Abstract
1. Spatial summation within cat retinal receptive fields was studied by recording from optic-tract fibres the responses of ganglion cells to grating patterns whose luminance perpendicular to the bars varied sinusoidally about the mean level.
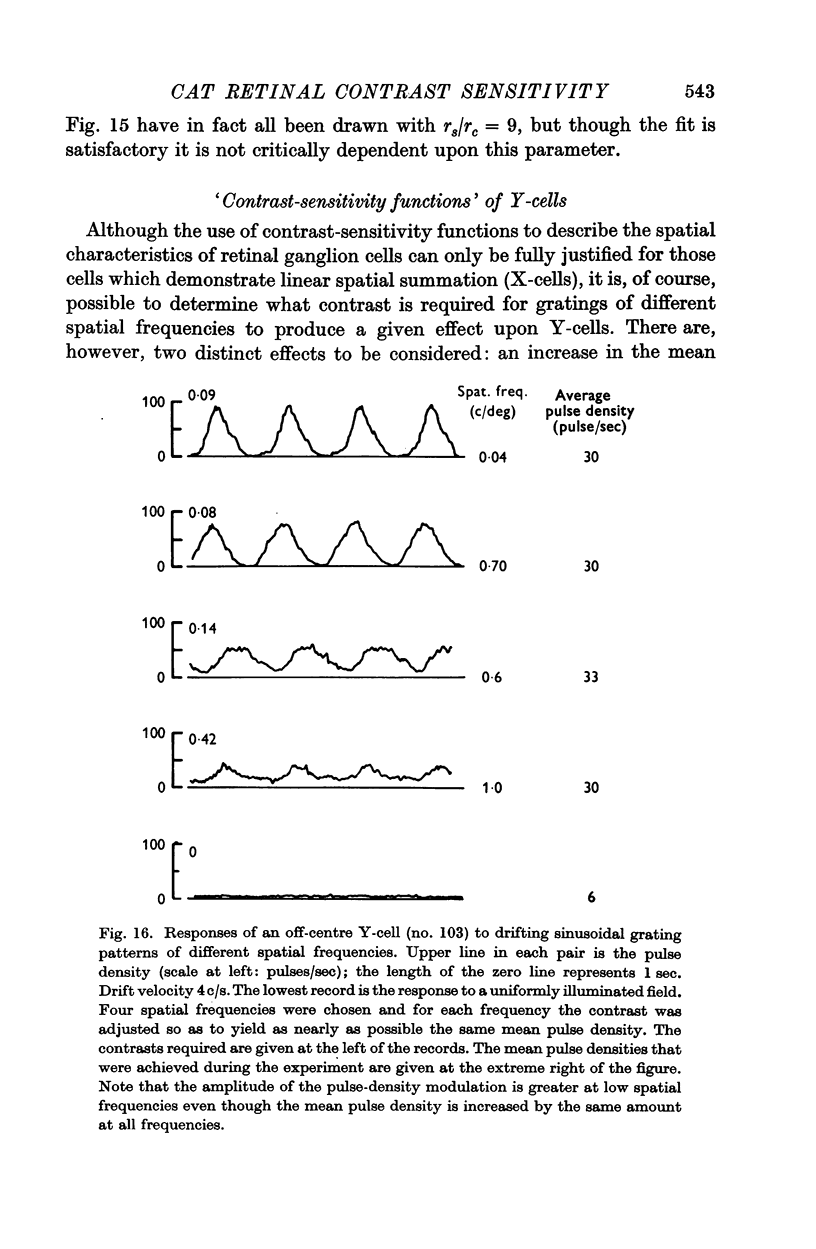
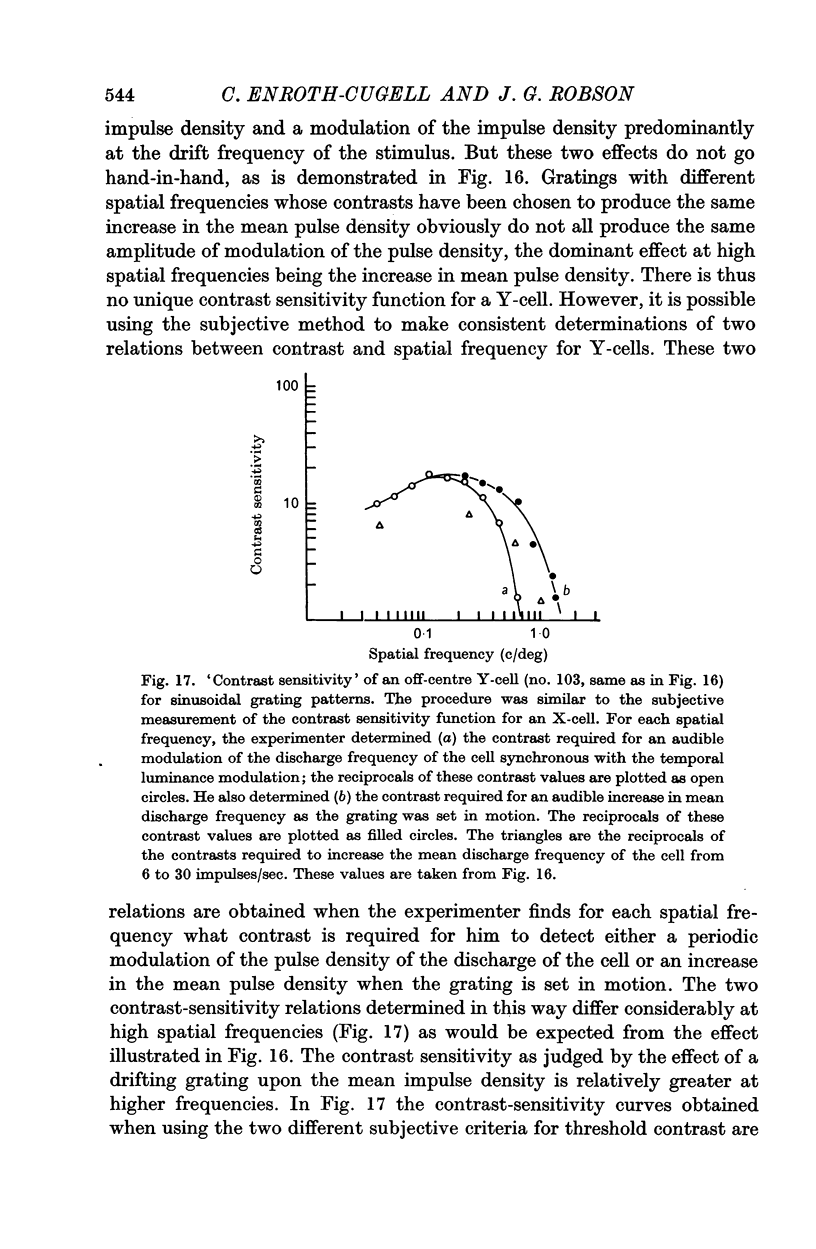
