Abstract
1. In cats anaesthetized with sodium pentobarbital 160 external intercostal muscle spindle afferents were identified by their pause in response to ventral root stimulation; the internal intercostal muscle was denervated.
2. In order to assignate the afferents to either primary or secondary endings they were tested for their responsiveness to vibration (Bianconi & Van Der Meulen, 1963). The maximal frequency which they were able to follow regularly for at least four cycles, termed `critical frequency', was determined.
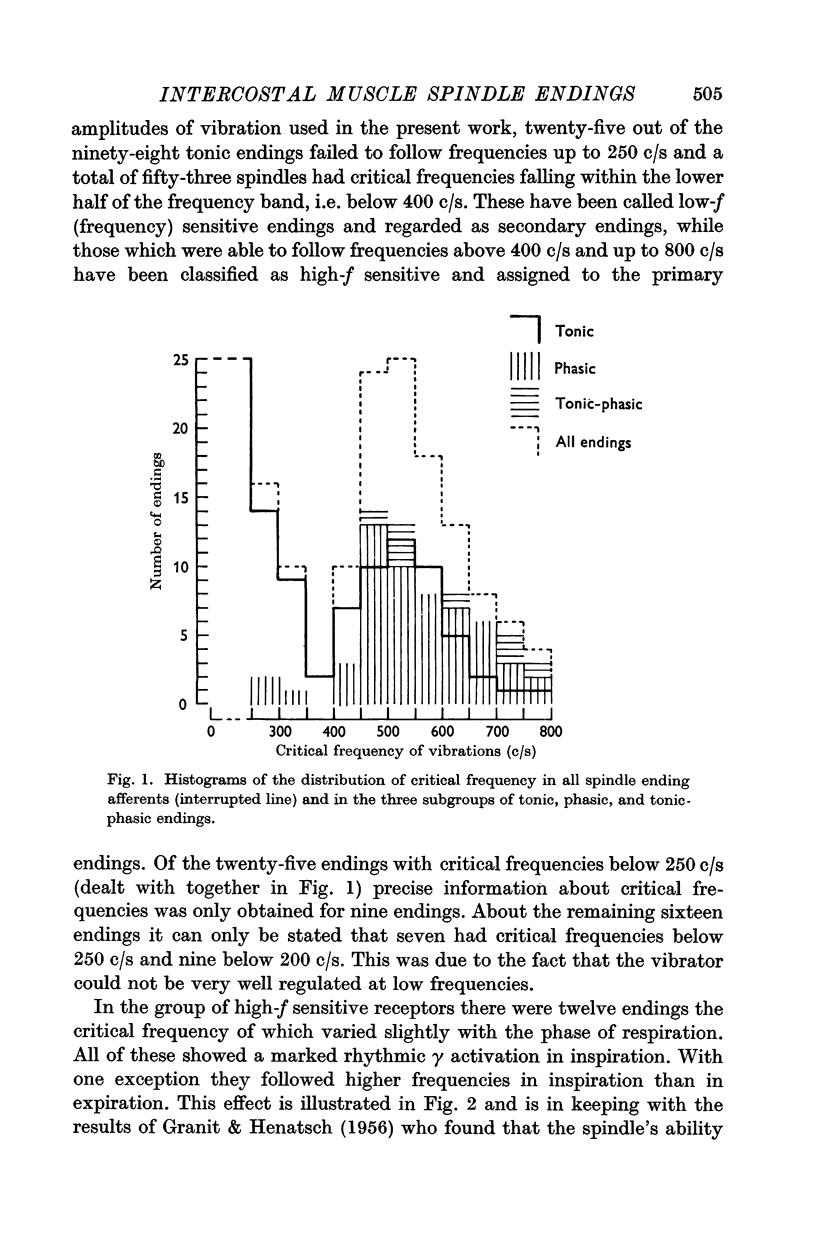
3. The endings fell into two groups: low-f (frequency) sensitive endings with critical frequencies below 400 c/s and high-f sensitive endings with critical frequencies above 400 c/s. The latter were regarded as primary endings and the former as secondary ones.
4. The manner in which the spindle endings resumed activity after a pause produced by shocks to the ventral root, i.e. whether `phasic' or `tonic' (Granit & Van Der Meulen, 1962) was studied in all the spindle afferents.
5. All the secondary (low-f sensitive) endings were `tonic' except three for which the determination of critical frequency was questionable. Both `tonic' and `phasic' properties were found among the primary (high-f sensitive) endings.
