Abstract
Competence for genetic transformation of Streptococcus pneumoniae is a transient physiological property inducible by a competence-stimulating peptide (CSP). A 68-kDa CSP-inactivating protein was previously obtained following lithium chloride (LiCl) extraction. By the same protocol, a CSP-inactivating protein was purified and identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry as an endopeptidase, PepO. Analysis of a pepO mutant provided no support for the hypothesis that PepO participates in competence regulation. To reconcile in vitro and in vivo data, we suggest that LiCl treatment results in the release of intracellular molecules, including PepO.
Competence for genetic transformation of the human pathogen Streptococcus pneumoniae is a transient physiological property regulated by a quorum-sensing system encoded by two genetic loci, comCDE and comAB. The 17-amino-acid competence-stimulating peptide (CSP) is the product of the comC gene (7) and is matured and exported through ComA/ComB (10). It acts via the two-component ComD/ComE signal transduction system (17). Genetic evidence strongly suggests that ComD, a histidine kinase of 51 kDa, is the CSP receptor (8). However, Alloing et al. (2) reported the capacity of a ΔcomCDE (noncompetent) mutant to delay the competence of wild-type cells in a mixed-culture experiment, which suggested a possible titration of CSP by a receptor different from ComD. Previous reports also pointed to the possible existence of an additional CSP receptor. Horne et al. (9) isolated from membrane fractions of noncompetent cells a protein of 68 kDa, called activation inhibitory protein (AIP) because of its ability to prevent the induction of competence (activation) following incubation with activator preparations (now known to contain CSP). Moreover, Fujii et al. (6) partially purified this putative receptor from living noncompetent cells by using a lithium chloride (LiCl) extraction protocol believed to cause limited solubilization of cell surface proteins.
In this study, using a similar extraction procedure, we identified the putative CSP receptor of Fujii et al. as a protein 67% identical to the PepO endopeptidase of Streptococcus thermophilus (3) and we investigated its role in competence development.
Release of a putative CSP-inactivating protein (CIP) from noncompetent cells treated with LiCl.
Proteins were released from noncompetent S. pneumoniae R800 wild-type cells by using the LiCl extraction procedure of Fujii et al. (6). Briefly, 2 liters of bacteria (3 × 107 cells per ml) grown in C+Y medium (2) (without bovine serum albumin; pH 7), i.e., under acid growth conditions known to prevent competence induction (14), was harvested by centrifugation after cooling to 4°C and resuspended in 0.1 volume of the same medium containing 2 mM 2-mercaptoethanol, 2 M LiCl, and 50 mM Tris-HCl (pH 7.8) instead of 50 mM K2HPO4. After incubation at 30°C for 30 min, the supernatant was concentrated (approximately 100-fold) by dialysis against buffer A (50 mM Tris-HCl [pH 7.8], 75 mM NaCl, 3 mM 2-mercaptoethanol) with a Centricon Plus-80 Biomax-5 centrifugal filter device (Millipore). Typically, this procedure releases about 2 mg of proteins (hereinafter termed LiCl extract), which represents ∼2 to ∼4% of total proteins.
To evaluate the relative amounts of CIP present in LiCl extracts, an aliquot (1 to 5 μ l) was added to 50 μ l of transformation medium (2) containing CSP (5 ng/ml), incubated for 5 min at 37°C, and mixed with 50 μ l of R243 (comA::ermAM) cell suspension for 15 min at 37°C to allow competence induction (6). Competence levels were measured by transformation with chromosomal DNA carrying a streptomycin resistance (Smr) marker. In our experiments, 1 μ g (∼1 μ l) of LiCl extract decreased the number of transformants to 0.01% of the control level (cells incubated with untreated CSP).
Identical results were obtained with LiCl extracts from R315 cells (R800, but with comCDE deleted), suggesting that ComD was not responsible for the observed effect (data not shown). This result prompted us to further characterize CIP.
Purification of CIP.
