Abstract
Bacillus anthracis produces the anthrax toxin proteins protective antigen (PA), lethal factor (LF), and edema factor (EF) in a growth phase-dependent manner when cultured in liquid medium. Expression of the toxin genes pagA, lef, and cya peaks in late log phase, and steady-state levels of the toxin proteins are highest during the transition into stationary phase. Here we show that an apparent transition state regulator negatively regulates toxin gene expression. We identified two orthologues of the B. subtilis transition state regulator abrB in the B. anthracis genome: one on the chromosome and one on the 182-kb virulence plasmid pXO1. The orthologue located on the chromosome is predicted to encode a 94-amino-acid protein that is 85% identical to B. subtilis AbrB. The hypothetical protein encoded on pXO1 is 41% identical to B. subtilis AbrB but missing 27 amino acid residues from the amino terminus compared to the B. subtilis protein. Deletion of the pXO1-encoded abrB orthologue did not affect toxin gene expression under the conditions tested. However, a B. anthracis mutant in which the chromosomal abrB gene was deleted expressed pagA earlier and at a higher level than the parent strain. Expression of a transcriptional pagA-lacZ fusion in the abrB mutant was increased up to 20-fold during early exponential growth compared to the parent strain and peaked in mid-exponential rather than late exponential phase. In contrast to the strong effect of abrB on pagA expression, lef-lacZ and cya-lacZ expression during early-log-phase growth was increased only two- to threefold in the abrB null mutant. Western hybridization analysis showed increased PA, LF, and EF synthesis by the mutant. As is true in B. subtilis, the B. anthracis abrB gene is negatively regulated by spo0A. Our findings tie anthrax toxin gene expression to the complex network of postexponential phase adaptive responses that have been well studied in B. subtilis.
Bacteria adjust their physiological state in response to environmental cues, shifting from periods of growth and multiplication to quiescent states and vice versa. In liquid batch culture, changes in growth rate are reflected as the different phases of the bacterial growth curve. Many of the nutrient factors and other environmental parameters that determine growth rate and cause the transition from one growth phase to another are known. However, the regulatory genes and networks controlling gene expression that is specific to certain growth phases are not well understood. Moreover, for pathogenic bacteria multiplying in the environment of a host organism, the significance of growth phase-specific gene expression with regard to pathogenesis is not clear.
Toxin synthesis by Bacillus anthracis, the causative agent of anthrax, peaks during the transition from exponential phase to stationary phase during growth in batch culture. B. anthracis produces three different toxin proteins, protective antigen (PA), lethal factor (LF), and edema factor (EF), encoded by pagA, lef, and cya, respectively (10, 23). The anthrax toxin genes are not part of an operon but are coordinately controlled. Toxin gene expression is highest when the bacterium is cultured at 37°C in a highly buffered synthetic medium containing bicarbonate. In this medium, toxin gene transcript levels and toxin protein levels peak at the end of exponential growth (21, 24, 25, 35).
For an increasing number of pathogenic bacteria, production of toxins and other virulence factors at the end of exponential growth when cells are at a high density has been attributed to quorum sensing. The individual cells of a bacterial population produce a diffusible signal molecule which, after crossing a concentration threshold, interacts with a histidine kinase sensor and a transcriptional activator to induce gene expression (11, 17, 48). To date, there are no reports of B. anthracis toxin gene control by quorum sensing. Under all growth conditions tested in our laboratory, addition of cell-free, spent media to B. anthracis cultures did not affect toxin gene expression or expression of the trans-acting virulence gene regulator atxA (7, 8, 44; T. M. Koehler, unpublished data).
Growth rate-dependent gene expression that appears to be unlinked to diffusible chemical signals has been studied in only a few bacteria. In Streptococcus pyogenes, the trans-acting regulatory protein Mga activates transcription of several genes, including emm (which encodes M protein) and scpA (which encodes C5a peptidase). Genes regulated by Mga are expressed in the exponential but not in the stationary phase. Transcription of mga itself is growth rate dependent: expression is maximal in exponential phase but decreased upon entry into stationary phase (26). The gene encoding the nucleoid-associated protein Fis in Escherichia coli has a very similar expression profile (5) and has recently been implicated in the regulation of Salmonella enterica serovar Typhimurium SPI-1 invasion genes (30, 49). In the nonpathogenic soil bacterium Bacillus subtilis, regulatory proteins that control growth phase-dependent gene expression have been termed “transition state regulators” (31, 40). The best studied of these is AbrB (37). AbrB is a pleiotropic regulator of gene expression with a role in mediating the transition from a quiescent state to one of growth and cell division and the transition from active growth into stationary phase (29).
In the current model for B. anthracis-host interaction during inhalation anthrax disease, bacterial spores enter the lungs via normal respiration. Once in the lung, the spores are phagocytosed by alveolar macrophages (32). Some B. anthracis spores survive the hostile environment of the macrophage and are transported to the mediastinum, which is the initial site of infection. The spores then germinate in the macrophages, becoming metabolically active vegetative cells. Ultimately, vegetative cells enter the bloodstream, reaching concentrations of up to 108 cells per ml (12, 13). Guidi-Rontani et al. (15) have reported that the toxin genes, pagA, lef, and cya, and the positive regulator atxA are expressed by vegetative B. anthracis cells shortly after germination in cultured macrophages. In studies using the murine macrophage-like cell line RAW264.7 infected with B. anthracis strains harboring transcriptional lacZ fusions, the toxin genes and atxA are expressed within 3 h after infection. The role of the toxin proteins at this very early stage of infection is not clear, but a report by Dixon et al. (9) indicates that toxin synthesis in the macrophage may have a role in release of the bacterium from the eukaryotic cell.
Anthrax toxin gene expression shortly after B. anthracis spores are phagocytosed by macrophages indicates that toxin synthesis occurs when the bacterium is transitioning from the dormant spore state to one of active growth. We hypothesized that the timing of toxin gene expression by B. anthracis is controlled by a transition state regulator, such as AbrB of B. subtilis. We searched the B. anthracis plasmid sequence databases and the incomplete chromosome sequence database of The Institute for Genomic Research (TIGR) for abrB orthologues in B. anthracis. We identified two potential abrB genes, one located on the pathogenicity island of pXO1 and another on the B. anthracis chromosome. Here we show that the chromosomal abrB gene is involved in timing of toxin gene expression in B. anthracis during batch culture. Evidence for the involvement of an abrB orthologue in the regulation of toxin synthesis in B. anthracis is the first report tying virulence factor expression to the regulatory network controlling the transition of bacterial cells into and out of exponential growth.
MATERIALS AND METHODS
Growth conditions.
