Abstract
1. The properties of fifty-two stretch receptors in the extraocular muscles were studied in thirty cats. Between ten and twenty-eight receptors were observed in each preparation.
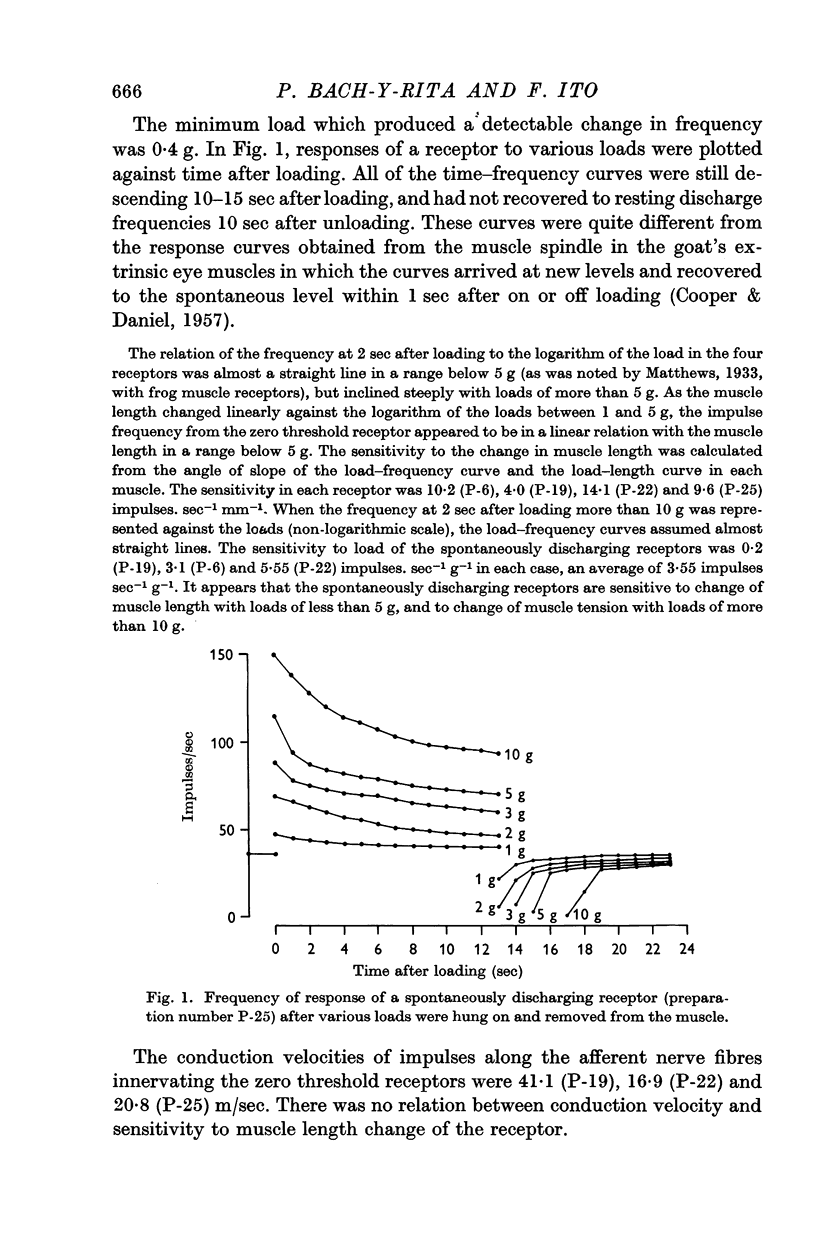