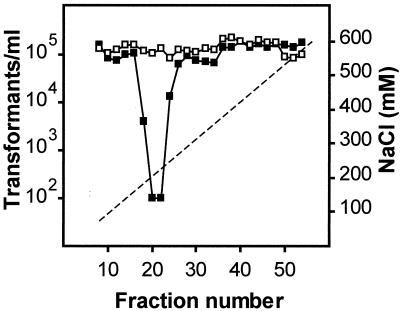
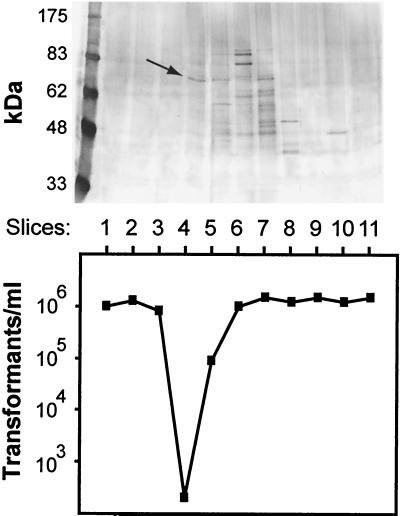
The putative CIP was purified by medium-pressure chromatography. Two milligrams of R800 LiCl extract was applied to an anion-exchange column (UNO Q-1; Bio-Rad). Bound proteins were eluted with 75 to 600 mM NaCl in buffer A. Fractions of 600 μ l were collected, and 5 μ l of every other fraction was assayed for the presence of CIP as described above. Fractions 18 to 24, eluted with 0.17 to 0.23 M NaCl, were shown to inactivate CSP (Fig. 1). These fractions were pooled, and proteins were separated by nondenaturing polyacrylamide gel electrophoresis (PAGE). The gel was sliced and eluted into buffer A. Aliquots were assayed for CIP and analyzed by sodium dodecyl sulfate (SDS)-PAGE. CIP was essentially present in slice 4 (Fig. 2, bottom panel) and appeared after SDS-PAGE as a single major band (apparent molecular mass, 68 kDa), as revealed by colloidal blue staining (Fig. 2, top panel).
FIG. 1.
Fractionation of CIP prepared from noncompetent wild-type (▪) or pepO mutant (□) cells treated with LiCl. Sixty fractions were collected, and every other fraction was assayed for CIP as described in the text. The dashed line indicates the NaCl gradient.
FIG. 2.
Identification of CIP. (Top panel) SDS-PAGE of slices obtained after purification of CIP by nondenaturing PAGE. Staining was performed with colloidal blue stain. The arrow indicates the 68-kDa major band. (Bottom panel) CIP activity profile after nondenaturing PAGE, analyzed by the number of Smr transformants obtained in a comA mutant strain per milliliter.
Identification of the 68-kDa protein as PepO.
The 68-kDa protein was excised from an SDS-polyacrylamide gel and digested with trypsin. The molecular masses of the peptides thus produced were measured by matrix-assisted laser desorption ionization-time of flight mass spectrometric analysis (12) and subsequently matched to entries in a computer-generated database obtained from the in silico trypsin digestion of an S. pneumoniae translated-nucleotide database (http://www.tigr.org). A single entry, SP1647 (18), was found to match the experimental data. The corresponding open reading frame (1,890 nucleotides) was predicted to encode a protein of 630 amino acids. A search of current databases with BLASTP (http://www.ncbi.nlm.nih.gov/BLAST/) revealed that the predicted protein had significant sequence homology to the pfam01431 M13 family of metalloendopeptidases (http://www.sanger.ac.uk/Software/Pfam) found among bacteria and mammals. The highest sequence identities were with the PepO endopeptidases of S. thermophilus (3), Streptococcus parasanguinis (5), and Lactococcus lactis (15) (67, 67, and 44%, respectively). The COOH terminus of the pneumococcal PepO contains the canonical zinc-dependent HEXXH consensus motif (amino acids 478 to 482), as well as a conserved consensus sequence EXXA/GD (amino acids 538 to 542), in which the glutamate serves as the third zinc ligand; both motifs are typical of this M13 peptidase family (19).
