Abstract
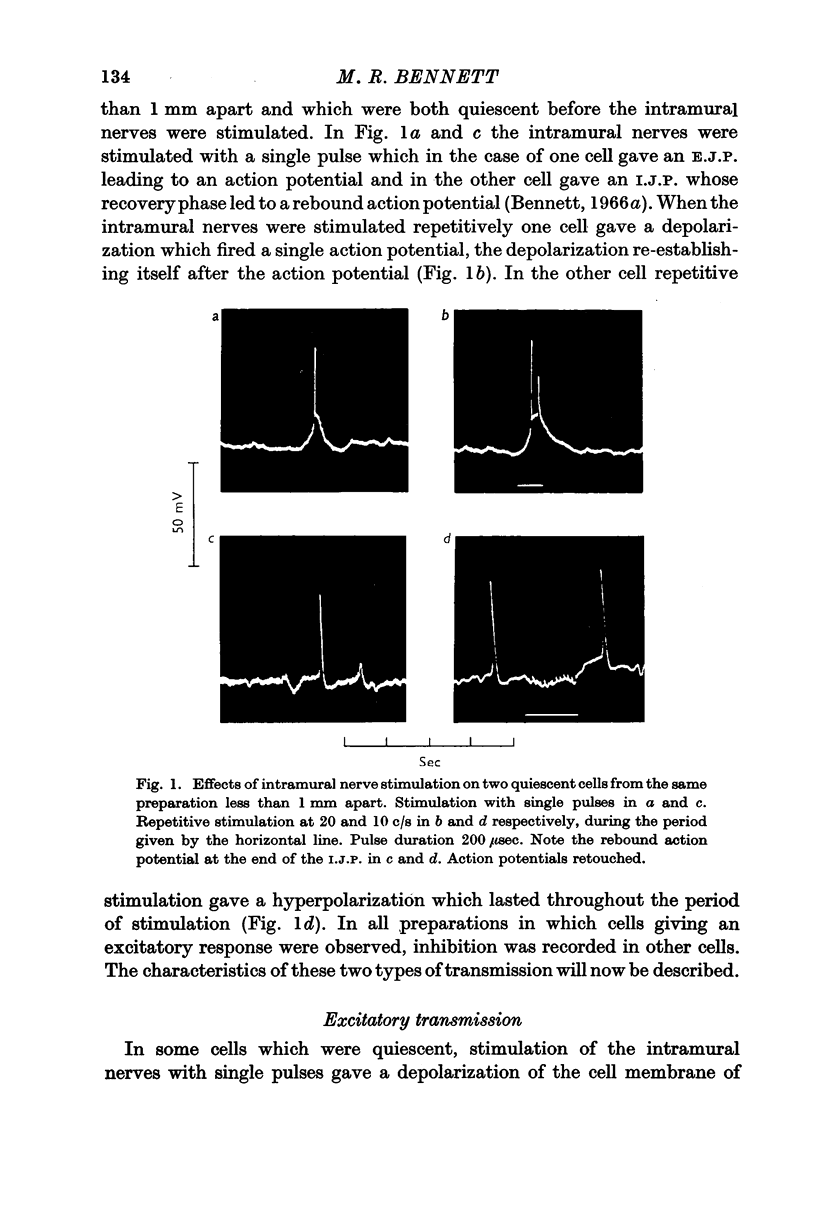
1. A study has been made of transmission from intramural excitatory nerves to the smooth muscle cells of the guinea-pig taenia coli.
2. Only ten cells out of eighty gave depolarizing (i.e. excitatory junction potentials, E.J.P.S) on stimulating the intramural nerves, the remaining cells gave hyperpolarizing responses (i.e. inhibitory junction potentials, I.J.P.S). E.J.P.S were recorded in cells which were less than 1 mm away from cells which gave I.J.P.S.