E. coli strains were grown in Luria-Bertani (LB) broth (2) and used as hosts for cloning. For extraction of chromosomal DNA, the B. anthracis strains were grown in brain heart infusion medium (BHI) (Difco, Detroit, Mich.). For extraction of pXO1 DNA, BHI was amended with 10% horse serum. For transductions and electroporations, B. anthracis strains were grown in BHI containing 0.5% glycerol. Culture supernatant samples and cell extracts for Western hybridization experiments were obtained from B. anthracis cultures grown in LB broth buffered with 100 mM HEPES (pH 8.0) and 0.8% (wt/vol) sodium bicarbonate, in an atmosphere containing 5% CO2 at 37°C. Cells for β-galactosidase assays were grown in buffered LB at 37°C in a 5% CO2 atmosphere. The following antibiotics were purchased from Sigma-Aldrich (St. Louis, Mo.) or Fisher Scientific and added to media (concentrations are indicated in parentheses) when appropriate: ampicillin (100 μg/ml), erythromycin (5 μg/ml), kanamycin (20 μg/ml), lincomycin (25 μg/ml), spectinomycin (100 μg/ml), and tetracycline (5 μg/ml).
DNA isolation and manipulation.
A method for extraction of plasmid DNA from B. anthracis cultures has been described (14). For isolation of chromosomal DNA, the protocol was slightly modified by omitting the centrifugation step that is intended to remove chromosomal DNA from plasmid DNA. Preparation of plasmid DNA from E. coli, transformation of E. coli, and recombinant DNA techniques were carried out by standard procedures (2). B. anthracis was electroporated with unmethylated plasmid DNA from E. coli GM1684 as described elsewhere (21). Restriction enzymes, T4 ligase, and Taq polymerase were purchased from Promega (Madison, Wis.) or Gibco BRL (Rockville, Md.).
Strain construction.
Strains, plasmids, and their relevant characteristics are listed in Table 1. Oligonucleotide primers are listed in Table 2. B. anthracis abrB (UT166), pXO1 open reading frame (ORF) 105 (UT141), and spo0A (UT157) mutants were constructed by replacement of coding sequences with an omega element conferring spectinomycin resistance, Ω-sp, using a method described previously (8). Generally, the Ω-sp element and DNA sequences directly upstream and downstream of the target gene were cloned into the vector pUTE29 such that B. anthracis sequences flanked the Ω-sp element. pUTE29 replicates in E. coli and B. anthracis and confers tetracycline resistance to both species but is unstable in B. anthracis when strains are grown without selection. pUTE29-derived constructs were electroporated into B. anthracis with selection for tetracycline resistance. Electroporants were inoculated into LB broth without antibiotics, incubated at 37°C, and transferred to fresh medium two times daily for several days. The cultures were screened for spectinomycin-resistant tetracycline-sensitive clones. Isolates in which a double-crossover recombination event had occurred were confirmed by PCR.
TABLE 1.
Plasmids and strains used in this study
| Plasmid or strain | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Plasmids | ||
| B. anthracis pXO1 | Tox+ | 42 |
| E. coli | ||
| pBSIIKS+ | Apr | Stratagene |
| pGEM-T Easy | Apr | Promega |
| pJRS312 | pUC18 carrying an Ω element with spectinomycin resistance marker aad9 (Ω-sp); Spr | J. R. Scott |
| pUTE387 | 1.9-kb product of inverse PCR (with ES2-ES3) containing 5′end of abrB and upstream sequences cloned into pGEM-T Easy; Apr | This work |
| pUTE460 | 556-bp PCR product (with ES4-ES16) containing abrB cloned into pGEM-T Easy; Apr | This work |
| pUTE474 | 1.9-kb SacII-PstI fragment from pUTE387 containing 5′ end of abrB and upstream sequences cloned into pBSIIKS+; Apr | This work |
| pUTE475 | 556-bp fragment from pUTE460 containing abrB cloned into pBSIIKS+; Apr | This work |
| Bifunctional | ||
| pHT304-18Z | Contains promoterless lacZ; Apr in E. coli, Emr in B. anthracis | 1 |
| pUTE29 | Apr in E. coli, Tcr in B. anthracis | 21 |
| pUTE379 | 2.3-kb BamHI fragment containing Ω-sp from pJRS312 flanked by (i) a 1.2-kb PCR product containing 3′ end of ORF 105 and downstream sequences (EB1-EB2) and (ii) a 1.3-kb PCR product containing 5′ end of ORF 105 and upstream sequences (EB3-EB4) cloned into pUTE29; Spr Tcr; used to construct UT141 ΔORF 105pXO1 | This work |
| pUTE408 | 2.3-kb BamHI fragment containing Ω-sp from pJRS312 flanked by (i) a 0.8-kb PCR product containing 3′ end of spo0A and downstream sequences (YC24/YC25) and (ii) a 1.0-kb PCR product containing 5′ end of spo0A and upstream sequences (YC22-YC23) cloned into pUTE29; Spr Tcr; used to construct UT157 Δspo0A | This work |
| pUTE411 | 1.2-kb PCR product containing promoter region of atxA (YC9-YC10) cloned into multiple cloning site of pHT304-18Z; atxA::lacZ; Apr Emr | This work |
| pUTE416 | 2.3-kb BamHI fragment containing Ω-sp from pJRS312 and 1.9-kb PCR product (ES8-M13fw, pUTE474 template) containing 5′end of abrB and upstream sequences cloned into pUTE417; Spr Tcr; used to construct UT166 ΔabrB | This work |
| pUTE417 | 1.6-kb BamHI-PstI PCR product (ES9-ES10) containing 3′end of abrB and downstream sequences cloned into pUTE29; Apr Tcr | This work |
| pUTE441 | 559-bp PCR product containing promoter region of abrB (ES21-ES22) cloned into multiple cloning site of pHT304-18Z; abrB::lacZ; Apr Emr | This work |
| pUTE448 | BamHI-SacI fragment from pUTE475 containing abrB cloned into pUTE29; Apr Tcr | This work |
| Strains | ||
| B. anthracis | ||
| RBAF140c | pagA-lacZ integrated adjacent to pagA locus; Emr Kmr | 35 |
| RBAF143c | lef-lacZ integrated adjacent to lef locus; Emr Kmr | 35 |
| RBAF144c | cya-lacZ integrated adjacent to cya locus; Emr Kmr | 35 |
| UM44b | pXO1+ Tox+ Ind− | 42 |
| UM44-1C9b | Plasmid-cured derivative of UM44; pXO1− Tox− Ind− Strr | C. Thorne |
| UT53b | UM44 ΔatxA; atxA is replaced by Ω km-2; Kmr | 8 |
| UT141b | UM44 electroporated with pUTE379; ΔORF 105pXO1; Ind− Spr | This work |
| UT157b | UM44 electroporated with pUTE408; Δspo0A; Ind− Spr | This work |
| UT166b | UM44 electroporated with pUTE416; ΔabrB; Ind− Spr | This work |
| UT168c | RBAF140 transduced with CP51 propagated on UT166; pagA::lacZ, ΔabrB; Emr Kmr Spr | This work |
| UT169c | RBAF143 transduced with CP51 propagated on UT166; lef::lacZ, ΔabrB; Emr Kmr Spr | This work |
| UT170c | RBAF144 transduced with CP51 propagated on UT166; cya::lacZ, ΔabrB; Emr Kmr Spr | This work |
| UT182c | RBAF140 transduced with CP51 propagated on UT141; pagA::lacZ, ΔORF 105pXO1; Emr Kmr Spr | This work |
| UT183c | RBAF143 transduced with CP51 propagated on UT141; lef::lacZ, ΔORF 105pXO1; Emr Kmr Spr | This work |
| UT184c | RBAF144 transduced with CP51 propagated on UT141; cya::lacZ, ΔORF 105pXO1; Emr Kmr Spr | This work |
| E. coli | ||
| DH5αF′ | F′ endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15] | 50 |
| GM1684 | F′ F-lacIq DM15 pro+/dam-4 Δ(lac-pro)X111thi-1 glnV44 (relA1) | R. Kolter |
| JM109 | F′ traD36 proAB23+lacIqlacZΔM15/ recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB) mcrA | 53 |
| TG1 | F′ traD36 lacIq Δ(lacZ)M15 proA+B+/supE Δ (hsdM-mcrB)5 (rK− mK+mcrB) thi Δ(lac-proAB) | 33 |
Abbreviations: Apr, ampicillin resistant; Emr, erythromycin resistant; Ind, indole; Kmr, kanamycin resistant; Spr, spectinomycin resistant; Strr, streptomycin resistant; Tcr, tetracycline resistant; Tox, anthrax toxin; pBSIIKS+, pBluescript II KS(+).