PepO has a theoretical isoelectric point of 4.72 and a predicted molecular mass of 71,899 Da, in good agreement with the pI of 4.2 and the molecular mass of 68 kDa determined for AIP by Horne et al. (9). In addition, PepO was eluted from UNO Q-1 with 0.19 M NaCl (see above), a value close to that reported for elution of AIP from DEAE-Sephadex (6). These observations suggested that PepO corresponds to the previously described AIP.
Is PepO a CIP?
To demonstrate that PepO was responsible for the inactivation of CSP by LiCl extracts, a mutant strain was constructed by disrupting the pepO gene by insertion-duplication mutagenesis (4). The insertion-duplication plasmid pKO1969 consisted of an internal region of the pepO gene cloned into a nonreplicative vector, pAS1, carrying an erythromycin resistance (Eryr) marker (16). S. pneumoniae R800 was transformed with pKO1969 DNA, and a representative Eryr transformant was retained as a pepO mutant (strain R361) after verification of its structure by PCR amplification of the chromosome-vector junctions. As shown in Fig. 1, no CIP was detected in LiCl extracts of strain R361. Moreover, examination of fractions 18 to 24 by nondenaturing PAGE did not reveal the presence of a 68-kDa band (data not shown). This led us to conclude that CIP, purified as a 68-kDa species, corresponded to PepO and that PepO was responsible for the inactivation of CSP by LiCl extracts. Our observations are fully consistent with PepO being the AIP previously characterized by Horne et al. (9) and subsequently by Fujii et al. (6). The lactococcal PepO is able to cleave substrates shorter than 20 amino acids at peptidic bonds on the amino side of hydrophobic amino acids, preferentially phenylalanine (F) (13). The CSP contains three F residues (7) and may therefore be a substrate for the pneumococcal PepO. Horne et al. (9) noticed that AIP preparations showed no significant protease activity. In this regard, it is of interest that enzymes of the zinc metalloendopeptidase family function exclusively as oligopeptidases (19).
Role of PepO in S. pneumoniae competence.
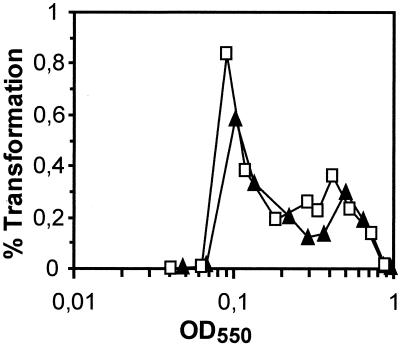
To study the possible involvement of PepO in competence development, we monitored the transformation profiles of cultures of R800 (wild type) and R361 (pepO mutant). Figure 3 shows that a peak of competence occurred at an optical density at 550 nm (OD550) of ∼0.1 for both strains, suggesting that neither quorum sensing nor the timing of competence development was affected in the pepO mutant. Finally, to determine whether PepO was responsible for the delayed competence observed in mixed cultures of wild-type and ΔcomCDE cells by Alloing et al. (2), we constructed a pepO ΔcomCDE mutant and cocultured it with a wild-type strain at a 2:1 or 3:1 ratio. The results were essentially identical to those obtained with mixtures of ΔcomCDE mutant and wild-type cells; i.e., in both cases, the presence of mutant cells delayed, or completely inhibited, the development of competence of the wild-type cells (data not shown). Therefore, PepO does not act as an additional receptor of CSP and could not titrate it. The postulated alternative CSP receptor remains to be identified. Alternatively, the competence delay observed under mixed-culture conditions may result from the existence of a mechanism for sensing growth conditions that impacts comCDE expression, as suggested by Alloing et al. (2).
FIG. 3.
