Abstract
1. Active transport of Na+ and K+ by Na-rich extensor digitorum and soleus muscles of rat was found to be increased considerably when muscles were innervated during enrichment with Na+ in K-free modified Krebs solution containing 160 mM-Na at 2° C and recovery in a similar fluid with 10 mM-K and 137 mM-Na at 37° C, bubbled with oxygen.
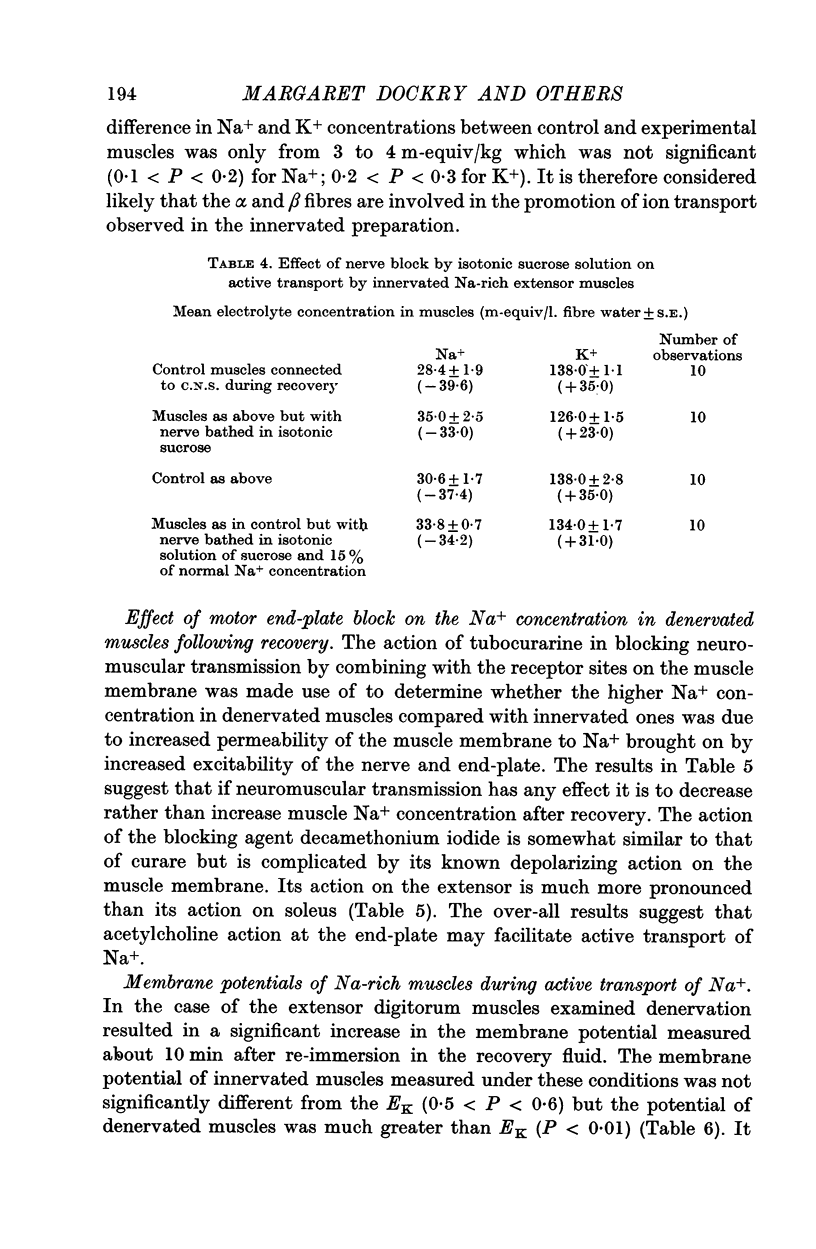