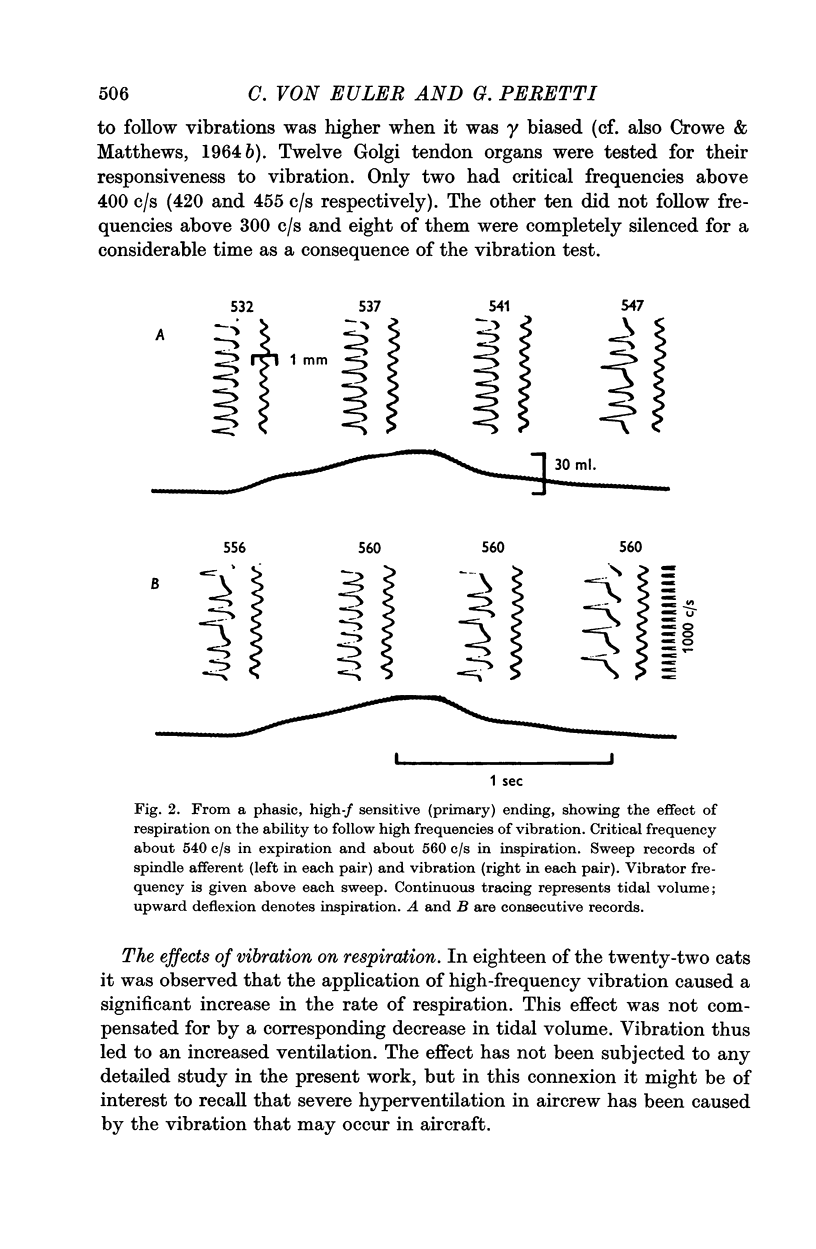
6. The majority of the secondary endings (74%) showed inspiratory rhythmic fusimotor activation in parallel with the skeletomotor contraction as did the primary endings (79%).
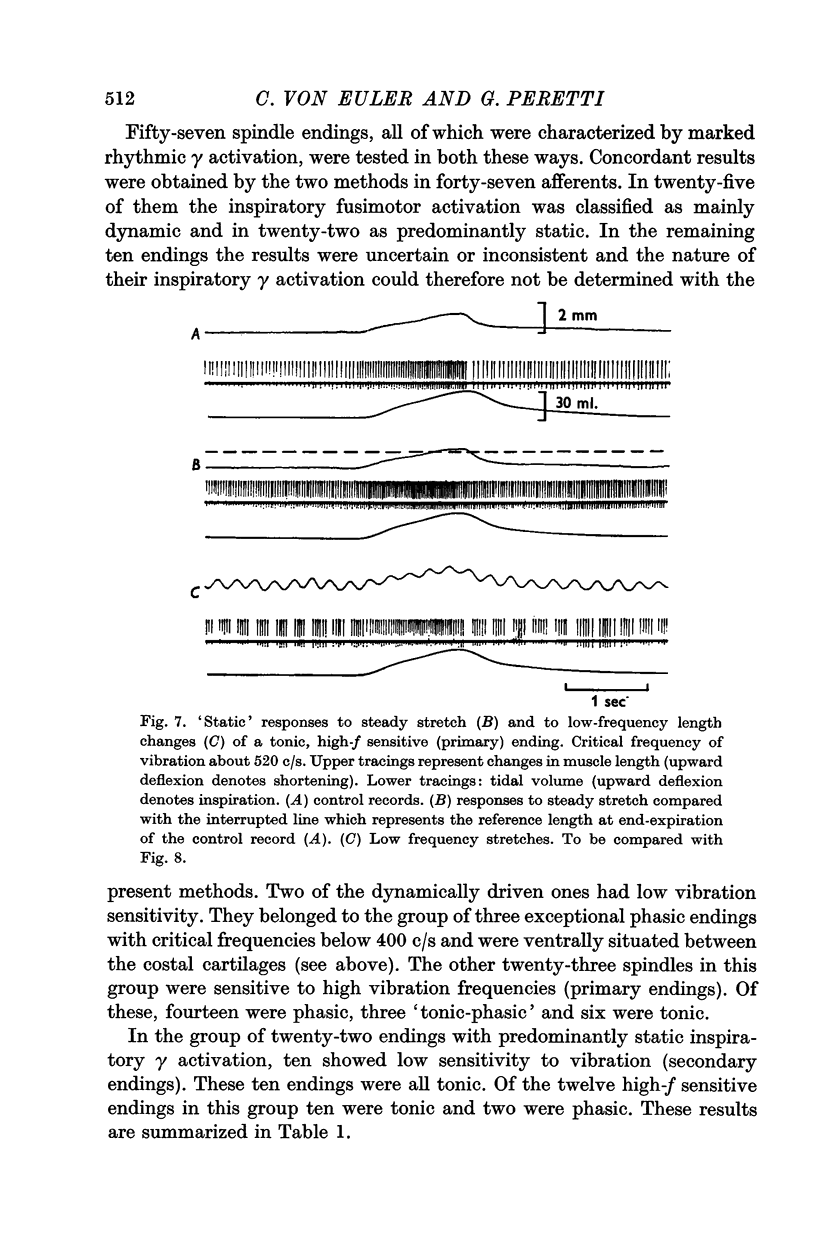
7. Fifty-seven spindle endings which all showed marked rhythmic inspiratory γ activation were tested for respiratory variations in their dynamic responses to steady stretch and length changes introduced at low repetition rates.
8. The results indicate that both `dynamic' and `static' γ fibres are represented among the rhythmic γ fibres controlling primary muscle spindle endings, whereas rhythmic activation of secondary endings seems to be mediated only by `static' fibres.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALNAES E., JANSEN J. K., RUDJORD T. FUSIMOTOR ACTIVITY IN THE SPINAL CAT. Acta Physiol Scand. 1965 Mar;63:197–212. doi: 10.1111/j.1748-1716.1965.tb04060.x. [DOI] [PubMed] [Google Scholar]
- BIANCONI R., van der MEULEN J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963 Jan;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- BOYD I. A. The innervation of mammalian neuromuscular spindles. J Physiol. 1958 Jan 23;140(1):14–5P. [PubMed] [Google Scholar]
- CORDA M., EKLUND G., VON EULER EXTERNAL INTERCOSTAL AND PHRENIC ALPHA-MOTOR RESPONSES TO CHANGES IN RESPIRATORY LOAD. Acta Physiol Scand. 1965 Mar;63:391–400. doi: 10.1111/j.1748-1716.1965.tb04079.x. [DOI] [PubMed] [Google Scholar]
- CORDA M., VONEULER C., LENNERSTRAND G. PROPRIOCEPTIVE INNERVATION OF THE DIAPHRAGM. J Physiol. 1965 May;178:161–177. doi: 10.1113/jphysiol.1965.sp007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRITCHLOW V., VON EULER INTERCOSTAL MUSCLE SPINDLE ACTIVITY AND ITS GAMMA MOTOR CONTROL. J Physiol. 