the sodium pump in this tissue.
2. Short tetani in 10 K(Na)ASW (artificial sea water in which Na is the major cation and the potassium concentration is 10 mM) increased the oxygen uptake which then declined exponentially. From the net influx of Na during the tetanus and the associated oxygen uptake, values between 1·9 and 3·4 were calculated for the Na: ∼ P ratio. After longer tetani, the recovery curve was S-shaped.
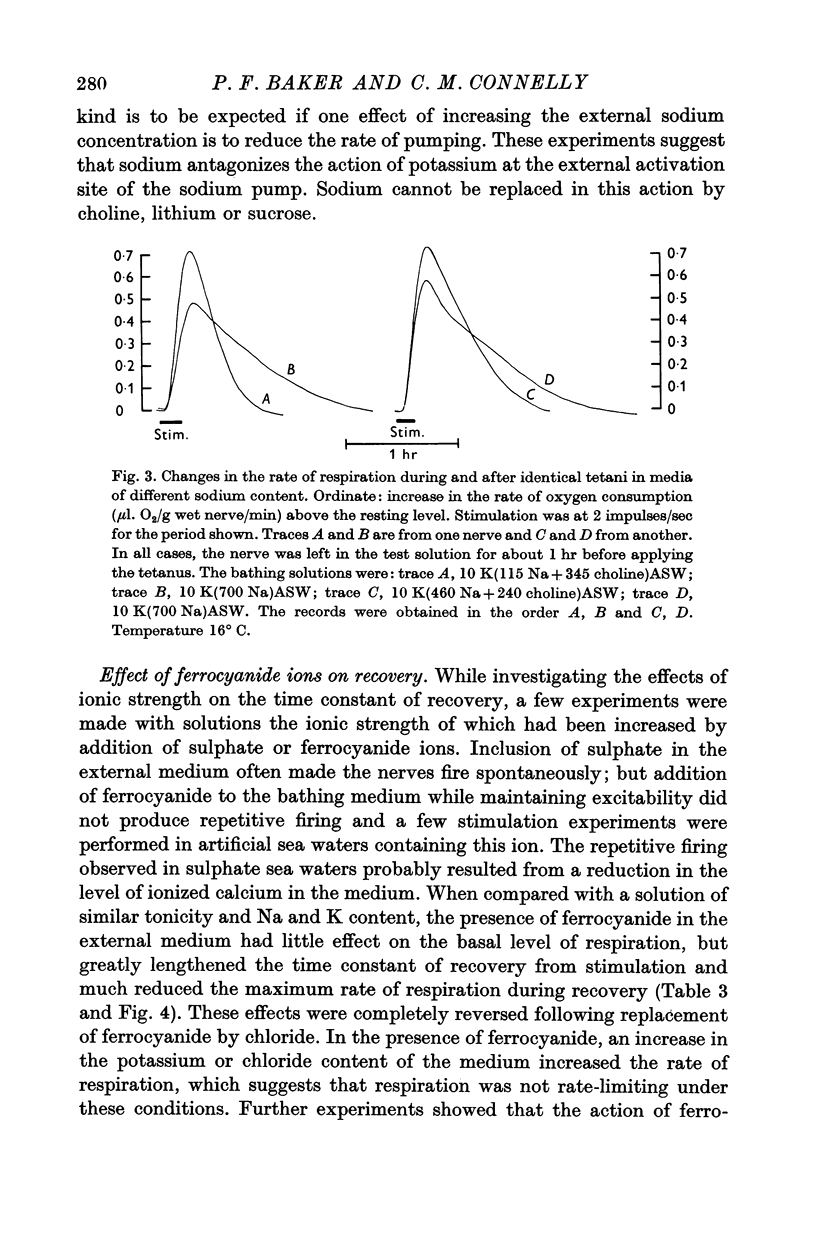
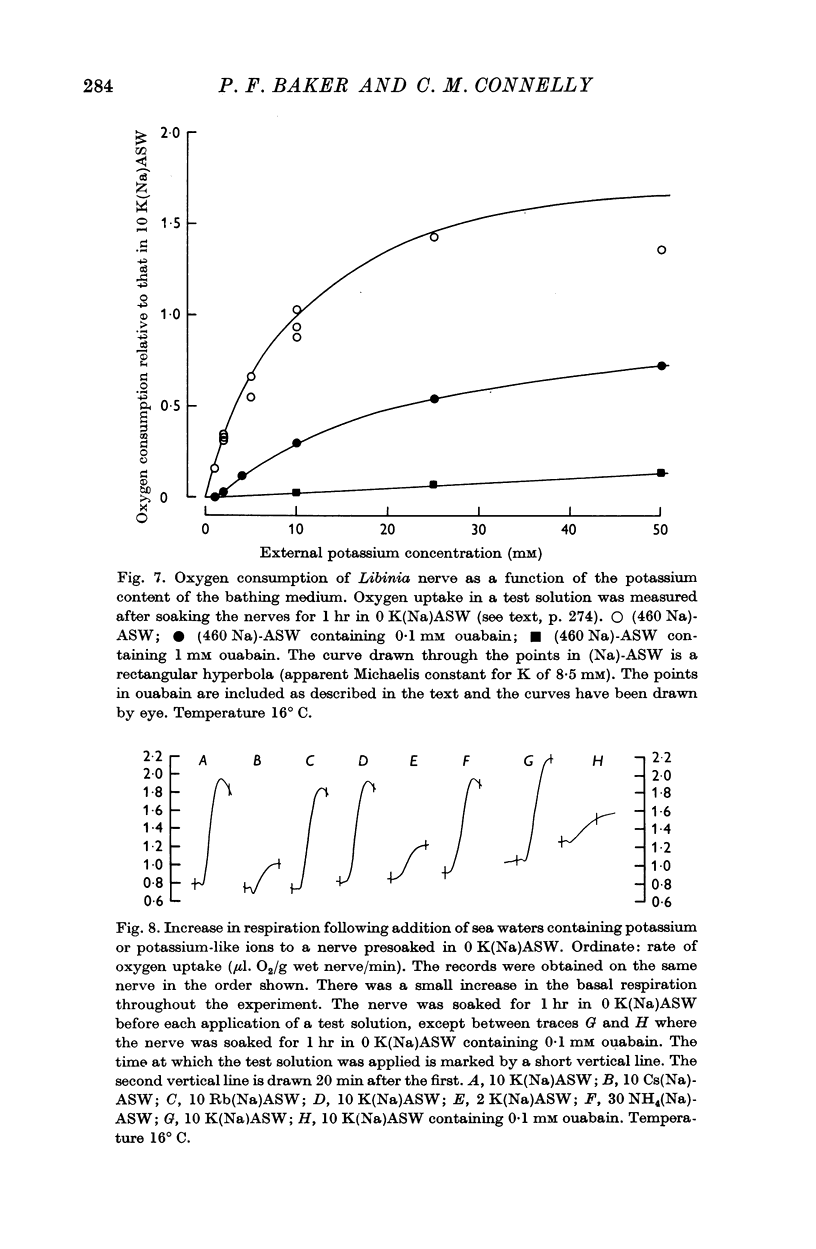
3. The pump was activated by potassium ions in the external medium and this activation was competitively inhibited by external sodium ions. The data are consistent with a Michaelis constant (Km) for external potassium of 1 mM and an inhibitor constant (Ki) for external sodium of 60 mM.
4. In activating the pump, K could be replaced by Tl+, Rb, NH4 and Cs ions; but, of the monovalent ions tested, sodium seemed to be unique in its inhibitory action.
5. In sea waters containing 460 mM-Na, ouabain behaved like a mixed inhibitor of the pump, reducing both the maximum velocity and the apparent affinity for external potassium. At a given ouabain concentration, reducing the sodium content of the medium was without effect on the maximum rate of pumping; but the apparent affinity for potassium increased more steeply than in a ouabain-free solution.
6. The rate of energy utilization associated with pumping was unaffected by inclusion of quite high concentrations of sulphydryl-blocking agents in the external medium.