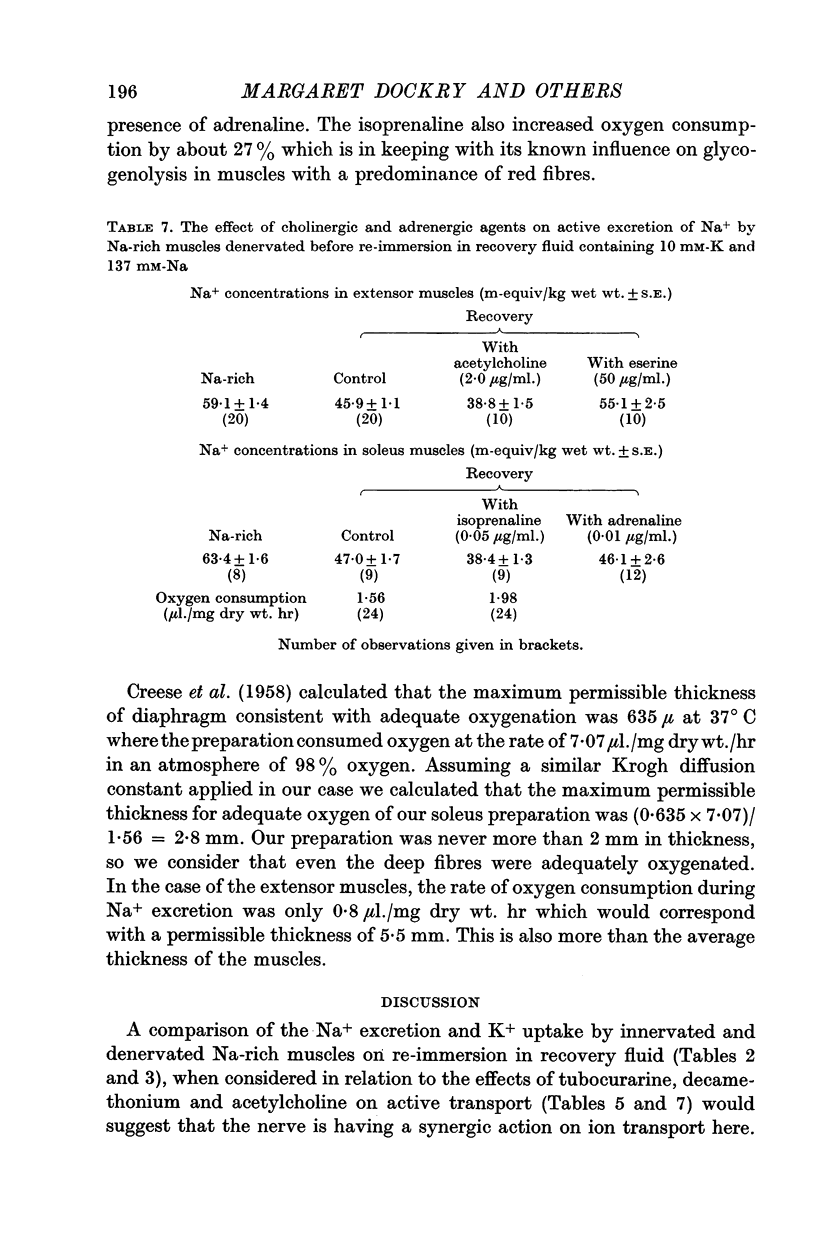
2. Addition of acetylcholine (2·0 μg/ml.) to recovery fluid containing denervated extensors increased active transport, whereas addition of eserine (50 μg/ml.), decamethonium (0·1 μg/ml.) and to a lesser extent tubocurarine (0·26 μg/ml.) inhibited active transport. Blocking of nerve conduction in innervated extensor inhibited K+ uptake more than Na+ excretion.