Weybridge strain derivative.
Sterne strain derivative.
TABLE 2.
Primers used in this studya
| Primer | Sequence (5′-3′)b (location) | Relevant property | Restriction site |
|---|---|---|---|
| EB1 | TTAGGACATGAGCTCATTATGACGACGG (from bp 1138 to 1111 after ORF 105 stop codon) | Outer primer used to amplify ORF 105 3′ region | SacI |
| EB2 | GCGGATCCGAAATCCAGCAACACTTAGTGAAGTAA (from bp 26 to 0 before ORF 105 stop codon) | Inner primer used to amplify ORF 105 3′ region | BamHI |
| EB3 | GCGGATTCCTACAATTCCTCATGTTCTTC (from bp 11 to −7 from ORF 105 start codon) | Inner primer used to amplify ORF 105 5′ region | BamHI |
| EB4 | CATTAAAGTCTTCACGTCGAAGATCAGC (from bp −1335 to −1308 from ORF 105 start codon) | Outer primer used to amplify ORF 105 5′ region | |
| ES2 | CACCAGTTACTTGGCAAGTCATG (from bp 174 to 152 after abrB start codon) | Outward facing primer used for inverse PCR to amplify abrB 5′ region | |
| ES3 | CATCGCGTTTCGACAAATCTCG (from bp 55 to 76 after abrB stop codon) | Outward facing primer used for inverse PCR to amplify abrB 5′ region | |
| ES4 | CCTGCTCCAGAAGAACAATGGT (from bp 190 to 169 after abrB stop codon) | Used to amplify abrB | |
| ES8 | ATGGATCCGTACGGCGTAATTCGATTGG (from bp 73 to 54 after abrB start codon) | Inner primer used to amplify abrB 5′ region | BamHI |
| ES9 | GCCGGATCCAAGCAAAGAAGGCGCTGA (from bp 61 to 44 before abrB stop codon) | Inner primer used to amplify abrB 3′ region | BamHI |
| ES10 | ATACTGCAGATCCGCCTTATTTCCAAACG (from bp 1523 to 1504 after abrB stop codon) | Outer primer used to amplify abrB 3′ region | PstI |
| ES16 | GTCCATAGGGTATTTTGTCGAA (from bp −84 to −52 to abrB start codon) | Used to amplify abrB | |
| ES21 | GCCAAGCTTGTTAATGCGTGATAAAA (from bp −320 to −300 to abrB start codon) | Used to amplify abrB promoter | HindIII |
| ES22 | ATGGATCCCTCAGCGCCTTCTTTGCTTA (from bp 239 to 220 after abrB start codon) | Used to amplify abrB promoter | BamHI |
| M13 Fw (−20) | GTAAAACGACGGCCAGTG | ||
| YC9 | AAAAAAGCTTATAACCCCCCTTACAATC (from bp −870 to −843 to atxA start codon) | Used to amplify atxA promoter | HindIII |
| YC10 | ATAAGGATCCTACTTGTAAGTGGAG (from bp 364 to 340 to atxA start codon) | Used to amplify atxA promoter | BamHI |
| YC22 | ATCGGTACCATGACATTTGTTCATCC (from bp −884 to −859 from spo0A start codon) | Outer primer used to amplify spo0A 5′ region | KpnI |
| YC23 | ATAGGATCCAATACGAGTACATCCG (from bp 209 to 185 from spo0A start codon) | Inner primer used to amplify spo0A 5′ region | BamHI |
| YC24 | TTCGGATCCGCAACAACAGTAGATGG (from bp 386 to 361 before spo0A stop codon) | Inner primer used to amplify spo0A 3′ region | BamHI |
| YC25 | TTCCTGCAGTATACTCCTCAATCCG (from bp 454 to 430 after spo0A stop codon) | Outer primer used to amplify spo0A 3′ region | PstI |
Purchased from Sigma Genosys or IDT.
Restriction enzyme recognition sites are underlined.
Mutations were transduced between B. anthracis strains using a temperature-sensitive mutant of the generalized transducing phage CP51 as described previously (19, 43). The genotypes of transductants were confirmed by PCR.
Cloning of the abrB locus.
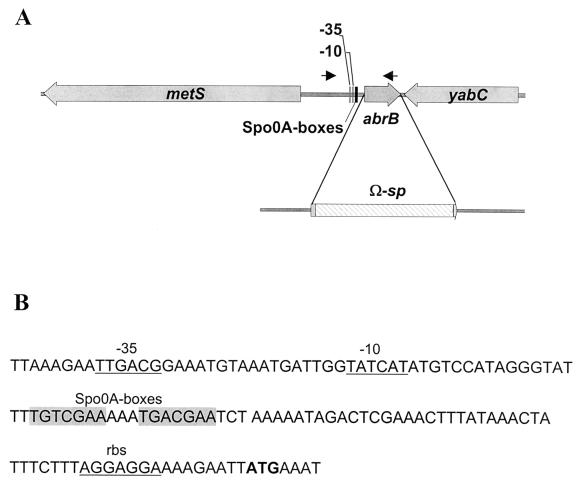
When this work was initiated, only partial sequence of the abrB locus was available in the TIGR database (http://www.tigr.org). The database revealed a DNA sequence containing 215 nucleotides (nt) of the 3′ end of the abrB coding sequence and 512 nt downstream of the gene. We cloned the 5′ end of the abrB gene and upstream sequences using the inverse PCR. B. anthracis chromosomal DNA was digested with EcoRI, for which there are no restriction sites in the target sequence; ligated; and used as a template for amplification with the oligonucleotides ES2 and ES3 (Table 2). The PCR product was cloned into pGEM-T Easy, giving rise to pUTE387, and sequenced with an Applied Biosystems PRISM 377 sequencer. A SacII-PstI fragment was subcloned into pBluescript II KS(+), generating pUTE474. pUTE474 was used as a template for PCR amplification with oligonucleotides ES8 and M13 forward. The PCR product was digested with BamHI and SacI. This fragment and the Ω-sp element from pJRS312 were cloned into pUTE417, giving rise to pUTE416. Thus, in pUTE416 the 2.3-kb Ω-sp cassette is flanked by 1.9 kb of DNA upstream of abrB, including 73 nt from the 5′ start of the abrB gene, and 1.6 kb of DNA downstream of abrB, including 61 nt from the 3′ end of the gene (Fig. 2A).