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F. AN EFFLUX OF NINHYDRIN-POSITIVE MATERIAL ASSOCIATED WITH THE OPERATION OF THE NA+ PUMP IN INTACT CRAB NERVE IMMERSED IN NA+-FREE SOLUTIONS. Biochim Biophys Acta. 1964 Sep 25;88:458–460. doi: 10.1016/0926-6577(64)90208-6. [DOI] [PubMed] [Google Scholar]
- BRINK F., Jr, BRONK D. W., CARLSON F. D., CONNELLY C. M. The oxygen uptake of active axons. Cold Spring Harb Symp Quant Biol. 1952;17:53–67. doi: 10.1101/sqb.1952.017.01.008. [DOI] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Shaw T. I. A comparison of the phosphorus metabolism of intact squid nerve with that of the isolated axoplasm and sheath. J Physiol. 1965 Sep;180(2):424–438. doi: 10.1113/jphysiol.1965.sp007710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. I. Partial inhibition of the active transport of cations in the giant axons of Loligo. J Physiol. 1960 Jul;152:591–600. doi: 10.1113/jphysiol.1960.sp006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRUMENTO A. S., MULLINS L. J. POTASSIUM-FREE EFFECT IN SQUID AXONS. Nature. 1964 Dec 26;204:1312–1313. doi: 10.1038/2041312b0. [DOI] [PubMed] [Google Scholar]
- Furusawa K. The depolarization of crustacean nerve by stimulation or oxygen want. J Physiol. 1929 Jul 25;67(4):325–342. doi: 10.1113/jphysiol.1929.sp002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS R., RODDY P. M., TITUS E. PREPARATION, ASSAY, AND PROPERTIES OF AN NA+- AND K+-REQUIRING ADENOSINE TRIPHOSPHATASE FROM BEEF BRAIN. J Biol Chem. 1965 May;240:2181–2187. [PubMed] [Google Scholar]
- GLYNN I. M. TRANSPORT ADENOSINETRIPHOSPHATASE' IN ELECTRIC ORGAN. THE RELATION BETWEEN ION TRANSPORT AND OXIDATIVE PHOSPHORYLATION. J Physiol. 1963 Nov;169:452–465. doi: 10.1113/jphysiol.1963.sp007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Uncoupling the sodium pump. Nature. 1965 Sep 4;207(5001):1098–1099. doi: 10.1038/2071098a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERKUT G. A., THOMAS R. C. AN ELECTROGENIC SODIUM PUMP IN SNAIL NERVE CELLS. Comp Biochem Physiol. 1965 Jan;14:167–183. doi: 10.1016/0010-406x(65)90017-4. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. SPECTROSCOPIC STUDIES OF FROG MUSCLE DURING SODIUM UPTAKE AND EXCRETION. J Physiol. 1963 Dec;169:862–878. doi: 10.1113/jphysiol.1963.sp007300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. SOME FURTHER OBSERVATIONS ON THE SODIUM EFFLUX IN FROG MUSCLE. J Physiol. 1965 May;178:305–325. doi: 10.1113/jphysiol.1965.sp007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn W. S., Brown R. H. Efficiency of energy conservation in oxidative phosphorylation. Biochim Biophys Acta. 1965 Jul 29;105(1):15–21. doi: 10.1016/s0926-6593(65)80171-0. [DOI] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- SCHATZMANN H. J. THE ROLE OF NA+ AND K+ IN THE OUABAIN-INHIBITION OF THE NA+ + K+-ACTIVATED MEMBRANE ADENOSINE TRIPHOSPHATASE. Biochim Biophys Acta. 1965 Jan 25;94:89–96. doi: 10.1016/0926-6585(65)90011-7. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. Studies on the Na ion and K ion activated ATP hydrolysing enzyme system. The role of SH groups. Biochem Biophys Res Commun. 1963 Jan 18;10:79–84. doi: 10.1016/0006-291x(63)90272-9. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- Wheeler K. P., Whittam R. Structural and enzymic aspects of the hydrolysis of adenosine triphosphate by membranes of kidney cortex and erythrocytes. Biochem J. 1964 Nov;93(2):349–363. doi: 10.1042/bj0930349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. Vectorial aspects of adenosine-triphosphatase activity in erythrocyte membranes. Biochem J. 1964 Nov;93(2):337–348. doi: 10.1042/bj0930337. [DOI] [PMC free article] [PubMed] [Google Scholar]