Competence profiles of R800 (wild type) and R361 (pepO mutant). To generate competence profiles of strain R800 (▴) and strain R361 (□), stocks of bacteria grown in Casamino Acids tryptone (CAT) medium (2) to an OD550 of 0.4 were diluted 1/100 in C+Y medium (pH 7.9) and grown at 37°C. Samples (0.1 ml) were withdrawn at 15-min intervals, diluted 10-fold into C+Y medium containing Smr chromosomal DNA, and incubated for 20 min at 30°C. The percentages of cells transformed were plotted relative to OD550s (both strains displayed identical growth curves [data not shown]).
Further discussion and conclusion.
Several pieces of evidence suggest that PepO is indeed the AIP previously detected by Horne et al. (9). First, while we could confirm that a CIP is released from noncompetent wild-type cells by LiCl treatment, no CIP could be recovered from a pepO mutant. Second, PepO was extracted from noncompetent cells by an identical procedure and showed physicochemical parameters, including pI and molecular mass, similar to those previously described for AIP (6, 9). Third, AIP was described to be active against the Streptococcus gordonii (Challis-Wicky) competence factor (9). Examination of the recently determined S. gordonii CSP sequence revealed the presence of two F residues (8), indicating that it could indeed be cleaved by the pneumococcal PepO. Nevertheless, our data do not support the hypothesis that AIP is a surface protein that participates in the control of competence induction. No typical signal sequence for export was found in the predicted sequence of PepO, suggesting that, like its orthologues from S. thermophilus (3), S. parasanguinis (5), and L. lactis (15), it is a cytoplasmic protein.
How could a cytoplasmic protein be released by LiCl? Although LiCl treatment had only a slight effect on cell viability under dilute conditions, as previously reported (6), we observed a significant reduction in colony-forming ability after LiCl treatment under preparative conditions (data not shown). Hussain et al. (11) also observed a decrease in viability in LiCl-treated Staphylococcus epidermidis. They reported a concomitant release of DNA and intracellular proteins and suggested that LiCl treatment, normally used for selective solubilization of cell surface proteins, did not fully preserve cell envelope integrity. Their conclusion that LiCl extraction may not be the method of choice for selective surface molecule extraction from staphylococci can be extended to streptococci.
Can PepO play a role in competence regulation? It has been shown that mammalian metallopeptidases are responsible for the splitting of regulatory neuropeptides, such as metenkephalin (19). Although no regulatory role has yet been demonstrated for bacterial endopeptidases, since PepO could cleave CSP, it may play a physiological role in the development of competence. As a cytoplasmic protein, PepO may affect only nonexported CSP (pre-CSP) or mature CSP internalized through the Ami-Ali oligopeptide permease (1). However, there is so far no evidence that mature CSP is internalized. In addition, the similarity of competence profiles of wild-type and pepO mutant strains (Fig. 3) provides no support for the hypothesis of an intracellular role for PepO in the regulation of competence.
Acknowledgments
We thank Philippe Rousseau for his help with medium-pressure chromatography and Garance Espagno for her participation in some of the experiments.
This research was financed by the Programme de Recherche Fondamentale en Microbiologie, Maladies Infectieuses et Parasitaires and by the European Union (grant BIO4-CT98-0424). Mathieu Bergé was the recipient of a Ph.D. thesis fellowship from the Ministère de la Recherche.