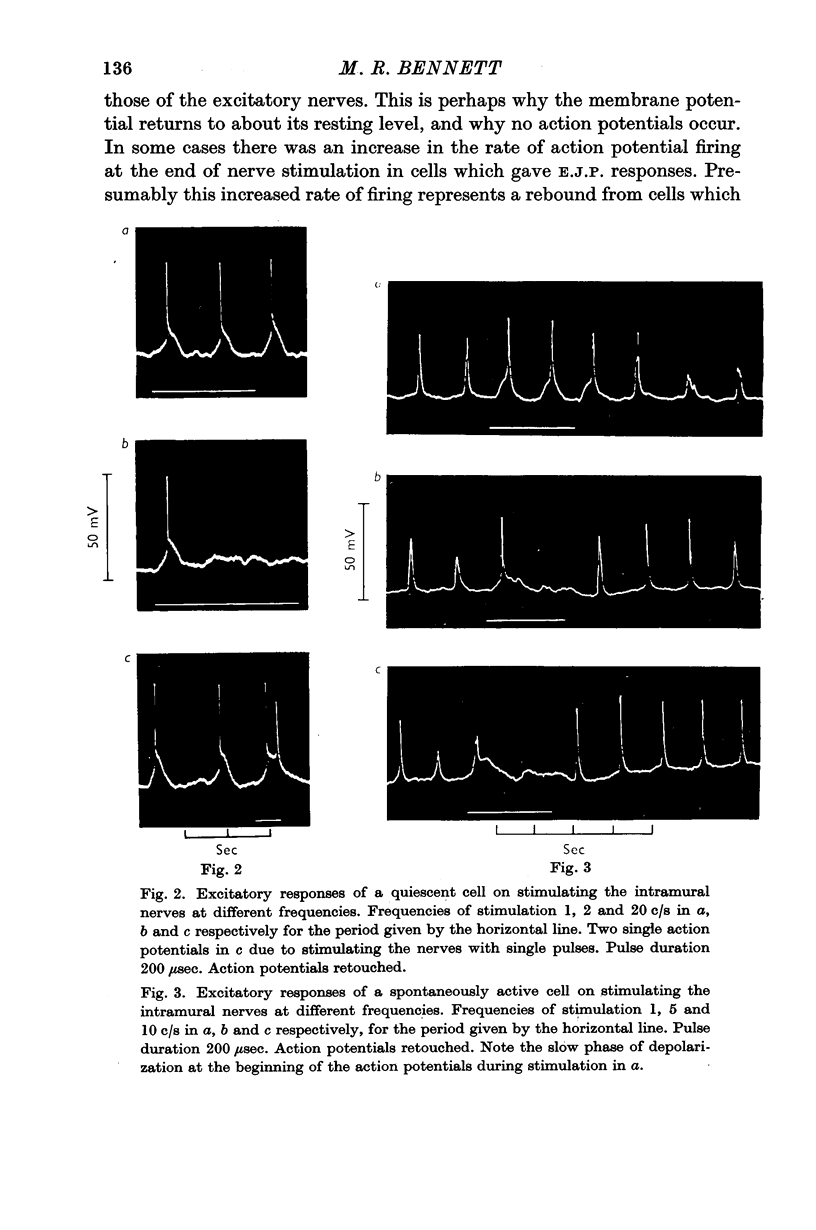
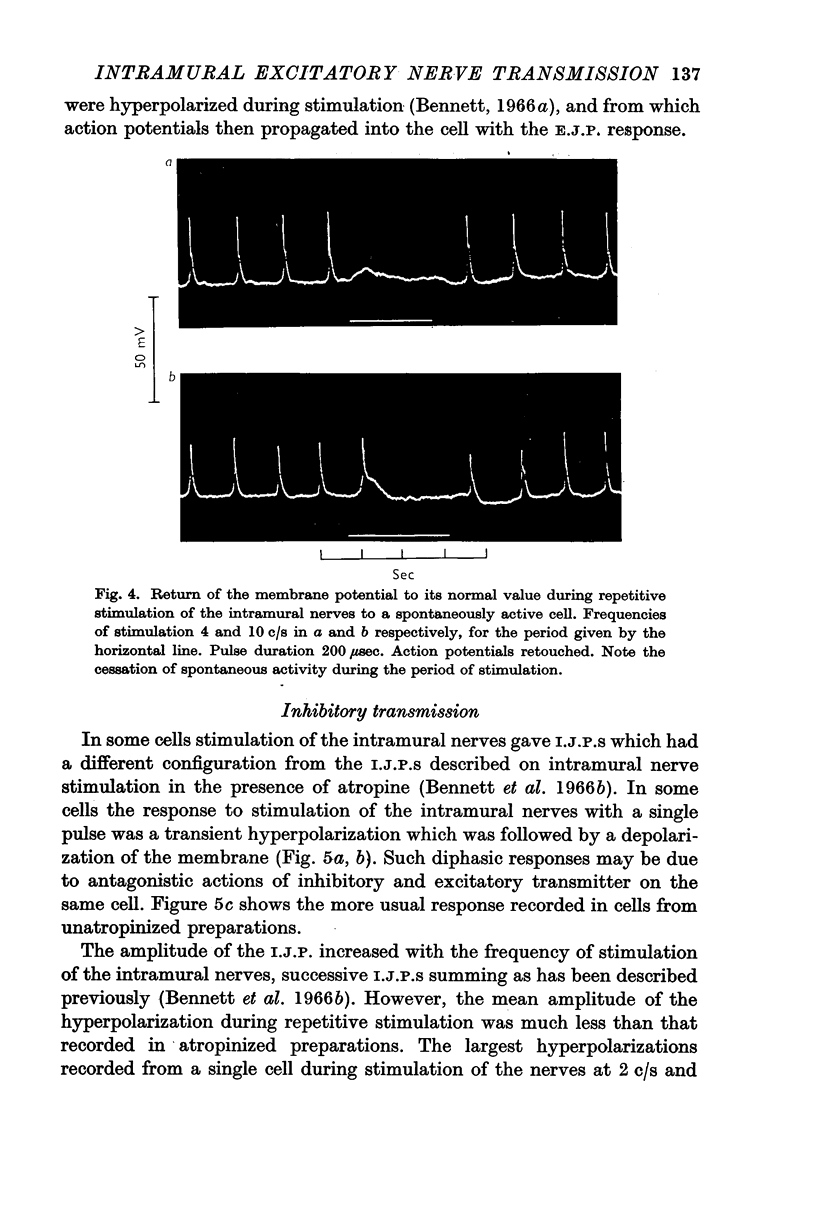
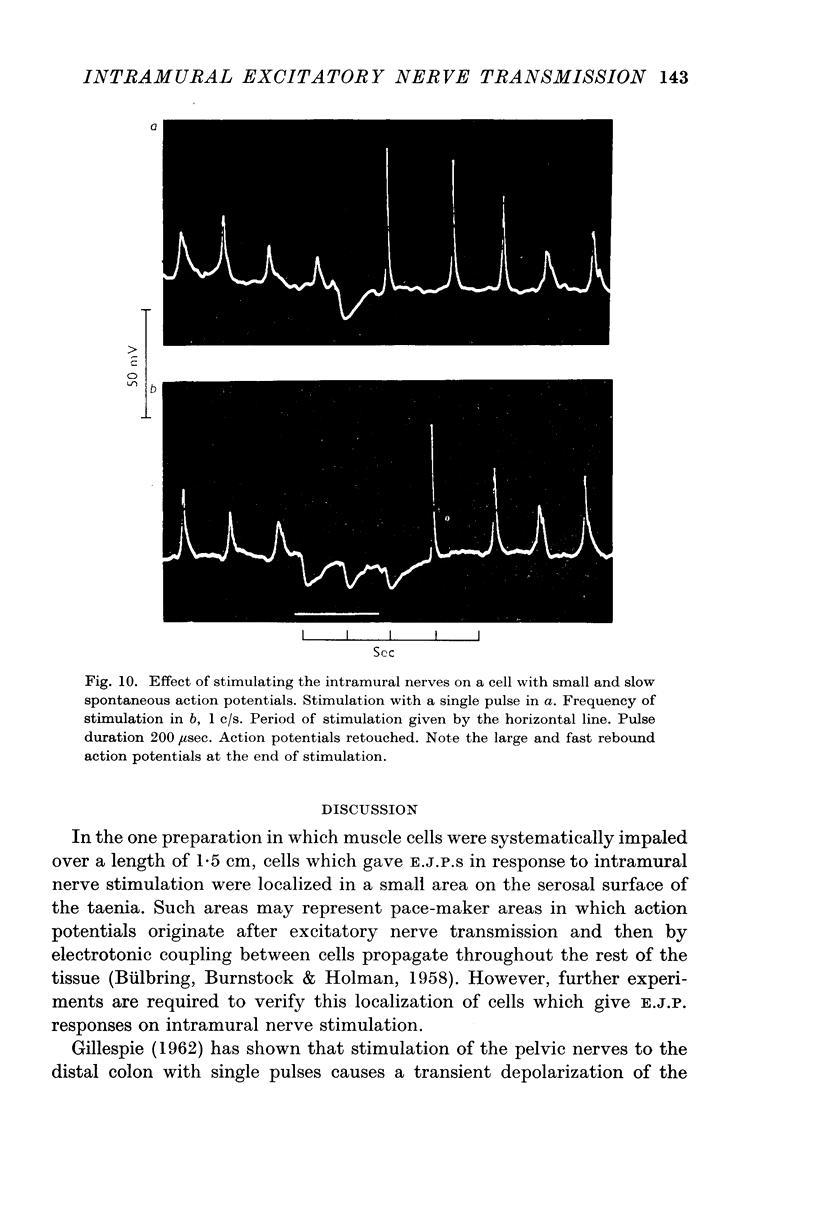
3. In some cells stimulation of the intramural nerves with single pulses of maximal strength gave E.J.P.S of about 20 mV amplitude after a latency of 100-200 msec. In quiescent cells these E.J.P.S gave rise to action potentials. Repetitive stimulation above 1 c/s depolarized the membrane for less than about 1 sec, and during the remainder of the stimulation no action potentials fired, even in spontaneous cells.