2. In four preparations, spontaneously discharging receptors were observed, with afferent fibre conduction velocities ranging from 16·9 to 41·1 m/sec.
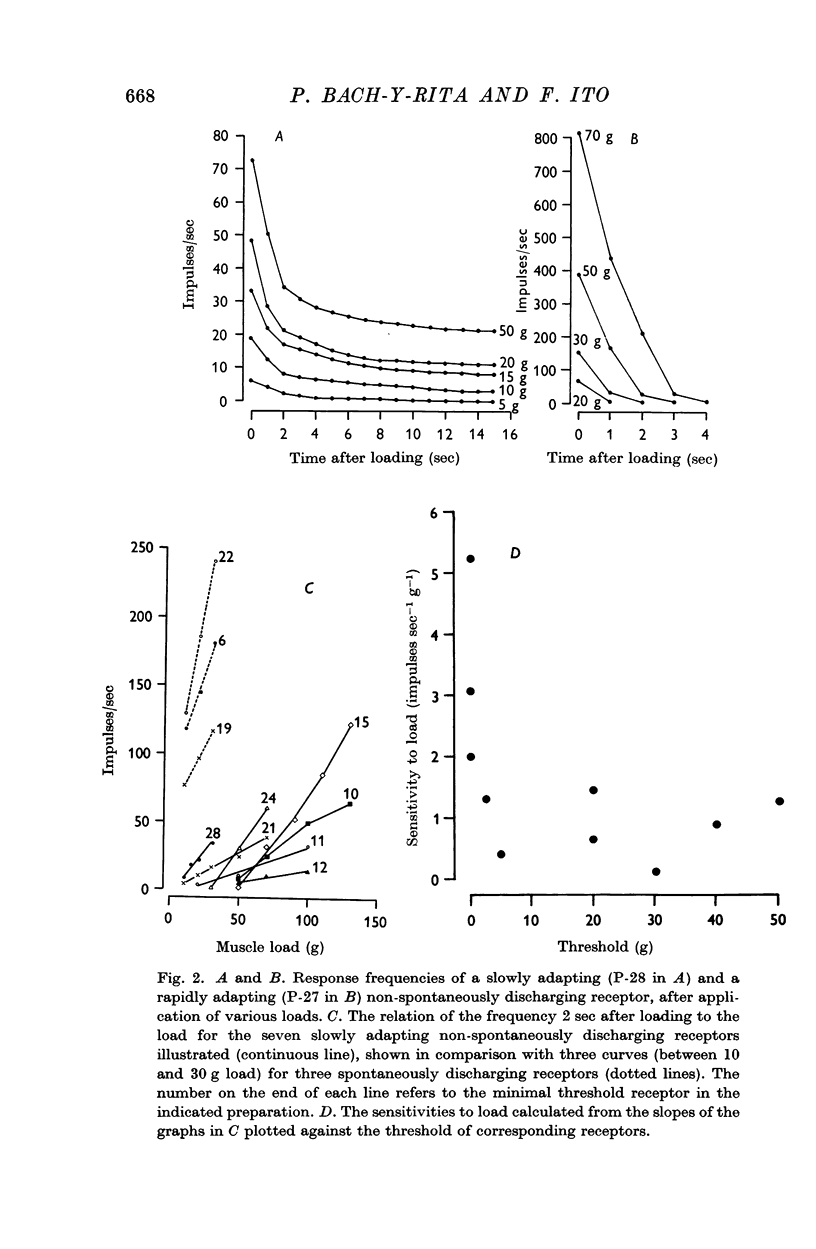
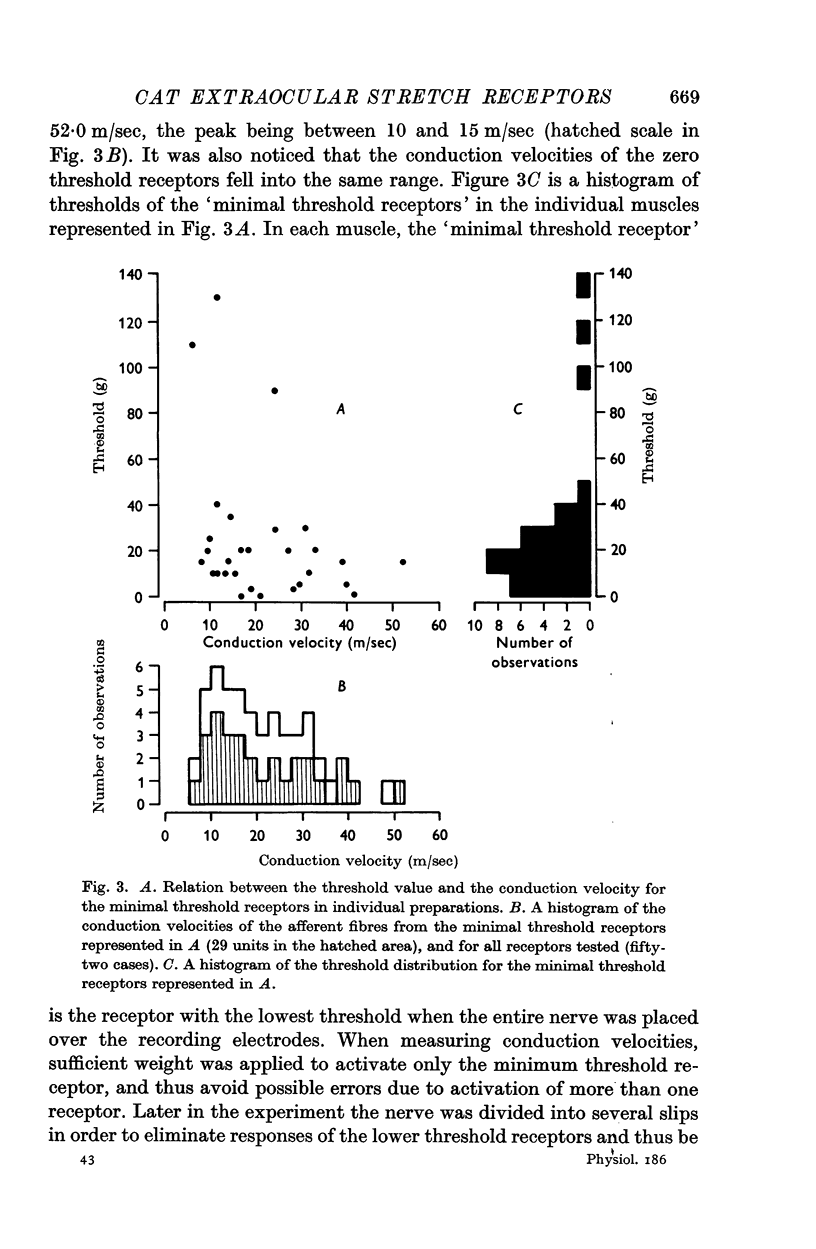