REFERENCES
- 1.Alloing, G., P. de Philip, and J. P. Claverys. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44–58. [DOI] [PubMed] [Google Scholar]
- 2.Alloing, G., B. Martin, C. Granadel, and J. P. Claverys. 1998. Development of competence in Streptococcus pneumoniae: pheromone auto-induction and control of quorum-sensing by the oligopeptide permease. Mol. Microbiol. 29:75–84. [DOI] [PubMed] [Google Scholar]
- 3.Chavagnat, F., J. Meyer, and M. G. Casey. 2000. Purification, characterisation, cloning and sequencing of the gene encoding oligopeptidase PepO from Streptococcus thermophilus A. FEMS Microbiol. Lett. 191:79–85. [DOI] [PubMed] [Google Scholar]
- 4.Claverys, J. P., H. Prats, H. Vasseghi, and M. Gherardi. 1984. Identification of Streptococcus pneumoniae mismatch repair genes by an additive transformation approach. Mol. Gen. Genet. 196:91–96. [DOI] [PubMed] [Google Scholar]
- 5.Froeliger, E. H., J. Oetjen, J. P. Bond, and P. Fives-Taylor. 1999. Streptococcus parasanguis pepO encodes an endopeptidase with structure and activity similar to those of enzymes that modulate peptide receptor signaling in eukaryotic cells. Infect. Immun. 67:5206–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujii, T., D. Naka, N. Toyoda, and H. Seto. 1987. LiCl treatment releases a nickase implicated in genetic transformation of Streptococcus pneumoniae. J. Bacteriol. 169:4901–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Håvarstein, L. S., G. Coomaraswamy, and D. A. Morrison. 1995. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 92:11140–11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Håvarstein, L. S., P. Gaustad, I. H. Nes, and D. A. Morrison. 1996. Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol. 21:865–871. [DOI] [PubMed] [Google Scholar]
- 9.Horne, D., S. Plotch, and A. Tomasz. 1977. Cell surface components implicated as attachment sites for the pneumococcal competence activator, p.11–34. In A. Portolès, R. López, and M. Espinosa (ed.), Modern trends in bacterial transformation and transfection. Elsevier/North-Holland Biomedical Press, Amsterdam, The Netherlands.
- 10.Hui, F. M., L. Zhou, and D. A. Morrison. 1995. Competence for genetic transformation in Streptococcus pneumoniae: organization of a regulatory locus with homology to two lactococcin A secretion genes. Gene 153:25–31. [DOI] [PubMed] [Google Scholar]
- 11.Hussain, M., G. Peters, G. S. Chhatwal, and M. Herrmann. 1999. A lithium chloride-extracted, broad-spectrum-adhesive 42-kilodalton protein of Staphylococcus epidermidis is ornithine carbamoyltransferase. Infect. Immun. 67:6688–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahm, H. W., and H. Langen. 2000. Mass spectrometry: a tool for the identification of proteins separated by gels. Electrophoresis 21:2105–2114. [DOI] [PubMed] [Google Scholar]
- 13.Lian, W., D. Wu, W. N. Konings, I. Mierau, and L. B. Hersh. 1996. Heterologous expression and characterization of recombinant Lactococcus lactis neutral endopeptidase (neprilysin). Arch. Biochem. Biophys. 333:121–126. [DOI] [PubMed] [Google Scholar]
- 14.Martin, B., M. Prudhomme, G. Alloing, C. Granadel, and J. P. Claverys. 2000. Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol. Microbiol. 38:867–878. [DOI] [PubMed] [Google Scholar]
- 15.Mierau, I., P. S. T. Tan, A. J. Haandrikman, J. Kok, K. J. Leenhouts, W. N. Konings, and G. Venema. 1993. Cloning and sequencing of the gene for a lactococcal endopeptidase, an enzyme with sequence similarity to mammalian enkephalinase. J. Bacteriol. 175:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortier-Barrière, I., A. de Saizieu, J. P. Claverys, and B. Martin. 1998. Competence-specific induction of recA is required for full recombination proficiency during transformation in Streptococcus pneumoniae. Mol. Microbiol. 27:159–170. [DOI] [PubMed] [Google Scholar]
- 17.Pestova, E. V., L. S. Håvarstein, and D. A. Morrison. 1996. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21:853–864. [DOI] [PubMed] [Google Scholar]
- 18.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498–506. [DOI] [PubMed] [Google Scholar]
- 19.Turner, A. J., R. E. Isaac, and D. Coates. 2001. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays 23:261–269. [DOI] [PubMed] [Google Scholar]