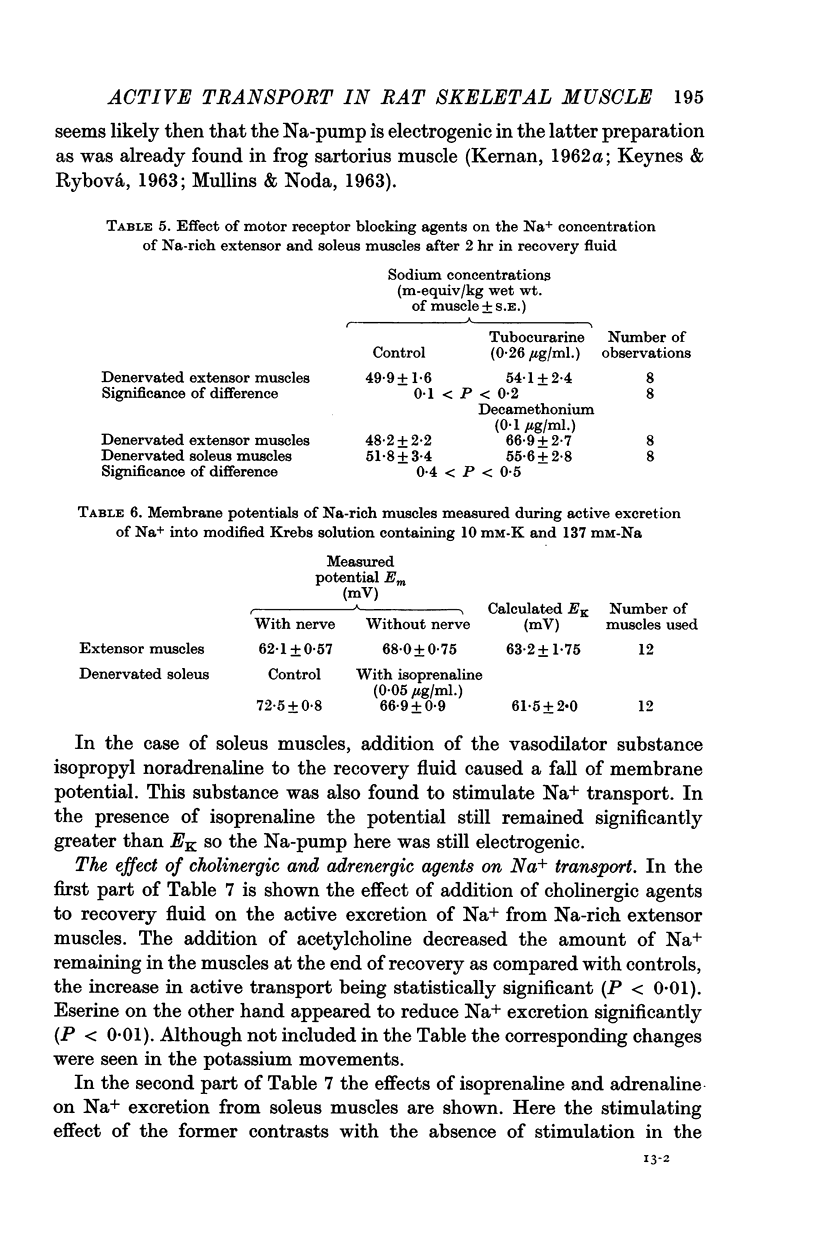
3. The membrane potential of Na-rich extensor muscles measured soon after re-immersion in recovery fluid was higher in denervated than in innervated muscles. In the latter it was close to the K-equilibrium potential (EK). It is suggested that denervation here makes the Na-pump electrogenic by decreasing K+ uptake either by decreased permeability or by inactivating a K-pump. Evidence is presented that the latter is more likely.
4. Addition of isoprenaline to Na-rich soleus muscles in recovery fluid increased active transport and reduced the membrane potential measured soon after re-immersion in recovery fluid. The Na-pump still remained electrogenic in the presence of isoprenaline. It was suggested that isoprenaline might also stimulate the Na-pump, perhaps through activation of lactic dehydrogenase.