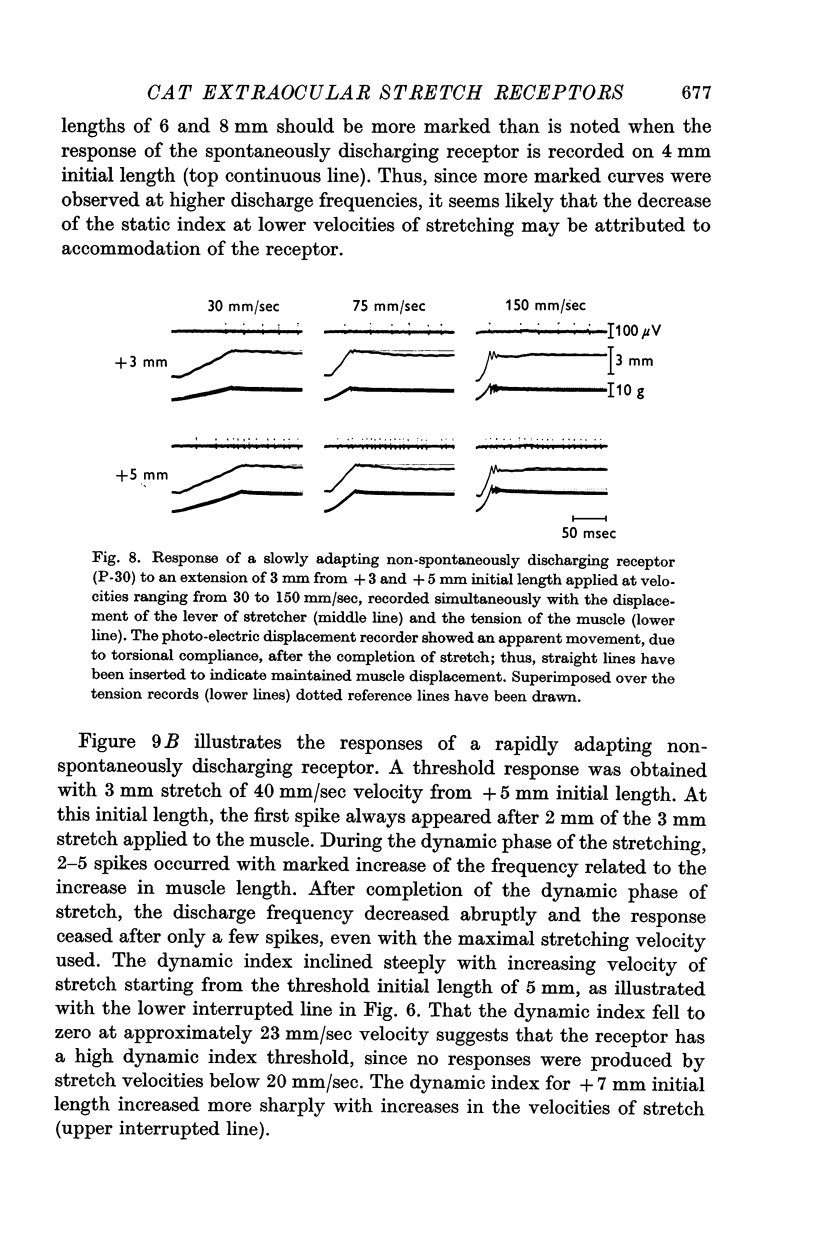
3. In the remaining twenty-six cats, the minimal threshold receptors ranged from 3 to 130 g, with a peak distribution between 10 and 20 g, and afferent fibre conduction velocities ranging from 6·5 to 