Abstract
1. Frequencies of miniature end-plate potentials (m.e.p.p.s) were recorded at neuromuscular junctions in rat diaphragm-phrenic nerve preparations in vitro.
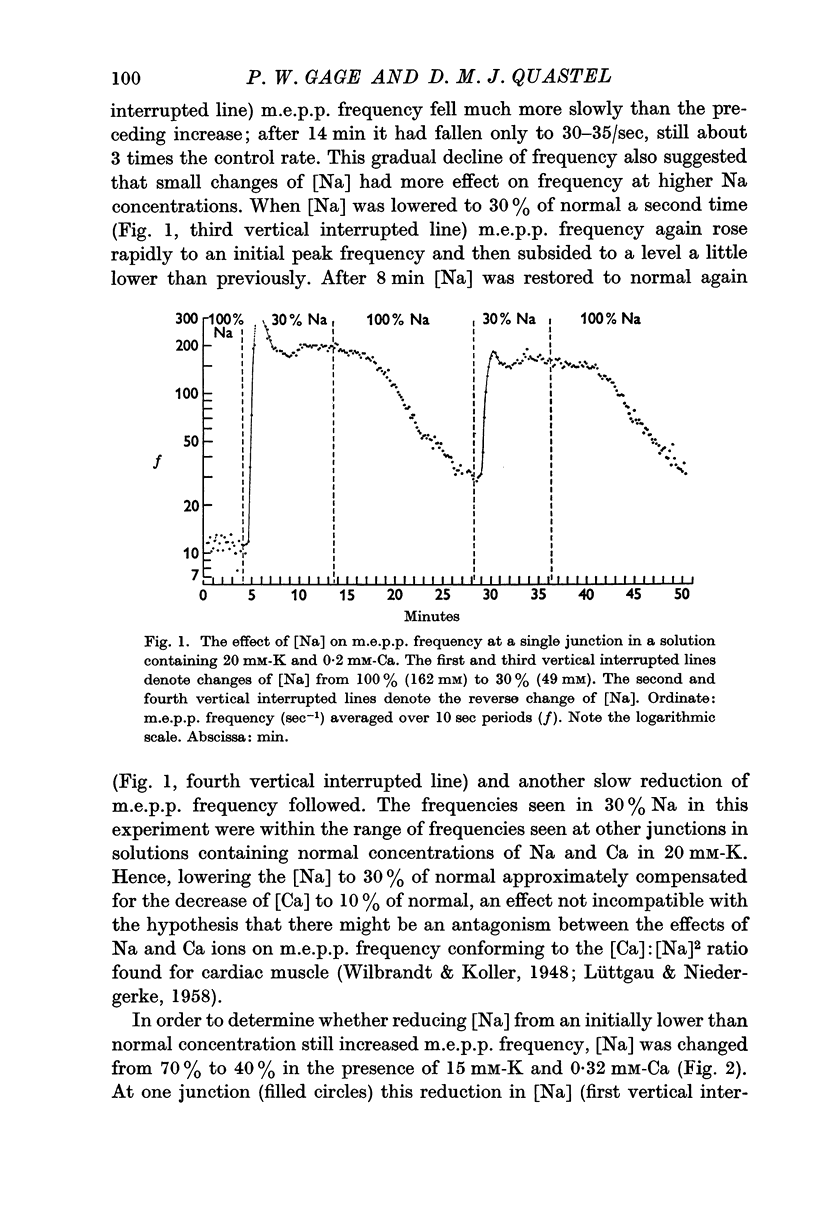
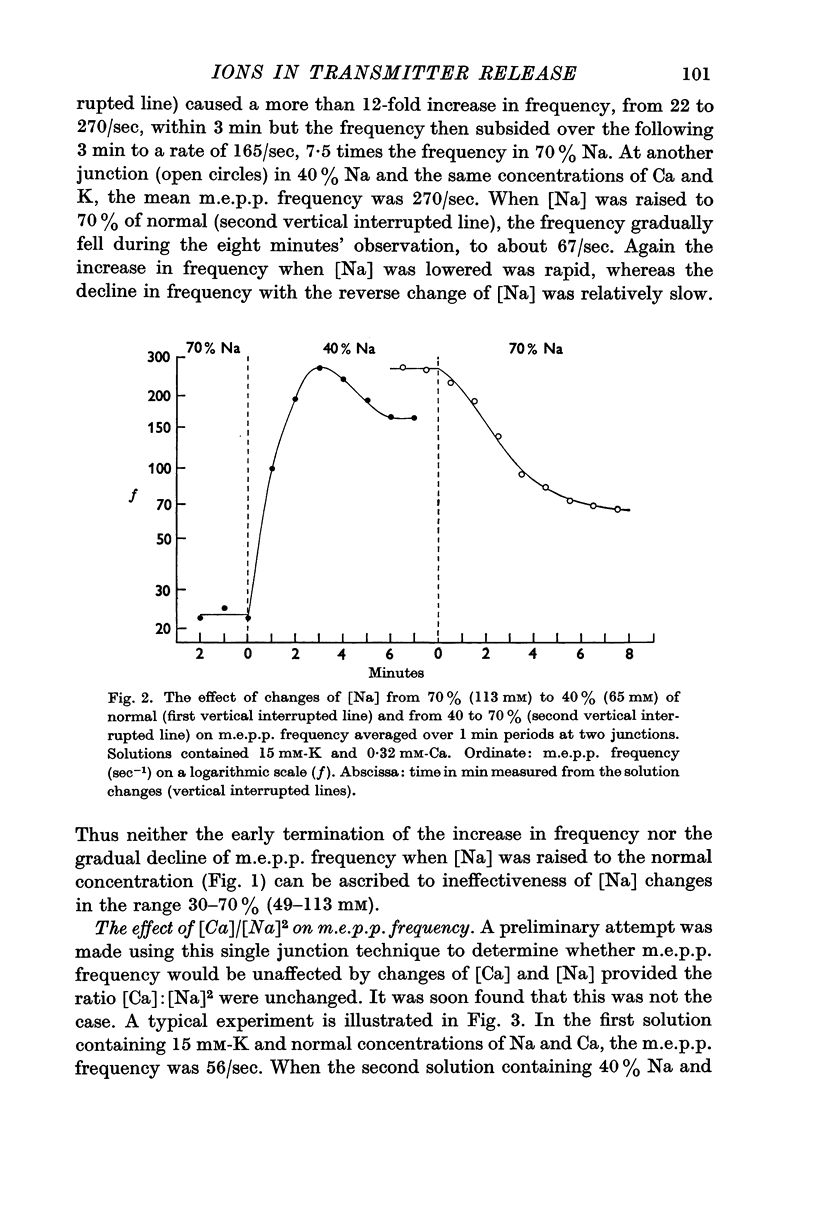
2. In the presence of raised [K] (15-20 mM) lowering [Na] caused a rapid increase in m.e.p.p. frequency whether [Ca] was low or normal. Raising [Na] towards the normal concentration (162 mM) caused a slow fall in frequency and raising [Ca] in the range 0·32-2 mM caused a slow increase in frequency. These effects were less in the normal [K] (5 mM).
3. Mean m.e.p.p. frequencies were determined for solutions containing 15 mM-K and combinations of [Ca] and [Na]. M.e.p.p. frequency varied inversely with [Na] when [Ca] was constant. In each of the three Na concentrations used (162, 113 and 65 mM) raising [Ca] in the range 0·32-2 mM increased m.e.p.p. frequency but when raised above 2-3 mM, Ca depressed frequency.
4. A model was proposed in which Ca affected transmitter release by changing the concentration in the presynaptic membrane of a complex CaX to which the rate of transmitter release was directly proportional. Higher concentrations of Ca depressed transmitter release by inactivating CaX. Sodium ions competitively depressed release either by competing with calcium ions for association with X or by reducing the affinity of X for Ca.
5. When [Na] was lowered in solutions containing raised [Mg] and [Ca], the increase of mean m.e.p.p. frequency was greater than that observed in raised [Ca] and normal [Mg] and was of the same order as the increases seen in low [Ca]. The result was interpreted to indicate either that Na and Mg do not compete with Ca at the same site or that Mg affects the affinity of X for Ca and Na.
6. The effect of lowering [Na] on m.e.p.p. frequency was a specific effect of Na ions. When LiCl was substituted for NaCl, the increase of m.e.p.p. frequency persisted. Changes in [Cl] had no effect on m.e.p.p. frequency.