FIG. 2.
(A) Schematic representation of the abrB region of the B. anthracis chromosome and replacement of 148 nt in the central portion of the 282-nt abrB coding region. The 73 nt at the 5′ end and 61 nt at the 3′ end of the gene are separated by an Ω-sp element. Black arrows mark the position and orientation of the primers used for PCR amplification of the region which was cloned into pHT304-18Z to generate a transcriptional abrB::lacZ fusion. (B) Nucleotide sequence at the 5′ end of the abrB coding sequence on the B. anthracis chromosome. Predicted areas of contact between the promoter sequence and the ςA subunit of RNA polymerase (−35, −10) and the ribosome binding site (rbs) are underlined. The predicted translational start codon is shown in bold type. The hypothesized binding sites for Spo0A are shaded.
β-Galactosidase assays.
Strains were cultured overnight at 28°C, with shaking at 250 rpm, in 50 ml of LB broth with appropriate antibiotics and 0.5% glycerol to suppress sporulation. One milliliter of the overnight culture was pelleted, and the cells were resuspended in 1 ml of LB broth. A 50-ml flask containing buffered LB broth was inoculated with this bacterial suspension to achieve an initial optical density at 600 nm (OD600) of 0.04 to 0.08. Cultures were incubated at 37°C in 5% CO2 with stirring (400 rpm). OD600 was measured at 1-h intervals, and duplicate samples were collected for β-galactosidase assays. The samples were frozen at −20°C until assaying for enzyme activity. β-Galactosidase assays were performed as described by Miller (27), using toluene to permeabilize the cells. At least three independent cultures were assayed for enzyme activity. The figures show data from representative experiments.
Western blot analysis.
Strains were cultured overnight and diluted into buffered LB broth the following morning as described above. Culture supernatant samples were collected and filtered through 0.2-μm-pore-size syringe filters. The samples were frozen immediately at −20°C. For detection of PA, LF, and EF, samples were applied to nitrocellulose membranes using vacuum blotting with a slot blot apparatus (Hoefer Scientific, San Francisco, Calif.). The membranes were blocked for 2 h with TBS-T (20 mM Tris base, 137 mM NaCl, 0.1% Tween 20 [pH 7.6]) containing 5% milk at 4°C. Subsequently, the membranes were incubated with rabbit anti-PA, anti-LF, or anti-EF serum diluted in TBS-T-5% milk overnight at 4°C. Membranes were washed in TBS-T and reacted with donkey anti-rabbit immunoglobulin G antibody conjugated to horseradish peroxidase (diluted 1:5,000 in TBS-T-5% milk) for 1 h at room temperature. Immunoreactive material was visualized on autoradiographs with enhanced chemiluminescence immunoblotting (Amersham). The autoradiographs were analyzed with the Scion Image Beta 4.02 software.
To investigate the accumulation of AbrB protein during growth, extracts were prepared from cells harvested at early exponential phase (OD600, 0.08 to 0.2), mid-exponential phase (OD600, 0.5 to 0.7), and stationary phase (OD600, 1.3 to 1.5). Cells were boiled for 10 min in sample buffer (63 mM Tris OH, 10% glycerol [vol/vol], 1% SDS [wt/vol)], 5% β-mercaptoethanol [vol/vol] [pH 6.8]). Protein concentrations were determined by using the Bio-Rad protein assay reagent with bovine serum albumin (Sigma) as the standard. Samples containing 15 μg of protein were resolved on 4 to 20% Tris-HCl Ready Gels (Bio-Rad) and transferred with a Semi-Dry Electroblotter (CLP) to Optitran nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) using Towbin buffer (0.025 M Tris base, 0.192 M glycine, 20% methanol [pH 8.3]). The membranes were treated as described above using rabbit antiserum raised against B. subtilis AbrB at a dilution of 1:1,000 in TBS-T-5% milk.
RESULTS
The B. anthracis genome harbors two apparent abrB genes.
BLAST searches of the preliminary B. anthracis chromosome sequence released by TIGR (http://www.tigr.org) and of the B. anthracis virulence plasmids pXO1 (28) and pXO2 (GenBank accession no. AF188935) revealed two predicted peptides highly homologous to B. subtilis AbrB. The hypothetical protein encoded by ORF 105 of pXO1, located 2.2 kb upstream of lef and within the boundaries of a pathogenicity island, is 41% identical and 64% similar to AbrB in B. subtilis. The predicted protein encoded by a chromosomal ORF is 85% identical and 93% similar to B. subtilis AbrB. Figure 1 shows an alignment of the predicted amino acid sequences of AbrB from several Bacillus species. All of the chromosomally encoded proteins (from B. subtilis, B. anthracis, Bacillus stearothermophilus, Bacillus halodurans, and Bacillus cereus) are highly conserved, whereas the hypothetical protein encoded by ORF 105 of B. anthracis plasmid pXO1 is lacking the first 27 residues of the N-terminal domain. In the B. subtilis protein, residues 1 to 53 are involved in DNA binding (46, 47, 52) and mutations of Arg23 and Arg24 result in loss of DNA-binding activity (39, 51). Since the hypothetical peptide encoded by ORF 105 is truncated in its potential DNA-binding domain, we surmise that a protein resulting from expression of ORF 105 would be nonfunctional.
FIG. 1.
Comparative analysis of AbrB peptide sequences. Black background indicates identical residues. Gray background signifies similar residues. The sequences were aligned with the procedure PILEUP in the Genetics Computer Group software package and plotted using BOXSHADE. Asterisks indicate amino acids that have been assigned a critical role following mutational analysis of the B. subtilis gene. Abbreviations: Bs, B. subtilis 168; Bachr, B. anthracis Ames chromosome; Bha, B. halodurans C-125; Bst, B. stearothermophilus ATCC 7953; Bc, B. cereus; BapXO1, B. anthracis Sterne pXO1.
In the B. subtilis genome, the abrB gene is flanked by the divergently transcribed metS and yabC genes (22). The sequence information from the B. anthracis chromosome shows identical organization (Fig. 2A). The DNA sequence immediately upstream of abrB on the chromosome of B. anthracis is comparable to the promoter region of the B. subtilis abrB gene (31). The region has the elements of a ςA-dependent promoter and contains Spo0A boxes identical to the ones found in B. subtilis (Fig. 2B) (18).
abrB is a negative regulator of toxin gene expression.