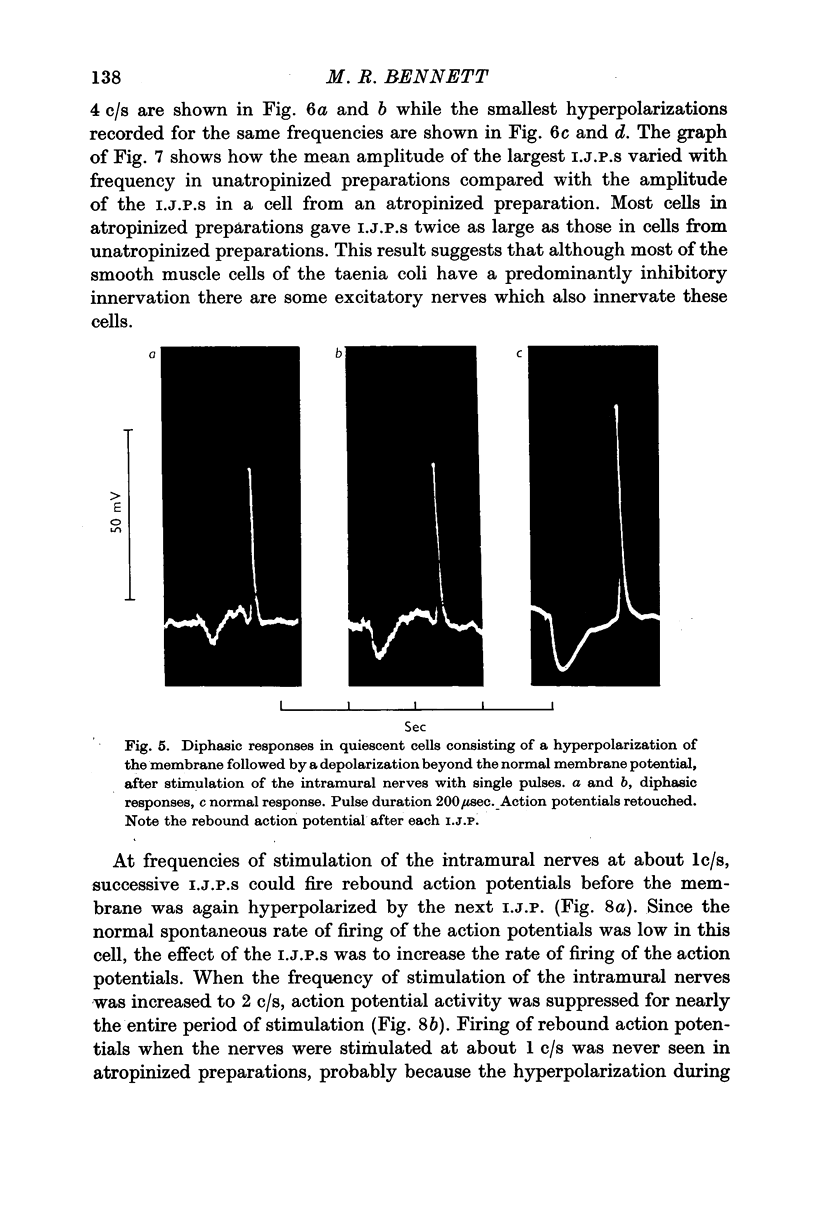
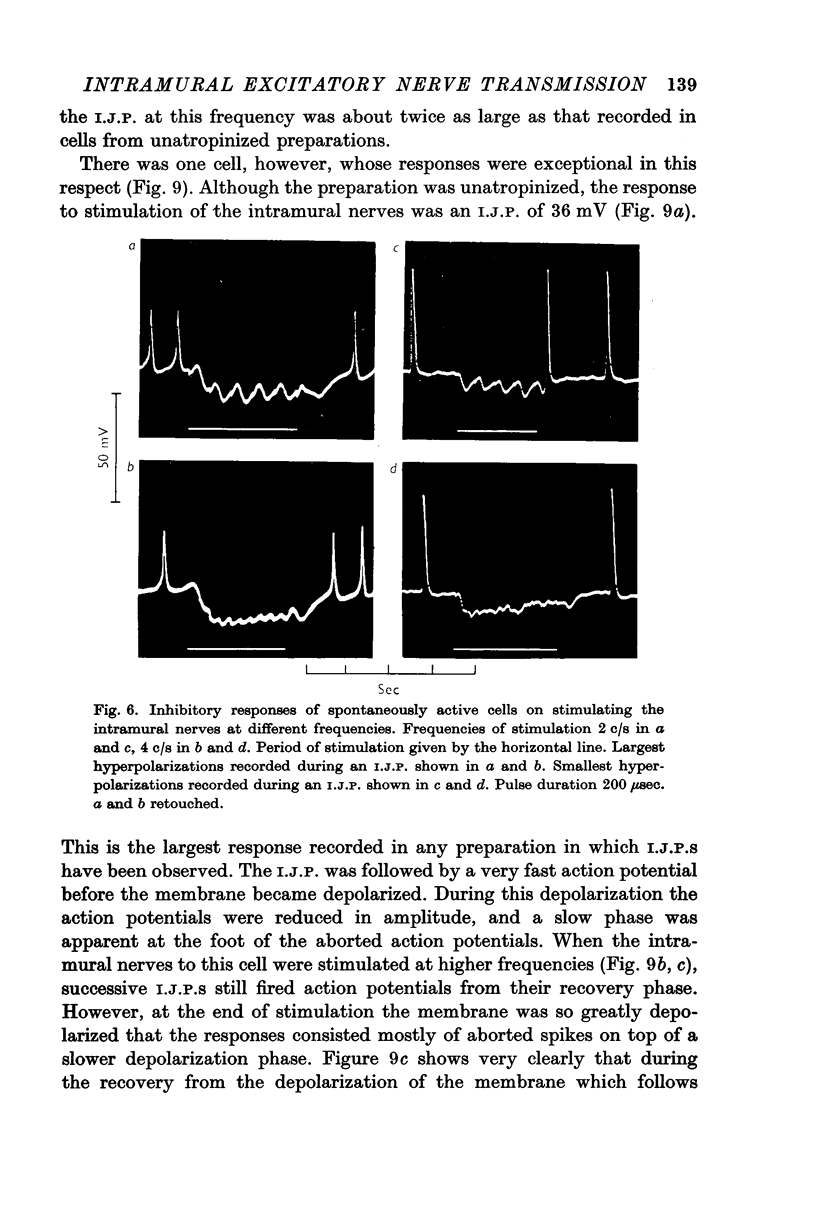
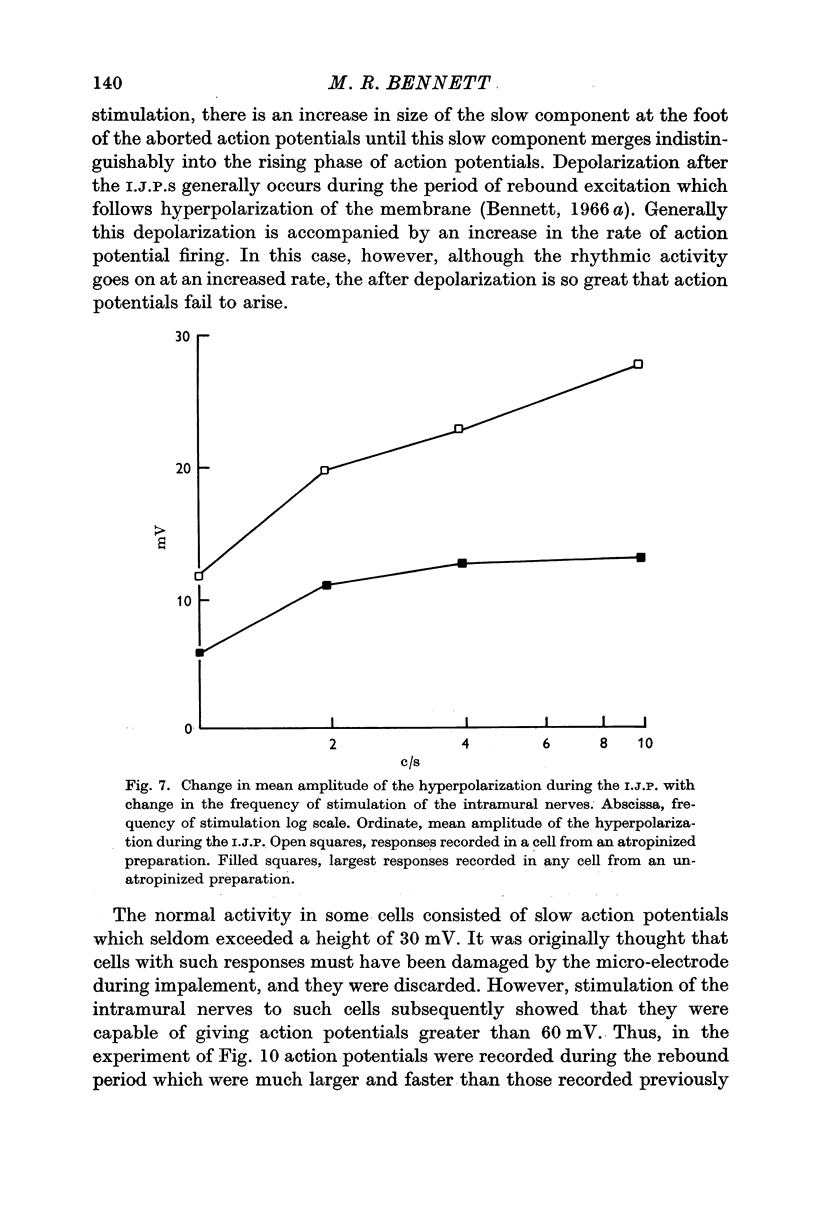
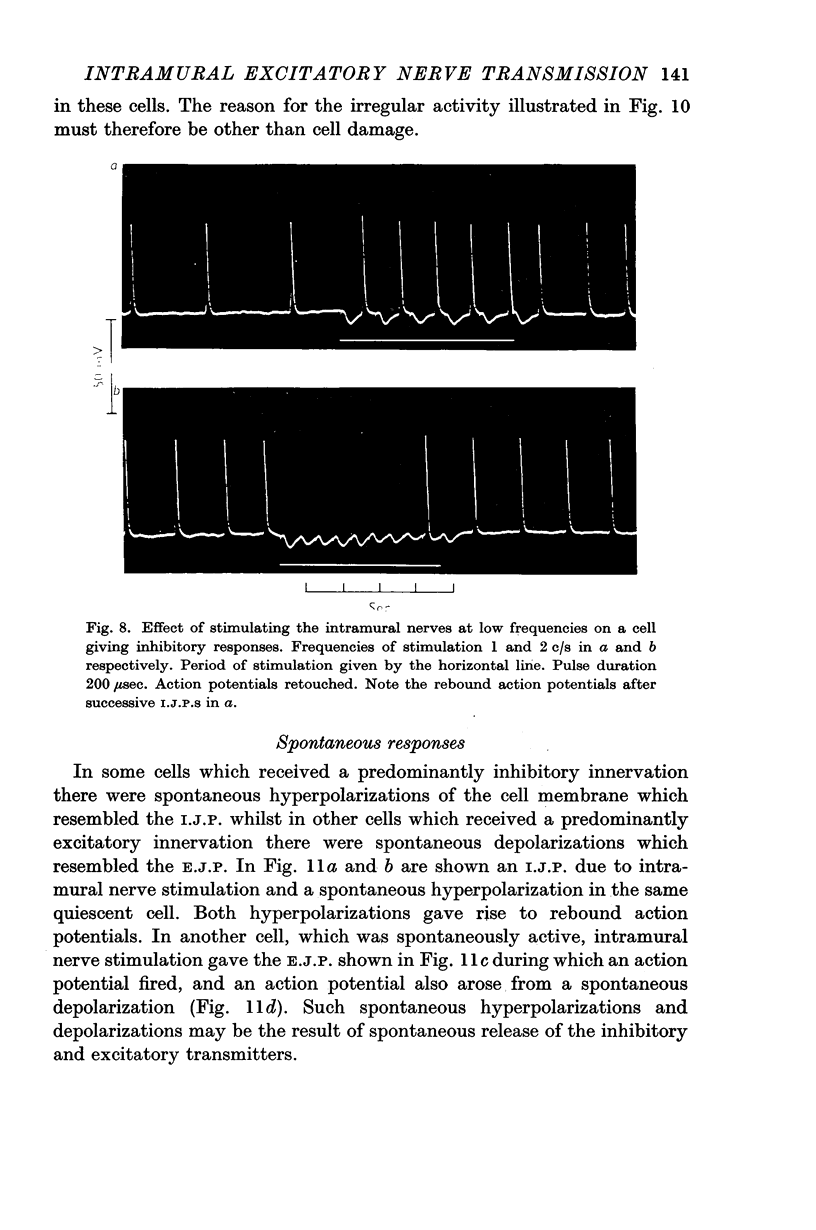