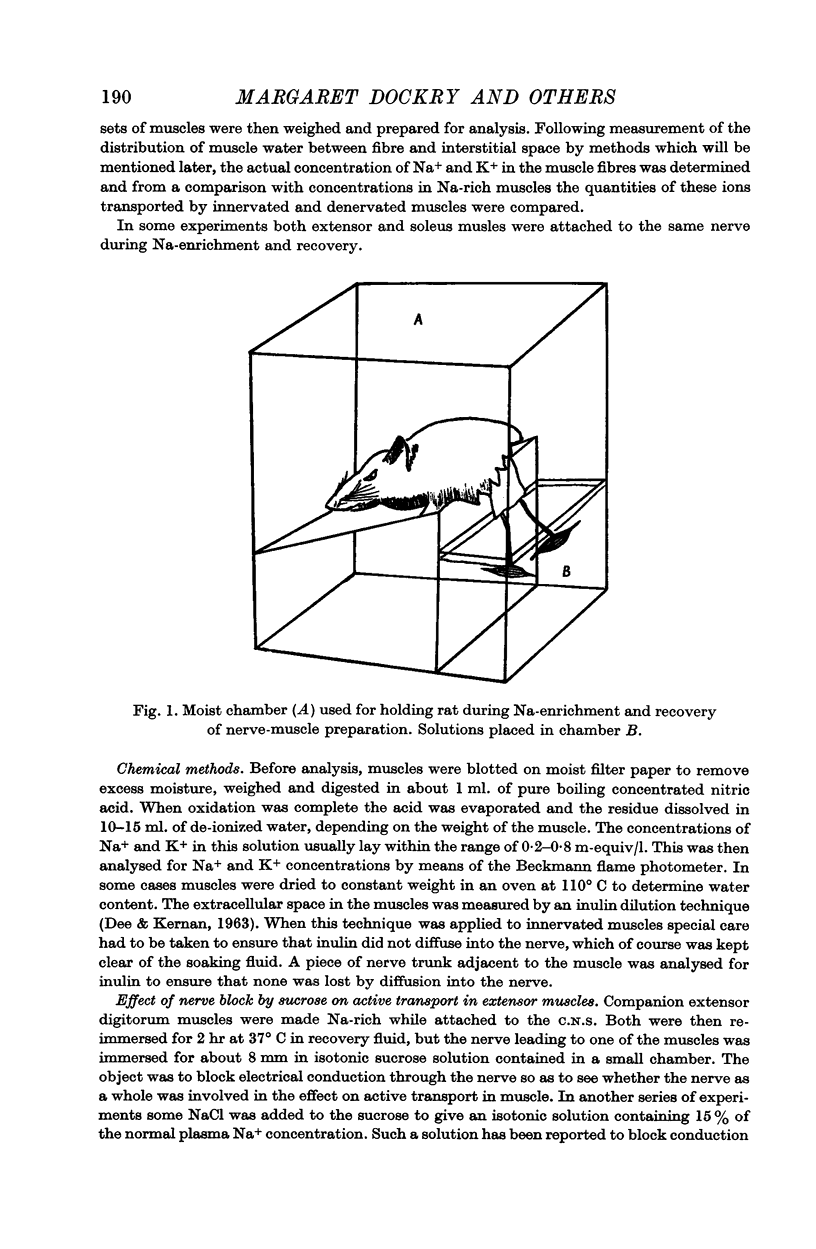
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLWOOD M. J., COBBOLD A. F. Lactic acid release by intraarterial adrenaline infusions before and after dibenyline, and its relationship to blood-flow changes in the human forearm. J Physiol. 1961 Jul;157:328–334. doi: 10.1113/jphysiol.1961.sp006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine on conduction in mammalian non-myelinated fibres and its prevention by an anticholinesterase. J Physiol. 1960 Jun;152:141–158. doi: 10.1113/jphysiol.1960.sp006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWMAN W. C., ZAIMIS E. The effects of adrenaline, noradrenaline and isoprenaline on skeletal muscle contractions in the cat. J Physiol. 1958 Nov 10;144(1):92–107. doi: 10.1113/jphysiol.1958.sp006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY M. J., CONWAY E. J., KERNAN R. P. Secretion of sodium ions by the frog's sartorius. J Physiol. 1959 Oct;148:51–82. doi: 10.1113/jphysiol.1959.sp006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J., KERNAN R. P., ZADUNAISKY J. A. The sodium pump in skeletal muscle in relation to energy barriers. J Physiol. 1961 Feb;155:263–279. doi: 10.1113/jphysiol.1961.sp006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOKE R. E., SEGAR W. E., CHEEK D. B., COVILLE F. E., DARROW D. C. The extrarenal correction of alkalosis associated with potassium deficiency. J Clin Invest. 1952 Aug;31(8):798–805. doi: 10.1172/JCI102665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREESE R., SCHOLES N. W., WHALEN W. J. Resting potentials of diaphragm muscle after prolonged anoxia. J Physiol. 1958 Feb 17;140(2):301–317. doi: 10.1113/jphysiol.1958.sp005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEE E., KERNAN R. P. Energetics of sodium transport in Rana pipiens. J Physiol. 1963 Mar;165:550–558. doi: 10.1113/jphysiol.1963.sp007078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESMEDT J. E. Electrical activity and intracellular sodium concentration in frog muscle. J Physiol. 1953 Jul;121(1):191–205. doi: 10.1113/jphysiol.1953.sp004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DETTBARN W. D. New evidence for the role of acetylcholine in conduction. Biochim Biophys Acta. 1960 Jul 15;41:377–386. doi: 10.1016/0006-3002(60)90034-2. [DOI] [PubMed] [Google Scholar]
- DRAHOTA Z., GUTMANN E. LONG-TERM REGULATORY INFLUENCE OF THE NERVOUS SYSTEM ON SOME METABOLIC DIFFERENCES IN MUSCLES OF DIFFERENT FUNCTION. Physiol Bohemoslov. 1963;12:339–348. [PubMed] [Google Scholar]