52·0 m/sec, the peak being between 10 and 15 m/sec. Of these receptors, nineteen were quickly adapting and seven were slowly adapting.
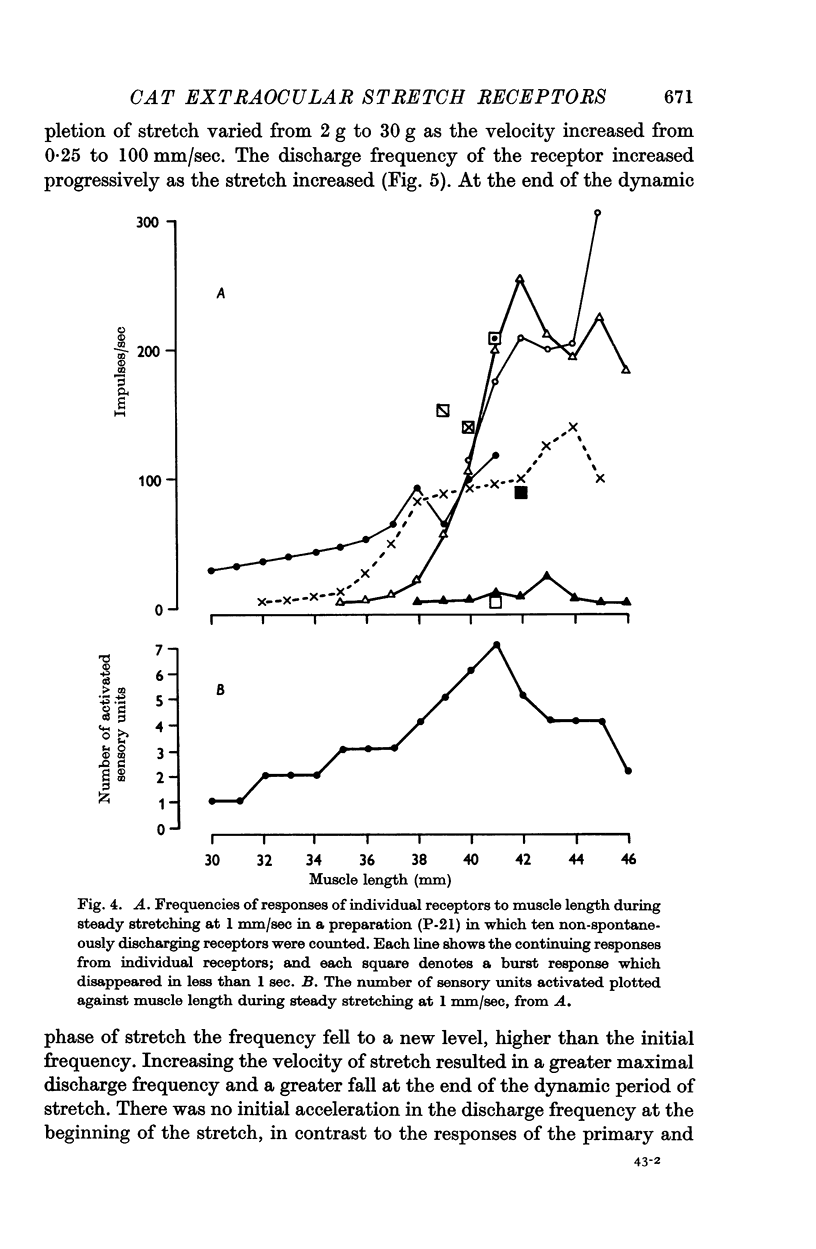
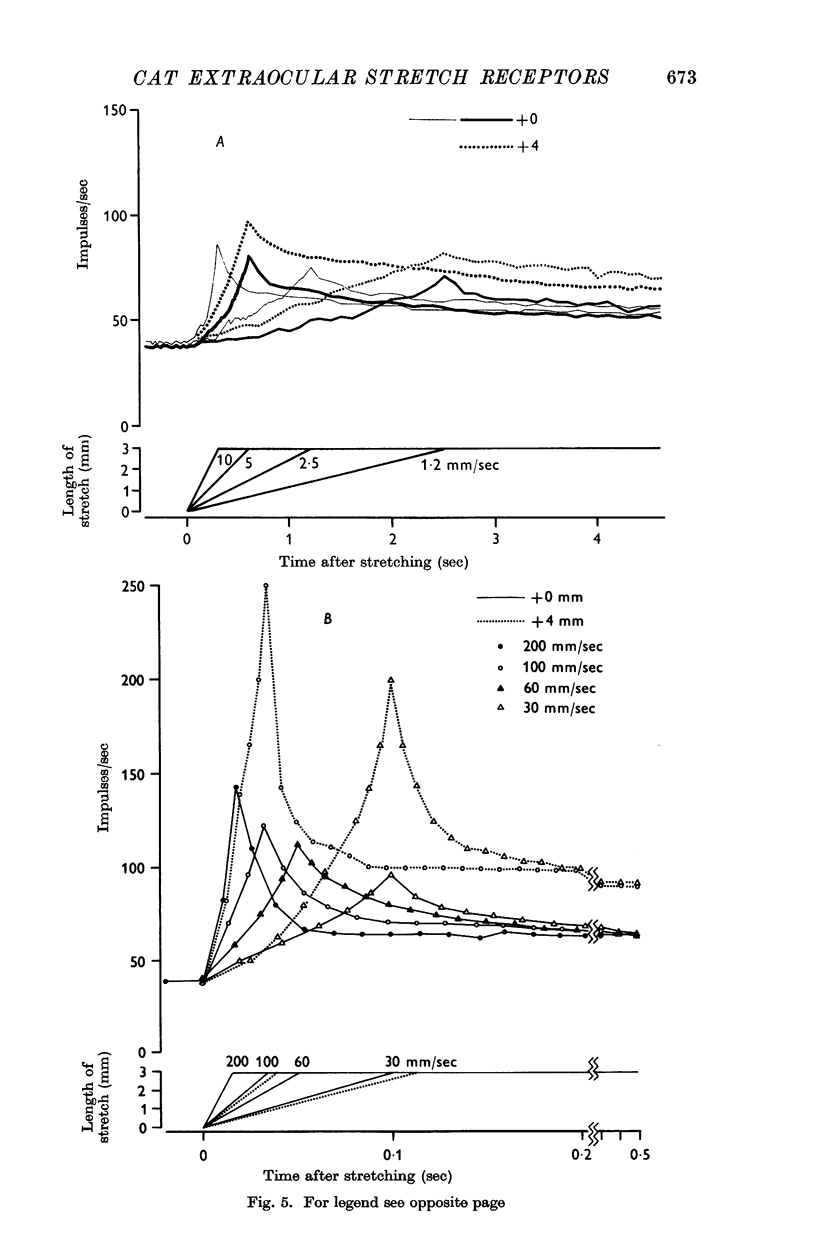