7. There was a linear relation between the mean logarithm of m.e.p.p. frequencies and [K], the slope of the relation increasing as [Na] was lowered. Conversely, lowering [Na] caused a greater increase in m.e.p.p. frequency as [K] was raised.
8. The variation of m.e.p.p. frequencies in a diaphragm was roughly proportional to a second or higher power of [Na] and inversely proportion to [Ca]. It was thought that this could be due to differences in chelation of Ca which were more apparent at low Ca concentrations.
9. The similarities between the effects of Na, Ca and K on m.e.p.p. frequency and the effects of these ions on Ca-influx in heart muscle led to the suggestion that transmitter release is proportional to the concentration of a negatively charged complex of a carrier X with one calcium ion (CaX) at the internal surface of the membrane and that changes in membrane potential affect transmitter release by changing the distribution or location of CaX in the membrane.
Full text
PDF


























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAM H. M., HARDWICK D. C., SPENCER K. E. Assay of histamine on the isolated guinea-pig intestine by the method of superfusion. Br J Pharmacol Chemother. 1954 Sep;9(3):360–366. doi: 10.1111/j.1476-5381.1954.tb01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R. I. THE ROLE OF SODIUM IONS IN THE METABOLISM OF ACETYLCHOLINE. Can J Biochem Physiol. 1963 Dec;41:2573–2597. [PubMed] [Google Scholar]
- Brown G. L., Feldberg W. The action of potassium on the superior cervical ganglion of the cat. J Physiol. 1936 Mar 9;86(3):290–305. doi: 10.1113/jphysiol.1936.sp003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Local activity at a depolarized nerve-muscle junction. J Physiol. 1955 May 27;128(2):396–411. doi: 10.1113/jphysiol.1955.sp005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., STARK L. The effect of calcium ions on the motor end-plate potentials. J Physiol. 1952 Apr;116(4):507–515. doi: 10.1113/jphysiol.1952.sp004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLEGO A., LORENTE DE NO R. On the effect of ammonium and lithium ions upon frog nerve deprived of sodium. J Gen Physiol. 1951 Nov;35(2):227–244. doi: 10.1085/jgp.35.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. After-potentials in mammalian non-myelinated nerve fibres. J Physiol. 1958 Dec 30;144(3):442–462. doi: 10.1113/jphysiol.1958.sp006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. An investigation of the post-tetanic potentiation of end-plate potentials at a mammalian neuromuscular junction. J Physiol. 1966 May;184(2):353–375. doi: 10.1113/jphysiol.1966.sp007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. Evidence for a Poisson distribution of miniature end-plate potentials and some implications. Nature. 1965 Oct 23;208(5008):395–396. doi: 10.1038/208395a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Dual effect of potassium on transmitter release. Nature. 1965 May 8;206(984):625–626. doi: 10.1038/206625a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Influence of sodium ions on transmitter release. Nature. 1965 Jun 5;206(988):1047–1048. doi: 10.1038/2061047a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., SCHMIDT R. F. An electrophysiological investigation of mammalian motor nerve terminals. J Physiol. 1963 Apr;166:145–167. doi: 10.1113/jphysiol.1963.sp007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., KOSTIAL K. Effect of magnesium and calcium ions on the release of acetylcholine. J Physiol. 1954 May 28;124(2):234–241. doi: 10.1113/jphysiol.1954.sp005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- KELLY J. S. ANTAGONISM BETWEEN NA+ AND CA2+ AT THE NEUROMUSCULAR JUNCTION. Nature. 1965 Jan 16;205:296–297. doi: 10.1038/205296a0. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Release of acetylcholine from a nerve terminal by electric pulses of variable strength and duration. Nature. 1965 Sep 4;207(5001):1097–1098. doi: 10.1038/2071097a0. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. II. The action potential and excitation. Pharmacol Rev. 1958 Jun;10(2):165–273. [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Changes in potassium concentration around motor nerve terminals, produced by current flow, and their effects on neuromuscular transmission. J Physiol. 1961 Jan;155:46–58. doi: 10.1113/jphysiol.1961.sp006612. [DOI] [PMC free article] [PubMed] [Google Scholar]