- Dydynska M., Harris E. J. Consumption of high-energy phosphates during active sodium and potassium interchange in frog muscle. J Physiol. 1966 Jan;182(1):92–109. doi: 10.1113/jphysiol.1966.sp007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Effects of sodium pump inhibitors on spontaneous acetylcholine release at the neuromuscular junction. J Physiol. 1965 Dec;181(3):498–505. doi: 10.1113/jphysiol.1965.sp007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Metabolic studies on the hyperpolarization following activity in mammalian non-myelinated nerve fibres. J Physiol. 1962 May;161:414–423. doi: 10.1113/jphysiol.1962.sp006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H. D., KEPCHAR J. H. Control of peripheral resistance in major systemic vascular beds. Physiol Rev. 1959 Jul;39(3):617–686. doi: 10.1152/physrev.1959.39.3.617. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- KERNAN R. P. The role of lactate in the active excretion of sodium by frog muscle. J Physiol. 1962 Jun;162:129–137. doi: 10.1113/jphysiol.1962.sp006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSCHNER L. B. Effect of cholinesterase inhibitors and atropine on active sodium transport across frog skin. Nature. 1953 Aug 15;172(4372):348–349. doi: 10.1038/172348a0. [DOI] [PubMed] [Google Scholar]
- Kernan R. P. Denervation and the electrogenesis of the sodium pump in frog skeletal muscle. Nature. 1966 Apr 30;210(5035):537–538. doi: 10.1038/210537a0. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., AWAD M. Z. THE CONTROL OF THE MEMBRANE POTENTIAL OF MUSCLE FIBERS BY THE SODIUM PUMP. J Gen Physiol. 1965 May;48:761–775. doi: 10.1085/jgp.48.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J., FRUMENTO A. S. The concentration dependence of sodium efflux from muscle. J Gen Physiol. 1963 Mar;46:629–654. doi: 10.1085/jgp.46.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J., NODA K. THE INFLUENCE OF SODIUM-FREE SOLUTIONS ON THE MEMBRANE POTENTIAL OF FROG MUSCLE FIBERS. J Gen Physiol. 1963 Sep;47:117–132. doi: 10.1085/jgp.47.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOFF J., KENNEDY T. J., Jr, BERLINER R. W. The effect of potassium in nephrectomized rats with hypokalemic alkalosis. J Clin Invest. 1953 Jun;32(6):538–542. doi: 10.1172/JCI102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRETER F. A., WOO G. CELL WATER, SODIUM, AND POTASSIUM IN RED AND WHITE MAMMALIAN MUSCLES. Am J Physiol. 1963 Dec;205:1290–1294. doi: 10.1152/ajplegacy.1963.205.6.1290. [DOI] [PubMed] [Google Scholar]
- ZAIMIS E. J. The action of decamethonium on normal and denervated mammalian muscle. J Physiol. 1951 Jan;112(1-2):176–190. doi: 10.1113/jphysiol.1951.sp004518. [DOI] [PMC free article] [PubMed] [Google Scholar]