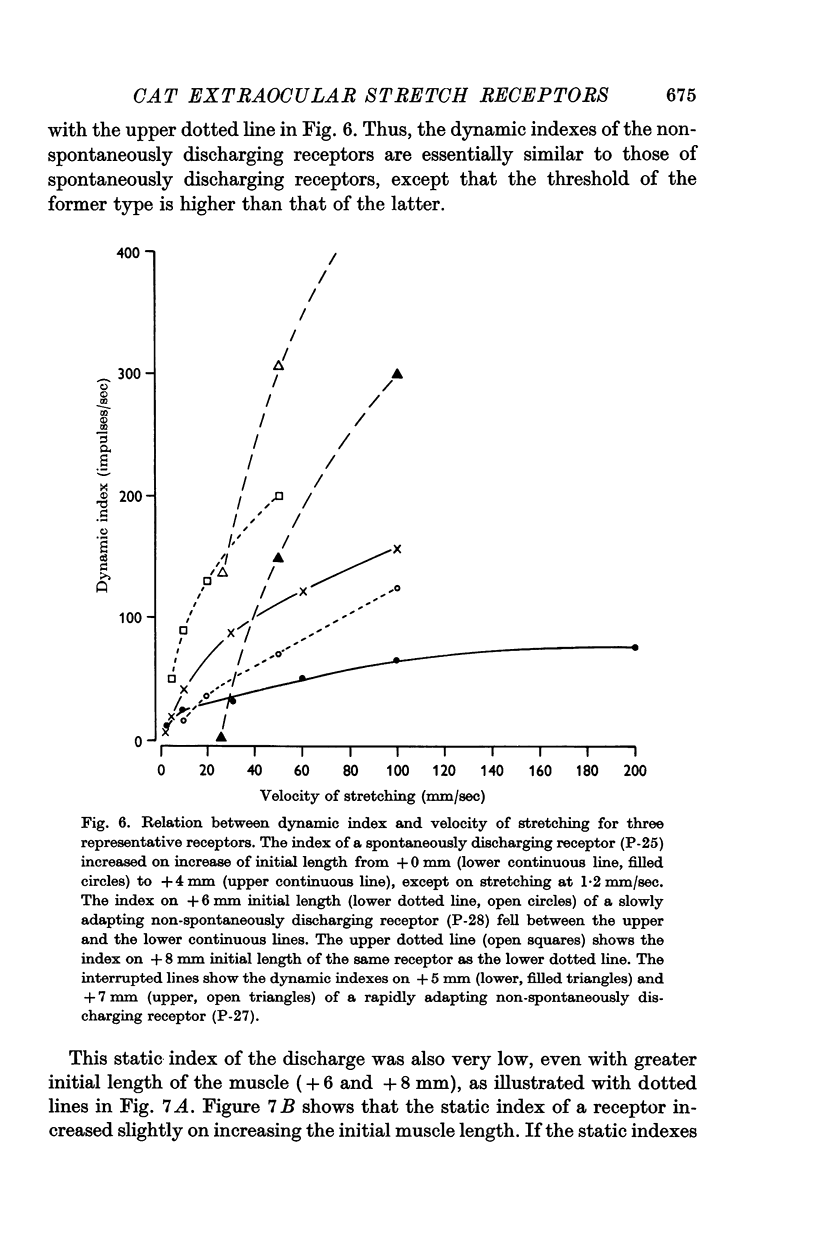
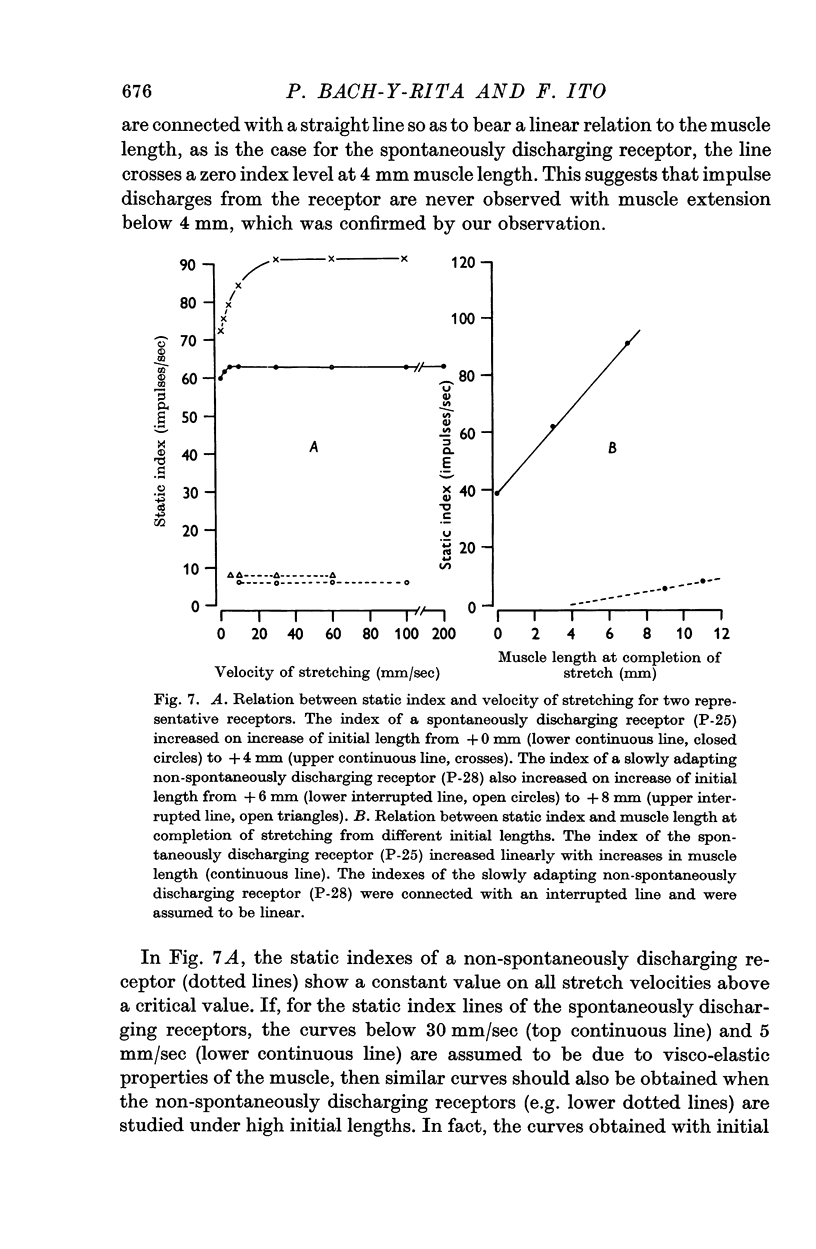
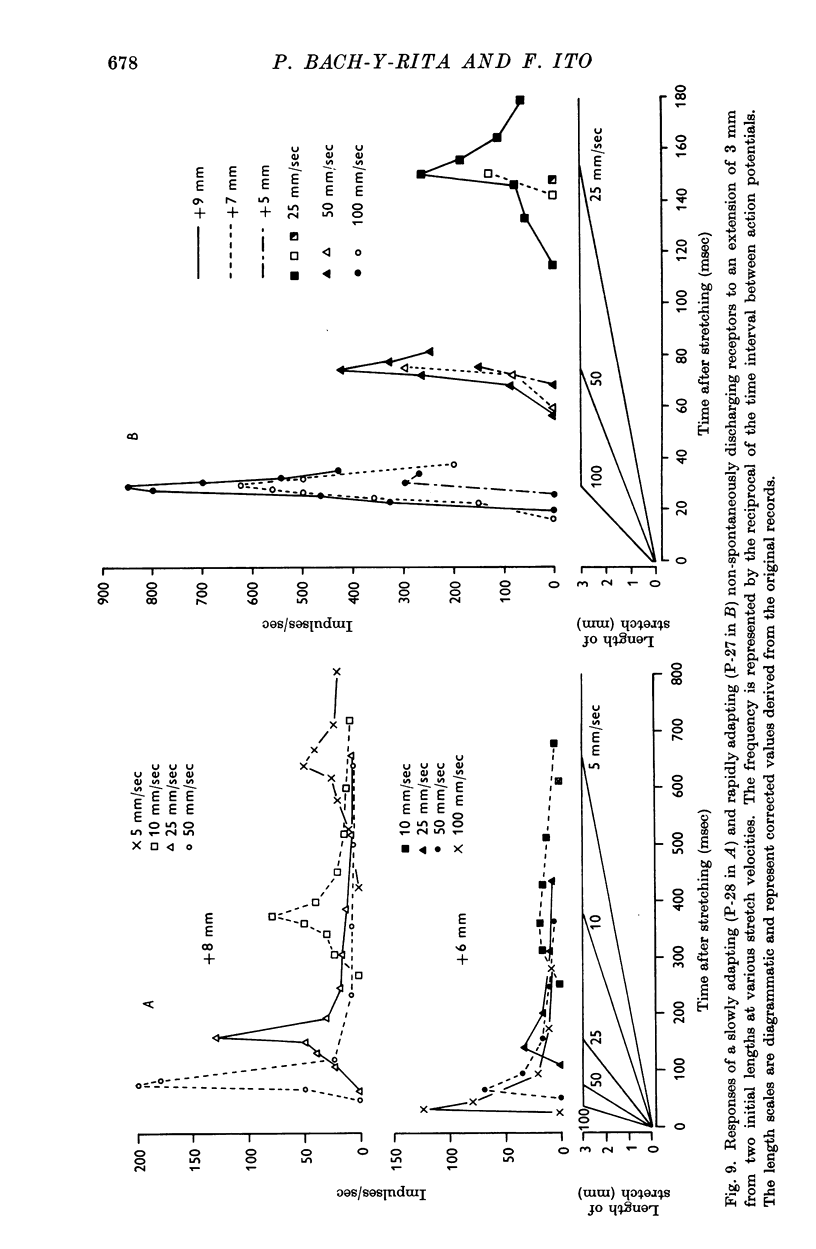
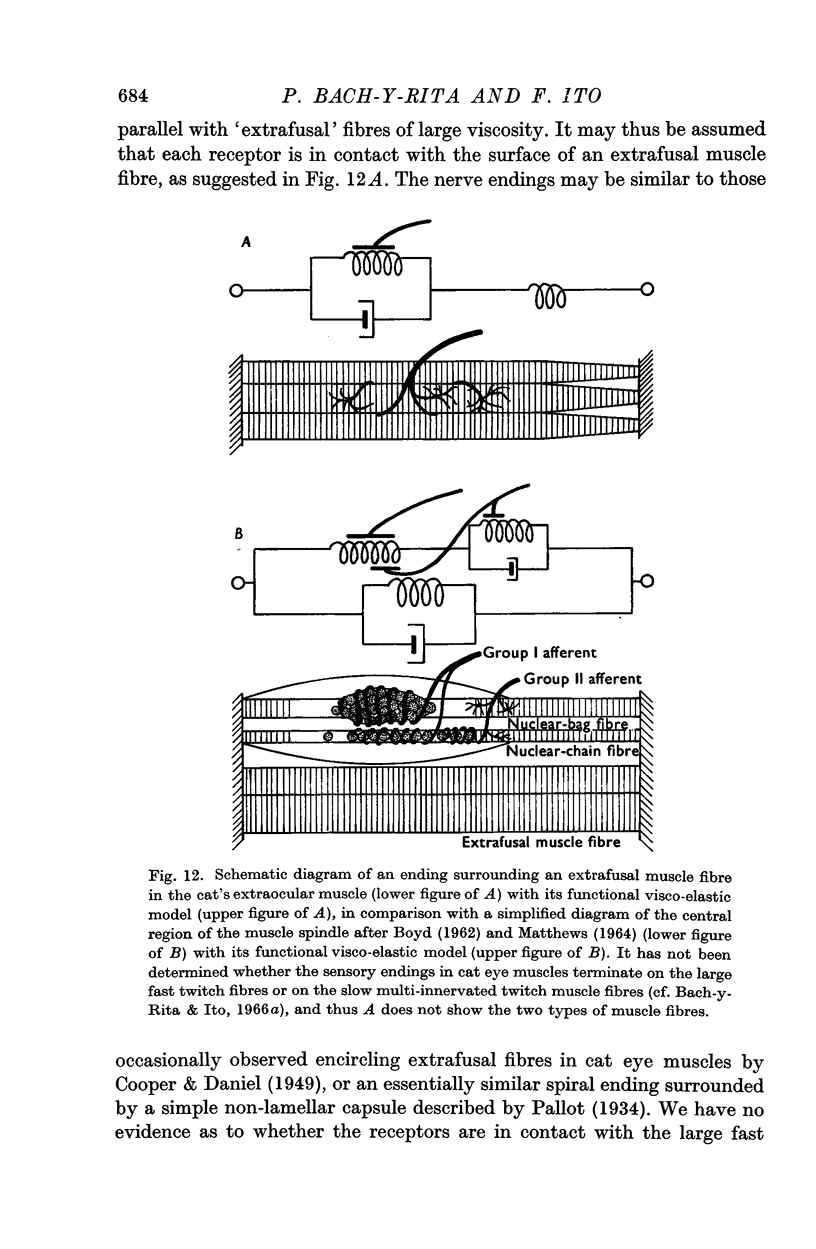
4. The dynamic and static indexes of all the receptors were essentially similar; they both increased markedly on increasing the initial length. This suggests that the receptors do not lie in contact with regions of reduced viscosity on the muscle fibres comparable to the equatorial region of the intrafusal muscle fibres.