To determine whether the B. anthracis abrB orthologues affect anthrax toxin gene expression, we created abrB null mutations in strains harboring toxin gene promoter-lacZ transcriptional fusions. Internal abrB sequences were deleted and replaced with Ω-sp. The Ω-sp DNA cassette harbors the spectinomycin resistance gene aad9 flanked on both sides by T4 transcription terminators and translational stop codons in all three reading frames. We measured β-galactosidase activity of the parent and abrB null mutants following growth at 37°C in a 5% CO2 atmosphere.
B. anthracis RBAF140 (35) carries a pagA::lacZ transcriptional fusion adjacent to the intact pagA locus. Strain UT168 was derived from RBAF140 and carries the chromosomal abrB null mutation depicted in Fig. 2A. As shown in Fig. 3, the parent and mutant strains grew similarly, yet expression of the reporter gene fusion in the abrB null mutant was up to 20-fold greater than that of the parent strain. The mutant exhibited a large increase in pagA::lacZ expression during early- and mid-exponential-phase growth, peaking in mid-exponential phase. β-Galactosidase activity of the parent strain increased more gradually, and peak levels were obtained upon entry into stationary phase. When a plasmid-encoded copy of abrB was introduced into UT168, pagA::lacZ expression was reduced to levels lower than those of the parent strain. The overcompensation was most likely due to the high copy number of the plasmid.
FIG. 3.
Growth curves (A) and β-galactosidase activity of pagA::lacZ (B) for parent strain RBAF140 (▪), abrB mutant UT168 (▴), abrB-complemented strain UT168(pUTE448) (○), and control UT168(pUTE29) (✻).
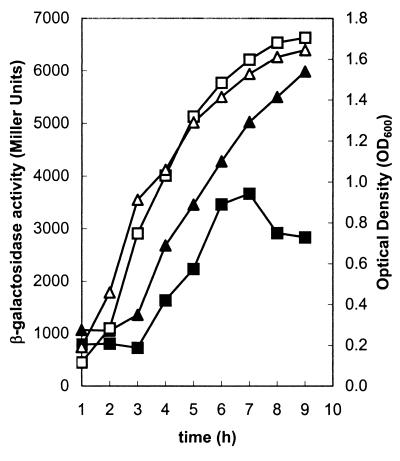
The chromosomal abrB null mutation exhibited a similar but less pronounced effect on expression of lef::lacZ and cya::lacZ transcriptional fusions. Figure 4 shows the β-galactosidase activities of the reporter strains RBAF143 (lef::lacZ) and RBAF144 (cya::lacZ) and the respective abrB mutants UT169 and UT170. The increase in reporter gene expression in the abrB mutants was only two- to threefold compared to the parent strains. However, we found consistently that the maximum difference between chromosomal abrB mutant and parent strains occurred during the first 5 h of growth, subsequent to dilution of stationary-phase cells into fresh medium. Thus, similar to the effect of the chromosomal abrB null mutation on expression of the pagA-lacZ fusion, lef-lacZ and cya-lacZ expression in abrB mutants was most elevated during early and mid-exponential growth phases.
FIG. 4.
Growth curves (open symbols) and β-galactosidase activity of toxin gene promoter-lacZ fusions (solid symbols). (A) Growth of parent RBAF143 (□) and abrB mutant UT169 (▵) and lef::lacZ expression of parent RBAF143 (▪) and abrB mutant UT169 (▴). (B) Growth of parent RBAF144 (□) and abrB mutant UT170 (▵) and cya::lacZ expression of parent RBAF144 (▪) and abrB mutant UT170 (▴).
To determine whether the abrB orthologue ORF 105 on pXO1 affected toxin gene expression, we constructed an ORF 105 deletion mutation in the toxin gene promoter-lacZ fusion strains. ORF 105 null mutants harboring a 166-nt internal deletion (Table 1) and carrying the wild-type chromosomal abrB gene were unaffected for toxin gene expression under the conditions tested (data not shown). Thus, of the two abrB orthologues found in the genome, only the chromosomal gene affects toxin gene expression.
abrB affects toxin protein levels.
When B. anthracis is grown in buffered LB broth at 37°C in 5% atmospheric CO2, toxin production by this bacterium is growth phase dependent. Levels of PA, LF, and EF peak during late exponential and early stationary phase (21, 24, 25). To determine whether elevated toxin gene transcription in the abrB null mutant resulted in a change in toxin protein synthesis, we assessed toxin levels in supernatants from cultures of parent and abrB null strains. Levels of the toxin proteins in supernatants from mid-exponential-phase cultures were examined using immunoblotting. Figure 5 shows that supernatant from a UT166 culture (abrB null mutant) contained significantly more PA, LF, and EF than supernatant from a culture of the parent strain, UM44. When plasmid pUTE448, harboring abrB, was introduced into the abrB null mutant, toxin protein levels were reduced to the amount detected for the parent strain UM44. UT166 containing the vector alone (pUTE29) produced toxin protein levels slightly below the ones for UT166 without the plasmid. Supernatant from a culture of the toxin-negative, pXO1-cured strain UM44-1C9 showed only very low levels of cross-reactive material.
FIG. 5.
Western blot analysis demonstrating abrB control of toxin production. Supernatant samples from mid-exponential-phase cultures were probed with antitoxin antisera as indicated. Lanes: UM44, parent; UT166, abrB mutant; UT166(pUTE448), abrB-complemented strain; UT166(pUTE29), abrB mutant; UM44-1C9, pX01−.
Negative regulatory effect of abrB on atxA::lacZ expression.
The main trans-acting regulator of anthrax toxin gene expression is the pXO1-encoded gene atxA. Transcription of all three toxin genes is decreased dramatically in an atxA null mutant (8); however, the molecular basis for atxA-dependent gene expression is not known. To determine whether an epistatic relationship exists between abrB and atxA, we monitored expression of atxA::lacZ and abrB::lacZ transcriptional fusions borne on a low-copy-number plasmid (4 ± 1 copies/chromosome) in parent and mutant strains grown under the same conditions used above.
Expression of the abrB::lacZ transcriptional fusion was unaffected by the presence of atxA. In repeated experiments, the β-galactosidase activity of the parent and atxA null strains harboring the plasmid-borne reporter (pUTE441) did not differ significantly throughout growth (Fig. 6A). Thus, abrB expression is independent of atxA.
FIG. 6.
(A) Growth curves for parent strain UM44 (□) and atxA mutant UT53 (▵), carrying a plasmid-encoded abrB::lacZ fusion, pUTE441, and β-galactosidase activity of UM44(pUTE441) (▪) and UT53(pUTE441) (▴). (B) Growth curves for parent strain UM44 (□) and abrB mutant UT166 (▵), carrying a plasmid-encoded atxA::lacZ fusion, pUTE411, and β-galactosidase activity of UM44(pUTE411) (▪) and UT166(pUTE411) (▴).
Expression of the atxA::lacZ fusion (pUTE411) was slightly elevated in the abrB null mutant compared to the parent strain (Fig. 6B). The expression profile was highly reproducible, with a distinct peak 4 h after diluting cells of an overnight culture into fresh medium. The peak at the 4-h time point coincides with the strong derepression of pagA observed during early exponential growth of the ΔabrB pagA::lacZ mutant. We conclude that abrB has a negative regulatory effect on atxA.
abrB is spo0A regulated.