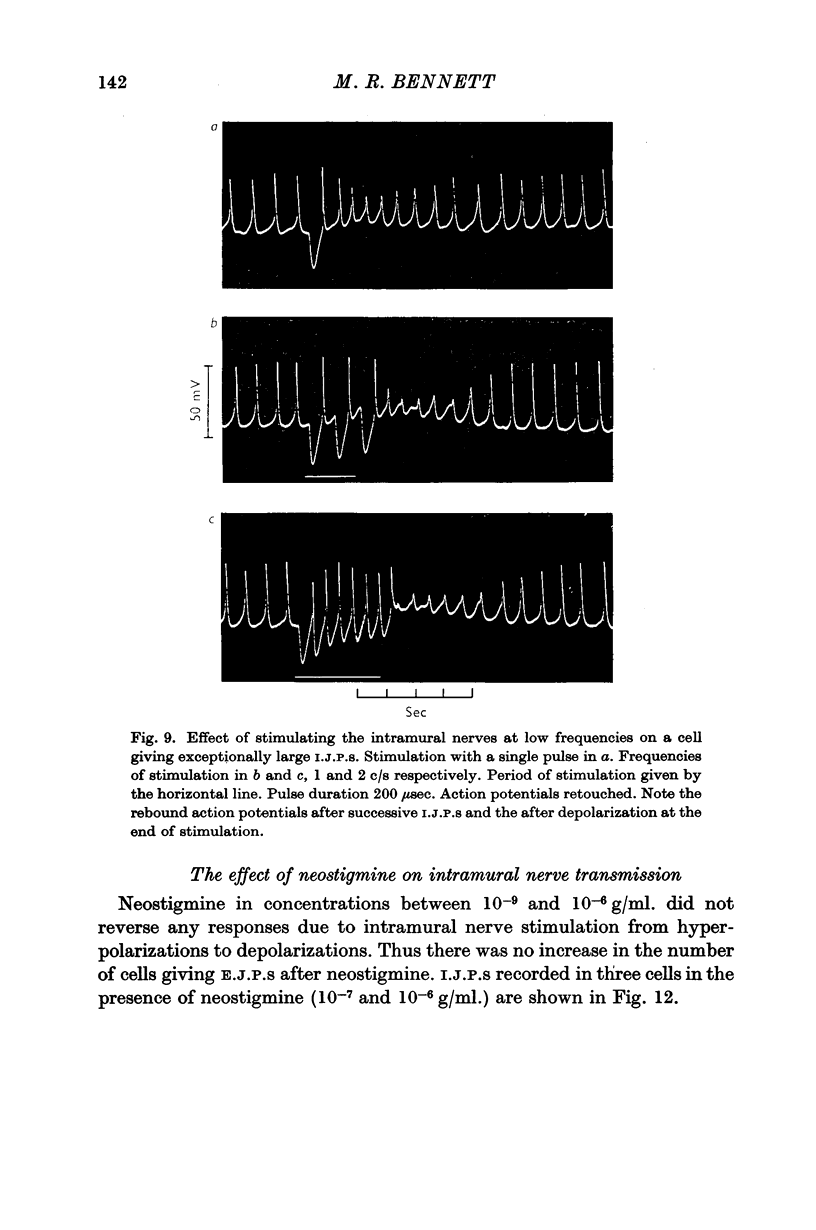
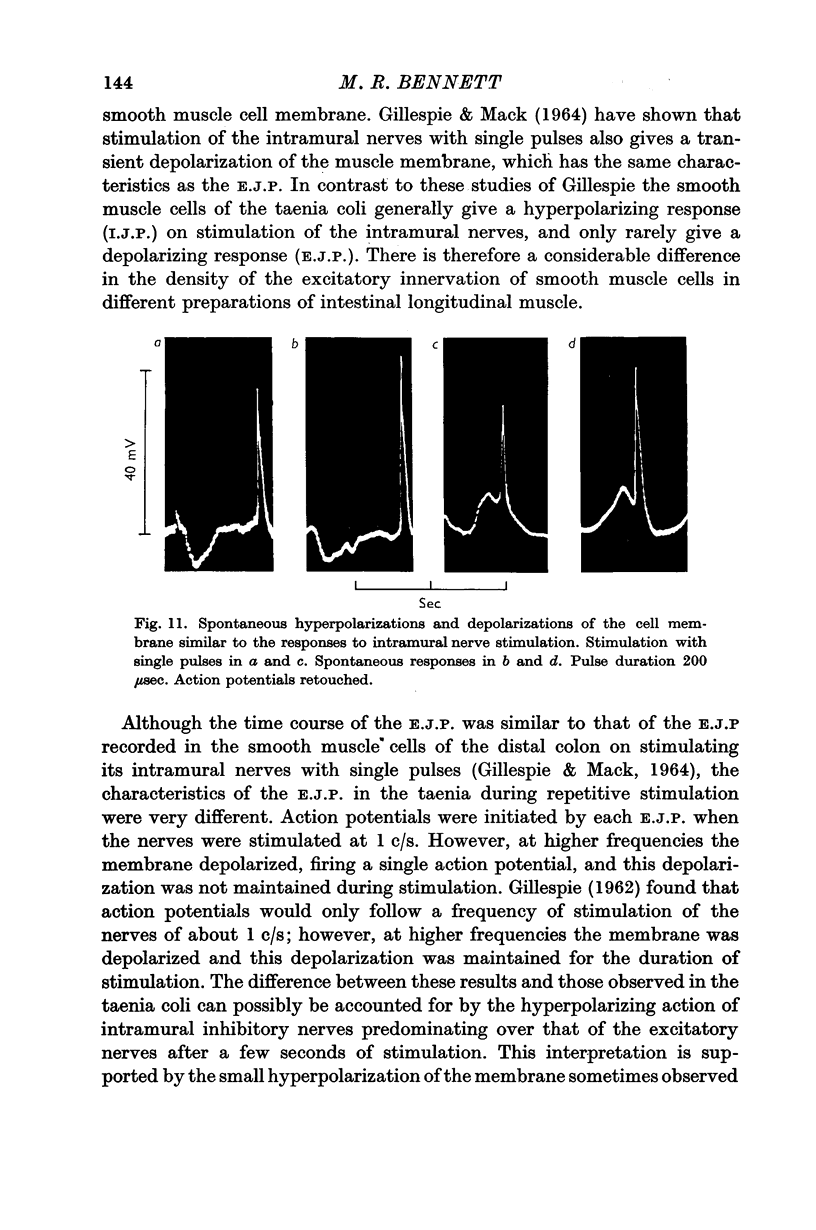
4. In some cells stimulation of the intramural nerves gave an I.J.P. The largest sized I.J.P.S were generally only about half the size of the I.J.P.S recorded in atropinized preparations. The decreased amplitude of the I.J.P.S enabled rebound action potentials to be fired by successive I.J.P.S when the intramural nerves were stimulated at about 1 c/s. At higher frequencies all spontaneous activity was suppressed.