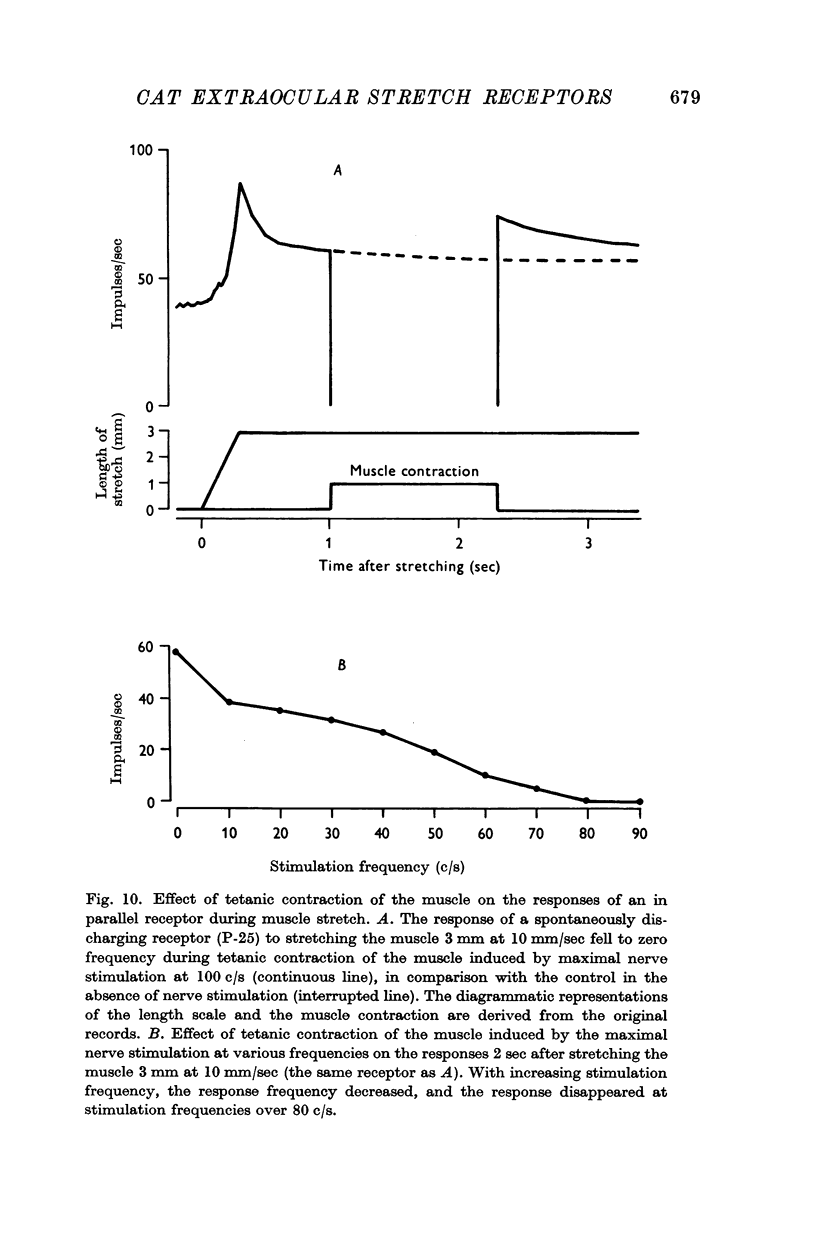
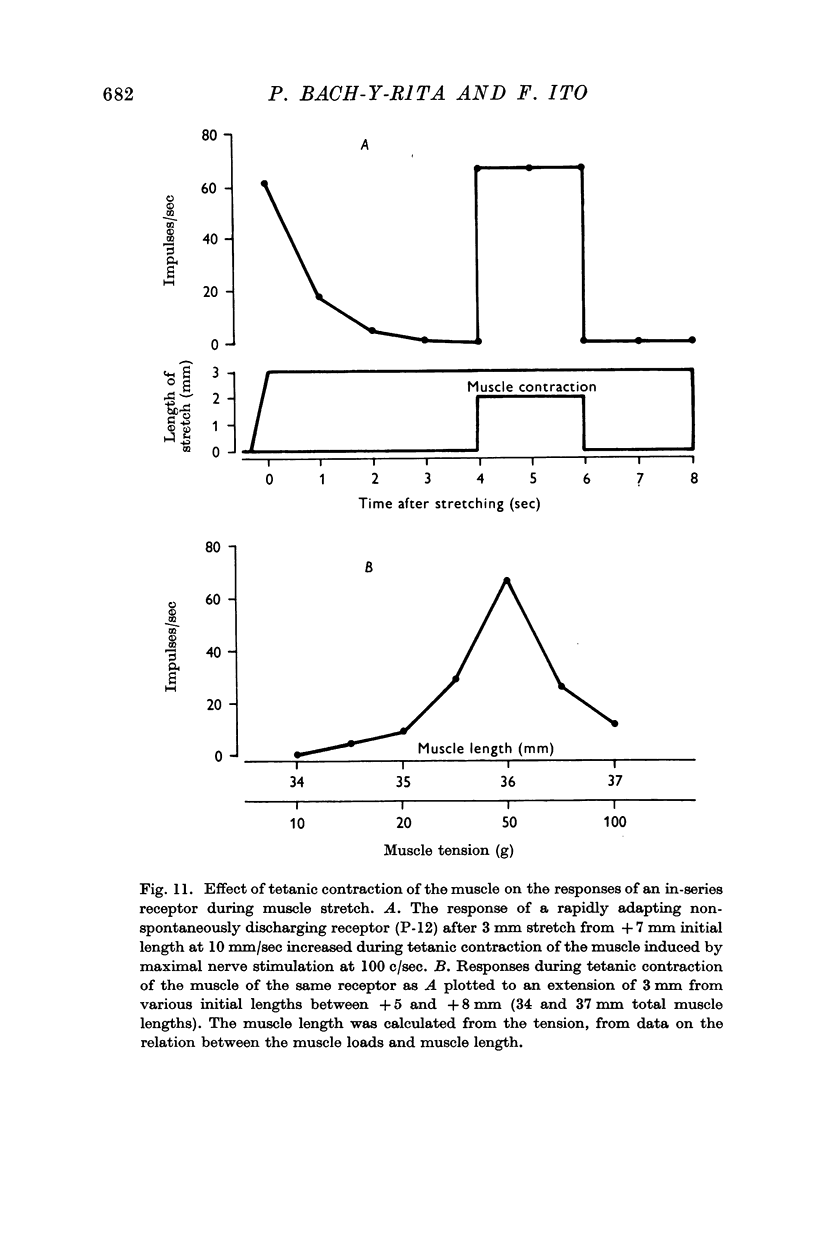
5. All of the receptors were located in the muscle; none was located in the tendon. Forty-seven of forty-nine receptors were in parallel and two receptors were in series with the contractile elements.
6. The properties of all the receptors studied appeared to be similar, suggesting that a single type of stretch receptor is located in the inferior oblique muscle of cats.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A., ROBERTS T. D. Proprioceptive discharges from stretch-receptors in the knee-joint of the cat. J Physiol. 1953 Oct;122(1):38–58. doi: 10.1113/jphysiol.1953.sp004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach-y-Rita P., Ito F. In vivo studies on fast and slow muscle fibers in cat extraocular muscles. J Gen Physiol. 1966 Jul;49(6):1177–1198. doi: 10.1085/jgp.0491177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER S., DANIEL P. M. Responses from the stretch receptors of the goat's extrinsic eye muscles with an intact motor innervation. Q J Exp Physiol Cogn Med Sci. 1957 Apr;42(2):222–231. doi: 10.1113/expphysiol.1957.sp001252. [DOI] [PubMed] [Google Scholar]
- COOPER S., DANIEL P. M., WHITTERIDGE D. Muscle spindles and other sensory endings in the extrinsic eye muscles; the physiology and anatomy of these receptors and of their connexions with the brain-stem. Brain. 1955;78(4):564–583. doi: 10.1093/brain/78.4.564. [DOI] [PubMed] [Google Scholar]