In B. subtilis, abrB mRNA can be detected during the lag phase of growth in batch culture. Transcript levels peak early in exponential phase and become undetectable in mid-exponential phase. The repression of abrB expression, beginning in exponential phase, is primarily due to negative control by phosphorylated Spo0A. As Spo0A-P levels increase, AbrB protein levels decrease. spo0A mutants exhibit high levels of abrB mRNA and AbrB protein in exponential phase and during the transition into stationary phase (29, 41).
Expression of the abrB::lacZ fusion was observed throughout growth in B. anthracis batch culture (Fig. 7). The presence of two well-conserved apparent Spo0A-binding sites upstream of the abrB-coding sequence suggested that the B. anthracis gene may be regulated in a manner similar to that of the B. subtilis abrB (4, 31, 36). The B. anthracis spo0A gene was identified previously by Brown et al. (6). We constructed a B. anthracis spo0A null mutant, UT157, by replacing an internal fragment of the spo0A gene with Ω-sp. As shown in Fig. 7, abrB::lacZ expression by UT157 did not decrease in mid-exponential and stationary phase, analogous to the situation described for a B. subtilis Δspo0A mutant (29).
FIG. 7.
Growth curves (open symbols) and abrB::lacZ expression (solid symbols) of parent strain UM44(pUTE441) (squares) and spo0A null mutant UT157(pUTE441) (triangles).
Relative AbrB protein levels in the B. anthracis strains were assessed using immunoblotting. Whole-cell extracts from cultures grown to early exponential, mid-exponential, and stationary phase were probed using antiserum generated in rabbits against purified B. subtilis AbrB (Fig. 8). In the parent strain UM44, the maximum amount of AbrB was present in mid-exponential phase, while only very low levels were found in early-log- and in stationary-phase cultures. In the Δspo0A mutant (UT157), high levels of AbrB protein persisted in stationary-phase cultures. No AbrB protein was detected in the abrB null mutant, UT166.
FIG. 8.
Detection of AbrB in B. anthracis total cell extracts using anti-AbrB serum raised against B. subtilis AbrB. Samples from cultures of B. anthracis strains UM44, UT157 (Δspo0A) and UT166 (ΔabrB) were taken at early exponential phase (ee), mid-exponential phase (me), and stationary phase (s). Approximately 15 μg of total protein was loaded into each well.
Taken together, these results indicate that the chromosomal abrB gene of B. anthracis is transcribed and regulated in a manner similar to that of the B. subtilis gene.
DISCUSSION
In this study, we have shown that the chromosomally encoded abrB gene of B. anthracis negatively regulates expression of the three anthrax toxin genes. The toxin genes, pagA, lef, and cya, are located noncontiguously on a 30-kb region of virulence plasmid pXO1 (28). Expression of the toxin genes was measured by monitoring the β-galactosidase activity of reporter strains harboring toxin gene promoter-lacZ fusions at the normal genetic loci on pXO1. In addition, toxin protein levels in culture supernatants were assessed using immunoblotting. For all three genes, promoter activity and protein levels were increased in the absence of a functional abrB gene. The pagA gene was the most strongly affected by abrB.
The B. anthracis abrB gene is an orthologue of the well-studied abrB gene of B. subtilis. AbrB has been called a “transition state regulator,” a term coined to describe regulatory proteins that repress or activate genes during the transition from active growth into stationary phase (37, 40). In B. subtilis, abrB affects expression of numerous genes upon entry of a culture into stationary phase and has also been implicated in altered gene expression during the transition from lag to exponential phase growth (29). Our results indicate that the B. anthracis abrB gene regulates toxin gene expression upon entry of the culture into exponential phase. In B. anthracis strains harboring toxin gene promoter-lacZ fusions, β-galactosidase activity increased gradually throughout exponential phase and reached maximum activity in late exponential phase. In contrast, the enzyme activity of reporter strains carrying an abrB null mutation increased sharply in early exponential phase and reached maximum activity in mid-exponential phase.
The chromosomal abrB genes of B. anthracis and B. subtilis seem to encode proteins of similar structure. The predicted amino acid sequences of the AbrB proteins are 85% identical and 93% similar. We found that antiserum raised against the B. subtilis AbrB protein cross-reacts with AbrB from B. anthracis. Moreover, the genes appear to have functional similarity. In a report by Baille et al. (3) the B. anthracis pagA gene was cloned in different B. subtilis mutants and toxin synthesis was assessed. A B. subtilis abrB mutant harboring pagA on a multicopy plasmid exhibited an eightfold increase in PA synthesis compared to the parent strain harboring the same plasmid construct (3).
In addition to the structural and functional similarity of the B. anthracis and B. subtilis AbrB proteins, the abrB genes of these species seem to be regulated similarly. In B. subtilis, expression of the abrB gene is transient and highly growth phase dependent. Highest levels of abrB transcript and protein are found during the transition from lag phase into exponential growth (29). After this early peak, levels drop sharply due to negative regulation by the phosphorylated form of response regulator Spo0A (29, 37). Our data show that the B. anthracis abrB gene is also spo0A regulated. The β-galactosidase activity of a B. anthracis strain harboring an abrB::lacZ fusion increased throughout growth, reaching maximum activity in late exponential phase. In a spo0A null B. anthracis mutant, β-galactosidase activity continued to increase in late exponential and stationary phase. The steady-state levels of AbrB protein in the parent and spo0A mutant B. anthracis strains echo the reporter gene fusion results. In the parent strain, AbrB levels decrease after mid-exponential phase. In the spo0A mutant, AbrB levels remain high during late exponential and stationary-phase growth. These data indicate that the B. anthracis abrB gene is negatively regulated by spo0A.
We are presently investigating the molecular mechanism for abrB-controlled anthrax toxin gene expression. The simplest model for negative regulation by AbrB is direct binding of the protein to each of the toxin gene promoters. In B. subtilis, AbrB has been shown to bind to the promoter regions of over 40 different genes (37). A DNA consensus sequence for AbrB binding in B. subtilis has been put forth by Strauch (38). The sequence, WAWWTTTWCAAAAAAW, is extremely AT rich, similar to the 67% AT in the B. anthracis genome (20, 28). Using only the 182-kb DNA sequence of the pXO1 plasmid, we found 16 potential AbrB binding sites when we allowed one mismatch and 122 potential sites when we allowed two mismatches. Although some potential binding sites are in the toxin gene promoter regions, we cannot predict with confidence whether AbrB directly binds to these sequences. Recently, Vaughn et al. (46) speculated that the unique structure of the N terminus of the B. subtilis AbrB protein confers DNA-binding specificity in the absence of a consensus sequence. These authors reported that the amino-terminal domain of the protein possesses a looped-hinge helix DNA-binding topology, representing a novel class of DNA binding proteins (46, 47).