2. Summation over the receptive fields of some cells (X-cells) was found to be approximately linear, while for other cells (Y-cells) summation was very non-linear.
3. The mean discharge frequency of Y-cells (unlike that of X-cells) was greatly increased when grating patterns drifted across their receptive fields.
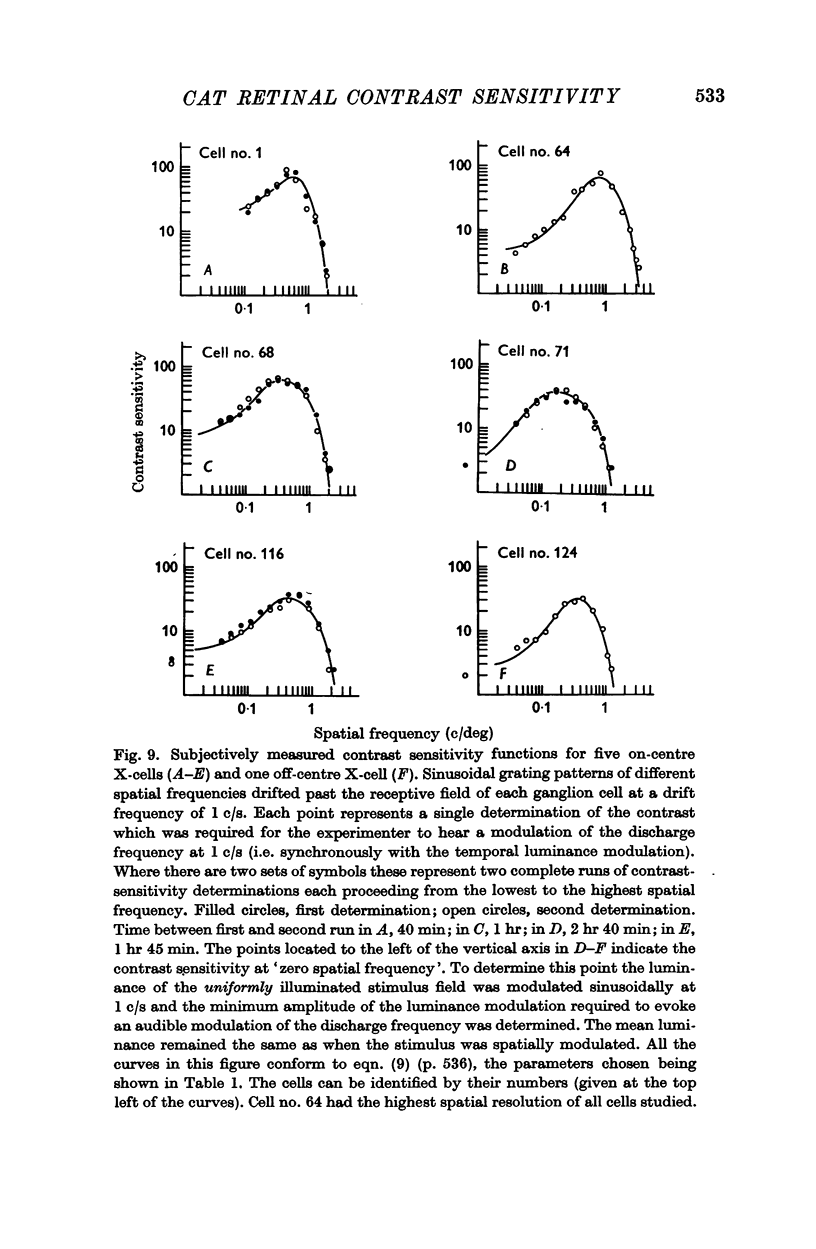