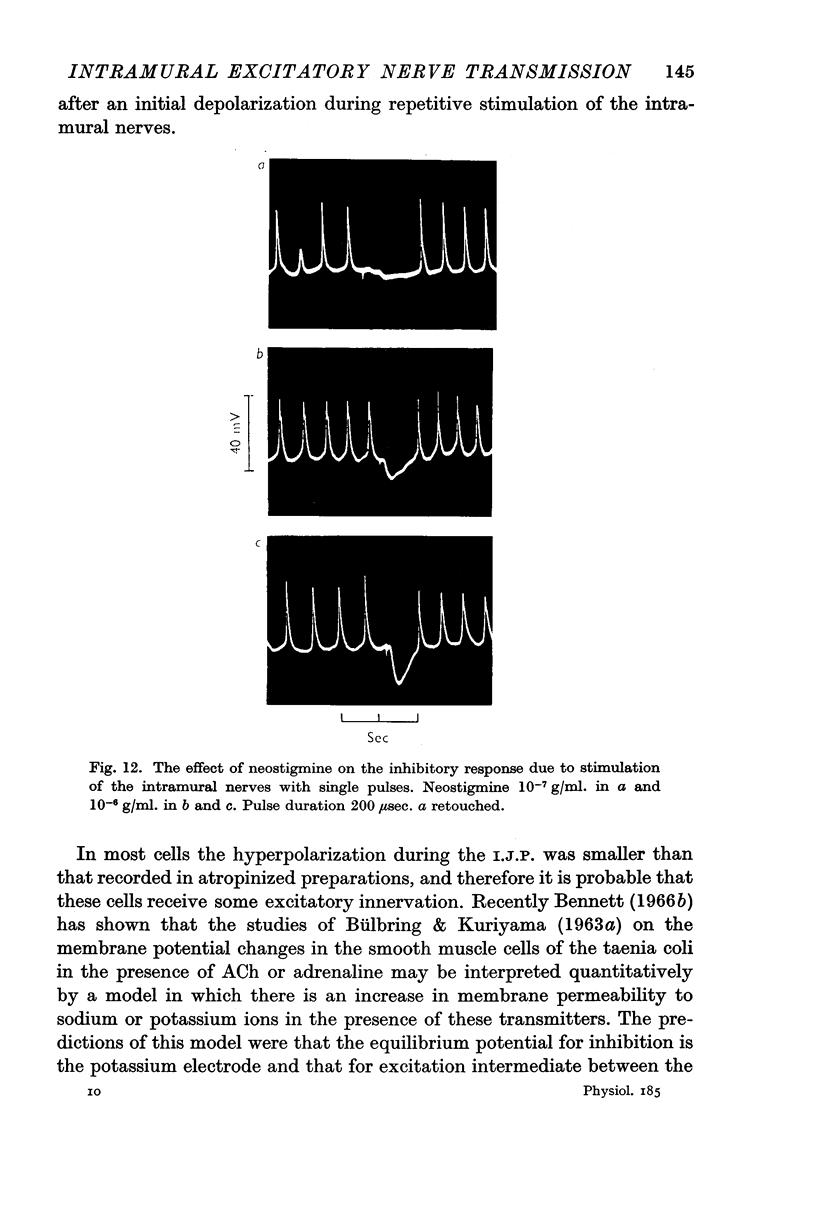
5. The effect of neostigmine (10-9-10-7 g/ml.) on the transmission was studied. There was no detectable increase in the number of cells giving E.J.P. responses in the presence of neostigmine.
6. The electrophysiological characteristics of intramural excitatory and inhibitory nerve transmission are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., SEARS T. A. THE VENTRO-BASAL COMPLEX OF THE THALAMUS: TYPES OF CELLS, THEIR RESPONSES AND THEIR FUNCTIONAL ORGANIZATION. J Physiol. 1964 Nov;174:370–399. doi: 10.1113/jphysiol.1964.sp007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUELBRING E., KURIYAMA H. THE EFFECT OF ADRENALINE ON THE SMOOTH MUSCLE OF GUINEA-PIG TAENIA COLI IN RELATION TO THE DEGREE OF STRETCH. J Physiol. 1963 Nov;169:198–212. doi: 10.1113/jphysiol.1963.sp007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., BURNSTOCK G., HOLMAN M. E. Excitation and conduction in the smooth muscle of the isolated taenia coli of the guinea-pig. J Physiol. 1958 Aug 6;142(3):420–437. doi: 10.1113/jphysiol.1958.sp006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in ionic environment on the action of acetylcholine and adrenaline on the smooth muscle cells of guinea-pig taenia coli. J Physiol. 1963 Apr;166:59–74. doi: 10.1113/jphysiol.1963.sp007090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. The effects of acetylcholine on membrane potential, spike frequency, conduction velocity and excitability in the taenia coli of the guinea-pig. J Physiol. 1958 Aug 29;143(1):165–182. doi: 10.1113/jphysiol.1958.sp006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. E. Transmission from perivascular inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):527–540. doi: 10.1113/jphysiol.1966.sp007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. Rebound excitation of the smooth muscle cells of the guinea-pig taenia coli after stimulation of intramural inhibitory nerves. J Physiol. 1966 Jul;185(1):124–131. doi: 10.1113/jphysiol.1966.sp007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Rand M. J. The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol. 1966 Feb;182(3):504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. Nerve-mediated excitation of the taenia of the guinea-pig caecum. J Physiol. 1966 Jul;185(1):148–159. doi: 10.1113/jphysiol.1966.sp007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. S. The electrical and mechanical responses of intestinal smooth muscle cells to stimulation of their extrinsic parasympathetic nerves. J Physiol. 1962 Jun;162:76–92. doi: 10.1113/jphysiol.1962.sp006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KELLERTH J. O., WILLIAMS T. D. INTRACELLULAR ASPECTS OF STIMULATING MOTONEURONES BY MUSCLE STRETCH. J Physiol. 1964 Nov;174:435–452. doi: 10.1113/jphysiol.1964.sp007496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSTERLITZ H. W., LEES G. M. PHARMACOLOGICAL ANALYSIS OF INTRINSIC INTESTINAL REFLEXES. Pharmacol Rev. 1964 Sep;16:301–339. [PubMed] [Google Scholar]