Whether or not AbrB binds directly to a specific nucleic acid sequence or structure, the differential effect of abrB on regulation of the three toxin genes may be indicative of divergent mechanisms for control of these genes. Previous studies of the toxin gene promoter regions have not revealed any obvious similarities in sequences or predicted structure (8). In B. subtilis, some of the effects of AbrB on gene expression are indirect, resulting from repression of additional regulatory genes by AbrB. For example, AbrB represses expression of the sigH (spo0H) gene, encoding the alternative sigma factor ςH. Consequently, genes transcribed by RNA polymerase containing ςH are affected in an abrB mutant (37). It is conceivable that in B. anthracis, abrB controls expression of another regulator important for expression of one or more of the toxin genes. It should be noted, however, that consensus sites for recognition by alternative sigma factors are not apparent in any of the toxin gene promoters.
Prior to this study, only one trans-acting regulator of the toxin genes was known. The atxA gene on pXO1 is essential for expression of all three toxin genes and also positively affects expression of the capsule biosynthesis genes (8, 16, 44, 45). atxA null mutants are avirulent in a mouse model for anthrax (8). The mechanism for atxA-mediated activation of gene expression is not known. The predicted amino acid sequence of the 56-kDa cytoplasmic AtxA protein does not indicate that it is a DNA-binding protein, and sequence-specific interactions between AtxA and the promoter regions of the toxin and capsule genes have not been demonstrated.
We tested for an epistatic relationship between abrB and atxA by monitoring expression of transcriptional atxA::lacZ and abrB::lacZ fusions in atxA and abrB null mutants. Our results indicate that abrB has a small negative effect on atxA expression. It is tempting to hypothesize that repression of toxin gene expression by abrB is due solely to negative regulation of atxA. However, results of previous studies render that model unlikely. Strains carrying multiple copies of the atxA gene produce elevated levels of AtxA but do not show increased PA synthesis (7). Also, the addition of the pagA promoter on a multicopy vector to a strain harboring the wild-type pagA gene on pXO1 does not affect PA synthesis (34). These data indicate that steady-state levels of AtxA are not limiting for toxin gene expression. Thus, the fourfold effect of abrB on atxA expression cannot explain the differences in toxin gene expression observed in an abrB mutant.
The role of regulatory genes for anthrax toxin synthesis during infection is critical to understanding B. anthracis pathogenesis. In inhalation anthrax, B. anthracis spores are phagocytosed by alveolar macrophages and can germinate intracellularly (15, 32). Ultimately, vegetative cells proliferate in the blood and other body tissues. The toxin proteins are synthesized and secreted by cells growing in the blood, and recently, evidence for atxA and toxin gene expression by newly vegetative cells in macrophages has been reported (15). Not surprisingly, in a mouse model for anthrax, atxA null mutant-infected animals show a significantly decreased antibody response to all three toxin proteins and atxA mutants are avirulent (8). The contribution of abrB to virulence has not been established. The newly discovered players in anthrax toxin gene expression, abrB and spo0A, may have a role in the timing of toxin synthesis during B. anthracis infection. Our findings link anthrax toxin gene expression to the multicomponent phosphorelay signaling system, well-studied in B. subtilis. Future experiments will address whether this signaling system plays a role in environmental sensing by B. anthracis during infection.
Acknowledgments
This work was supported by Public Health Service grant AI33537 from the National Institutes of Health.
We thank Yahua Chen for construction of the spo0A mutant and critical reading of the manuscript and Linda Husmann and June R. Scott for providing plasmid pJRS312. We are grateful to J. Hoch and M. Strauch for supplying anti-AbrB antiserum and to R. John Collier for antitoxin antisera.
REFERENCES
- 1.Agaisse, H., and D. Lereclus. 1994. Structural and functional analysis of the promoter region involved in full expression of the cryIIIA toxin gene of Bacillus thuringiensis. Mol. Microbiol. 13:97–107. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M. 1993. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, N.Y.
- 3.Baillie, L., A. Moir, and R. Manchee. 1998. The expression of the protective antigen of Bacillus anthracis in Bacillus subtilis. J. Appl. Microbiol. 84:741–746. [DOI] [PubMed] [Google Scholar]
- 4.Baldus, J. M., B. D. Green, P. Youngman, and C. P. Moran, Jr. 1994. Phosphorylation of Bacillus subtilis transcription factor Spo0A stimulates transcription from the spoIIG promoter by enhancing binding to weak 0A boxes. J. Bacteriol. 176:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball, C. A., R. Osuna, K. C. Ferguson, and R. C. Johnson. 1992. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J. Bacteriol. 174:8043–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, D. P., L. Ganova-Raeva, B. D. Green, S. R. Wilkinson, M. Young, and P. Youngman. 1994. Characterization of spo0A homologues in diverse Bacillus and Clostridium species identifies a probable DNA-binding domain. Mol. Microbiol. 14:411–426. [DOI] [PubMed] [Google Scholar]
- 7.Dai, Z., and T. M. Koehler. 1997. Regulation of anthrax toxin activator gene (atxA) expression in Bacillus anthracis: temperature, not CO2/bicarbonate, affects AtxA synthesis. Infect. Immun. 65:2576–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai, Z., J.-C. Sirard, M. Mock, and T. M. Koehler. 1995. The atxA gene product activates transcription of the anthrax toxin genes and is essential for virulence. Mol. Microbiol. 16:1171–1181. [DOI] [PubMed] [Google Scholar]
- 9.Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell. Microbiol. 2:453–463. [DOI] [PubMed] [Google Scholar]
- 10.Duesbery, N. S., and G. F. Vande Woude. 1999. Anthrax toxins. Cell Mol. Life Sci. 55:1599–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunny, G. M., and S. C. Winans. 1999. Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 12.Friedlander, A. M., S. L. Welkos, M. L. Pitt, J. W. Ezzell, P. L. Worsham, K. J. Rose, B. E. Ivins, J. R. Lowe, G. B. Howe, P. Mikesell, et al. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239–1243. [DOI] [PubMed] [Google Scholar]
- 13.Fritz, D. L., N. K. Jaax, W. B. Lawrence, K. J. Davis, M. L. Pitt, J. W. Ezzell, and A. M. Friedlander. 1995. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab. Investig. 73:691–702. [PubMed] [Google Scholar]
- 14.Green, B. D., L. Battisti, T. M. Koehler, C. B. Thorne, and B. E. Ivins. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun. 49:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidi-Rontani, C., M. Weber-Levy, E. Labruyère, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9–17. [DOI] [PubMed] [Google Scholar]
- 16.Guignot, J., M. Mock, and A. Fouet. 1997. AtxA activates the transcription of genes harbored by both Bacillus anthracis virulence plasmids. FEMS Microbiol. Lett. 147:203–207. [DOI] [PubMed] [Google Scholar]
- 17.Hardman, A. M., G. S. Stewart, and P. Williams. 1998. Quorum sensing and the cell-cell communication dependent regulation of gene expression in pathogenic and non-pathogenic bacteria. Antonie Leeuwenhoek 74:199–210. [DOI] [PubMed] [Google Scholar]
- 18.Hoch, J. A. 1993. spoO genes, the phosphorelay, and the initiation of sporulation, p.747–755. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. ASM Press, Washington, D.C.