- COOPER S., DANIEL P. M., WHITTERIDGE D. Nerve impulses in the brainstem of the goat; short latency responses obtained by stretching the extrinsic eye muscles and the jaw muscles. J Physiol. 1953 Jun 29;120(4):471–490. doi: 10.1113/jphysiol.1953.sp004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER S., FILLENZ M. Afferent discharges in response to stretch from the extraocular muscles of the cat and monkey and the innervation of these muscles. J Physiol. 1955 Feb 28;127(2):400–413. doi: 10.1113/jphysiol.1955.sp005266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. The isometric responses of mammalian muscles. J Physiol. 1930 Jun 27;69(4):377–385. doi: 10.1113/jphysiol.1930.sp002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSOKAWA H. Proprioceptive innervation of striated muscles in the territory of cranial nerves. Tex Rep Biol Med. 1961;19:405–464. [PubMed] [Google Scholar]
- ITO F., TOYAMA K., ITO R. A COMPARATIVE STUDY ON STRUCTURE AND FUNCTION BETWEEN THE EXTRAFUSAL RECEPTOR AND THE SPINDLE RECEPTOR IN THE FROG. Jpn J Physiol. 1964 Feb 15;14:12–33. doi: 10.2170/jjphysiol.14.12. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The central control of the dynamic response of muscle spindle receptors. J Physiol. 1962 May;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. MUSCLE SPINDLES AND THEIR MOTOR CONTROL. Physiol Rev. 1964 Apr;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Participation by pressure-pain receptors of mammalian muscles in the flexion reflex. J Physiol. 1961 May;156:498–514. doi: 10.1113/jphysiol.1961.sp006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON D. A. THE MECHANICS OF HUMAN SACCADIC EYE MOVEMENT. J Physiol. 1964 Nov;174:245–264. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASAKI K. Electrophysiological studies on oculomotor neurons of the cat. Jpn J Physiol. 1963 Jun 15;13:287–302. doi: 10.2170/jjphysiol.13.287. [DOI] [PubMed] [Google Scholar]