1963 Oct;168:820–847. doi: 10.1113/jphysiol.1963.sp007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. FURTHER STUDIES OF STATIC AND DYNAMIC FUSIMOTOR FIBRES. J Physiol. 1964 Oct;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda M., von Euler C., Lennerstrand G. Reflex and cerebellar influences on alpha and on 'rhythmic' and 'tonic' gamma activity in the intercostal muscle. J Physiol. 1966 Jun;184(4):898–923. doi: 10.1113/jphysiol.1966.sp007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKLUND G., VON EULER, RUTKOWSKI S. SPONTANEOUS AND REFLEX ACTIVITY OF INTERCOSTAL GAMMA MOTONEURONES. J Physiol. 1964 May;171:139–163. doi: 10.1113/jphysiol.1964.sp007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D. Gamma control of dynamic properties of muscle spindles. J Neurophysiol. 1956 Jul;19(4):356–366. doi: 10.1152/jn.1956.19.4.356. [DOI] [PubMed] [Google Scholar]
- GRANIT R., VAN DERMEULEN J. P. The pause during contraction in the discharge of the spindle afferents from primary end organs in cat extensor muscles. Acta Physiol Scand. 1962 Jun-Jul;55:231–244. doi: 10.1111/j.1748-1716.1962.tb02436.x. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The central control of the dynamic response of muscle spindle receptors. J Physiol. 1962 May;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- MERTON P. A. Slowly conducting muscle spindle afferents. Acta Physiol Scand. 1953 Jun 26;29(1):87–88. doi: 10.1111/j.1748-1716.1953.tb01003.x. [DOI] [PubMed] [Google Scholar]
- SUMI T. The segmental reflex relations of cutaneous afferent inflow to thoracic respiratory motoneurons. J Neurophysiol. 1963 May;26:478–493. doi: 10.1152/jn.1963.26.3.478. [DOI] [PubMed] [Google Scholar]