- 19.Hoffmaster, A. R., and T. M. Koehler. 1997. The anthrax toxin activator gene atxA is associated with CO2-enhanced non-toxin gene expression in Bacillus anthracis. Infect. Immun. 65:3091–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koehler, T. M. 2000. Bacillus anthracis, p.519–528. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 21.Koehler, T. M., Z. Dai, and M. Kaufman-Yarbray. 1994. Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J. Bacteriol. 176:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249–256. [DOI] [PubMed] [Google Scholar]
- 23.Leppla, S. H. 1995. Anthrax toxins, p.543–572. In J. Moss, B. Iglewski, M. Vaughan, and A. T. Tu (ed.), Handbook of natural toxins, vol. 8. Marcel Dekker, New York, N.Y.
- 24.Leppla, S. H. 1988. Production and purification of anthrax toxin. Methods Enzymol. 165:103–116. [DOI] [PubMed] [Google Scholar]
- 25.Leppla, S. H. 1991. Purification and characterization of adenylyl cyclase from Bacillus anthracis. Methods Enzymol. 195:153–168. [DOI] [PubMed] [Google Scholar]
- 26.McIver, K. S., and J. R. Scott. 1997. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J. Bacteriol. 179:5178–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Okinaka, R. T., K. Cloud, O. Hampton, A. R. Hoffmaster, K. K. Hill, P. Keim, T. M. Koehler, G. Lamke, S. Kumano, J. Mahillon, D. Manter, Y. Martinez, D. Ricke, R. Stevensson, and P. J. Jackson. 1999. Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J. Bacteriol. 181:6509–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Reilly, M., and K. M. Devine. 1997. Expression of AbrB, a transition state regulator from Bacillus subtilis, is growth phase dependent in a manner resembling that of Fis, the nucleoid binding protein from Escherichia coli. J. Bacteriol. 179:522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osuna, R., D. Lienau, K. T. Hughes, and R. C. Johnson. 1995. Sequence, regulation, and functions of fis in Salmonella typhimurium. J. Bacteriol. 177:2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perego, M., G. B. Spiegelman, and J. A. Hoch. 1988. Structure of the gene for the transition state regulator. abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol. Microbiol. 2:689–699. [DOI] [PubMed] [Google Scholar]
- 32.Ross, J. M. 1957. The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol. LXXIII:485–494. [Google Scholar]
- 33.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Sirard, J. C., M. Mock, and A. Fouet. 1995. Molecular tools for the study of transcriptional regulation in Bacillus anthracis. Res. Microbiol. 146:729–737. [DOI] [PubMed] [Google Scholar]
- 35.Sirard, J. C., M. Mock, and A. Fouet. 1994. The three Bacillus anthracis toxin genes are coordinately regulated by bicarbonate and temperature. J. Bacteriol. 176:5188–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauch, M., V. Webb, G. Spiegelman, and J. A. Hoch. 1990. The Spo0A protein of Bacillus subtilis is a repressor of the abrB gene. Proc. Natl. Acad. Sci. USA 87:1801–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strauch, M. A. 1993. AbrB, a transition state regulator, p.757–764. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. ASM Press, Washington, D.C.
- 38.Strauch, M. A. 1995. Delineation of AbrB-binding sites on the Bacillus subtilis spo0H, kinB, ftsAZ, and pbpE promoters and use of a derived homology to identify a previously unsuspected binding site in the bsuB1 methylase promoter. J. Bacteriol. 177:6999–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strauch, M. A., and M. Ayazifar. 1995. Bent DNA is found in some, but not all, regions recognized by the Bacillus subtilis AbrB protein. Mol. Gen. Genet. 246:756–760. [DOI] [PubMed] [Google Scholar]
- 40.Strauch, M. A., and J. A. Hoch. 1993. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol. Microbiol. 7:337–342. [DOI] [PubMed] [Google Scholar]
- 41.Strauch, M. A., M. Perego, D. Burbulys, and J. A. Hoch. 1989. The transition state transcription regulator AbrB of Bacillus subtilis is autoregulated during vegetative growth. Mol. Microbiol. 3:1203–1209. [DOI] [PubMed] [Google Scholar]
- 42.Thorne, C. B. 1985. Genetics of Bacillus anthracis, p.56–62. In L. Leive (ed.), Microbiology. American Society for Microbiology, Washington, D.C.
- 43.Thorne, C. B. 1968. Transducing bacteriophage for Bacillus cereus. J. Virol. 2:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchida, I., J. M. Hornung, C. B. Thorne, K. R. Klimpel, and S. H. Leppla. 1993. Cloning and characterization of a gene whose product is a trans-activator of anthrax toxin synthesis. J. Bacteriol. 175:5329–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida, I., S. Makino, T. Sekizaki, and N. Terakado. 1997. Cross-talk to the genes for Bacillus anthracis capsule synthesis by atxA, the gene encoding the trans-activator of anthrax toxin synthesis. Mol. Microbiol. 23:1229–1240. [DOI] [PubMed] [Google Scholar]
- 46.Vaughn, J. L., V. Feher, S. Naylor, M. A. Strauch, and J. Cavanagh. 2000. Novel DNA binding domain and genetic regulation model of Bacillus subtilis transition state regulator AbrB. Nat. Struct. Biol. 7:1139–1146. [DOI] [PubMed] [Google Scholar]
- 47.Vaughn, J. L., V. A. Feher, C. Bracken, and J. Cavanagh. 2001. The DNA-binding domain in the Bacillus subtilis transition-state regulator AbrB employs significant motion for promiscuous DNA recognition. J. Mol. Biol. 305:429–439. [DOI] [PubMed] [Google Scholar]
- 48.Williams, P., M. Camara, A. Hardman, S. Swift, D. Milton, V. J. Hope, K. Winzer, B. Middleton, D. I. Pritchard, and B. W. Bycroft. 2000. Quorum sensing and the population-dependent control of virulence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson, R. L., S. J. Libby, A. M. Freet, J. D. Boddicker, T. F. Fahlen, and B. D. Jones. 2001. Fis, a DNA nucleoid-associated protein, is involved in Salmonella typhimurium SPI-1 invasion gene expression. Mol. Microbiol. 39:79–88. [DOI] [PubMed] [Google Scholar]
- 50.Woodcock, D. M., P. J. Crowther, J. Doherty, S. Jefferson, E. DeCruz, M. Noyer-Weidner, S. S. Smith, M. Z. Michael, and M. W. Graham. 1989. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 17:3469–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu, K., D. Clark, and M. A. Strauch. 1996. Analysis of abrB mutations, mutant proteins, and why abrB does not utilize a perfect consensus in the −35 region of its sigma A promoter. J. Biol. Chem. 271:2621–2626. [DOI] [PubMed] [Google Scholar]
- 52.Xu, K., and M. A. Strauch. 2001. DNA-binding activity of amino-terminal domains of the Bacillus subtilis AbrB protein. J. Bacteriol. 183:4094–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119. [DOI] [PubMed] [Google Scholar]