4. In twenty-one X-cells the relation between the contrast and spatial frequency of drifting sinusoidal gratings which evoked the same small response was measured. In every case it was found that the reciprocal of this relation, the contrast sensitivity function, could be satisfactorily described by the difference of two Gaussian functions.
5. This finding supports the hypothesis that the sensitivities of the antagonistic centre and surround summating regions of ganglion cell receptive fields fall off as Gaussian functions of the distance from the field centre.
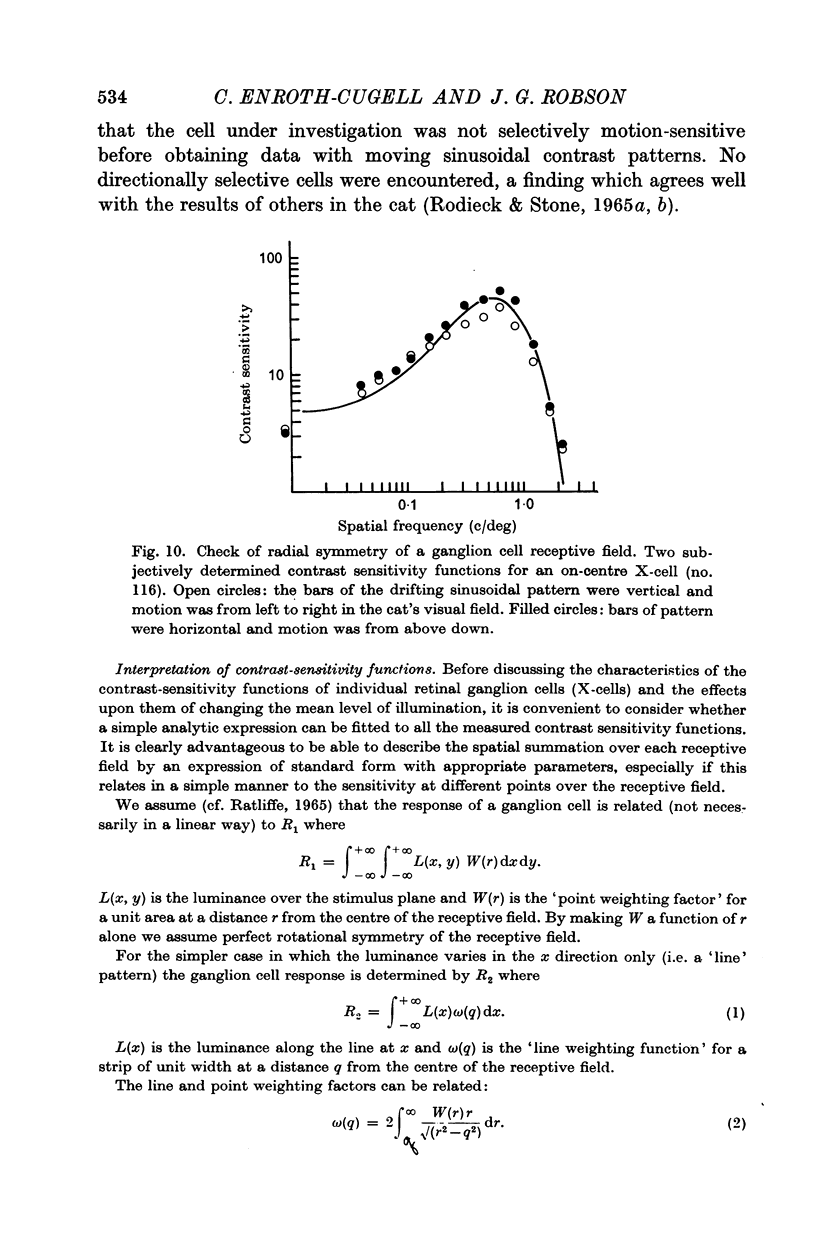
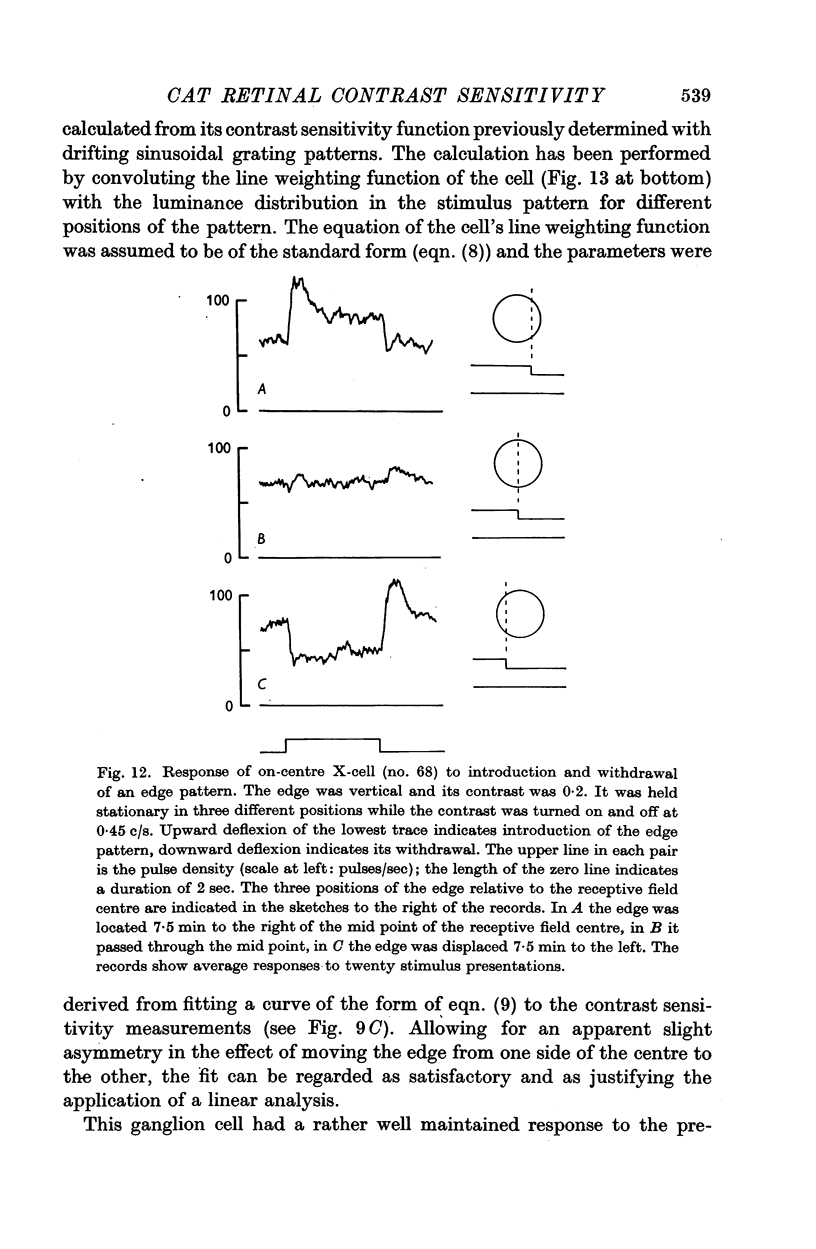
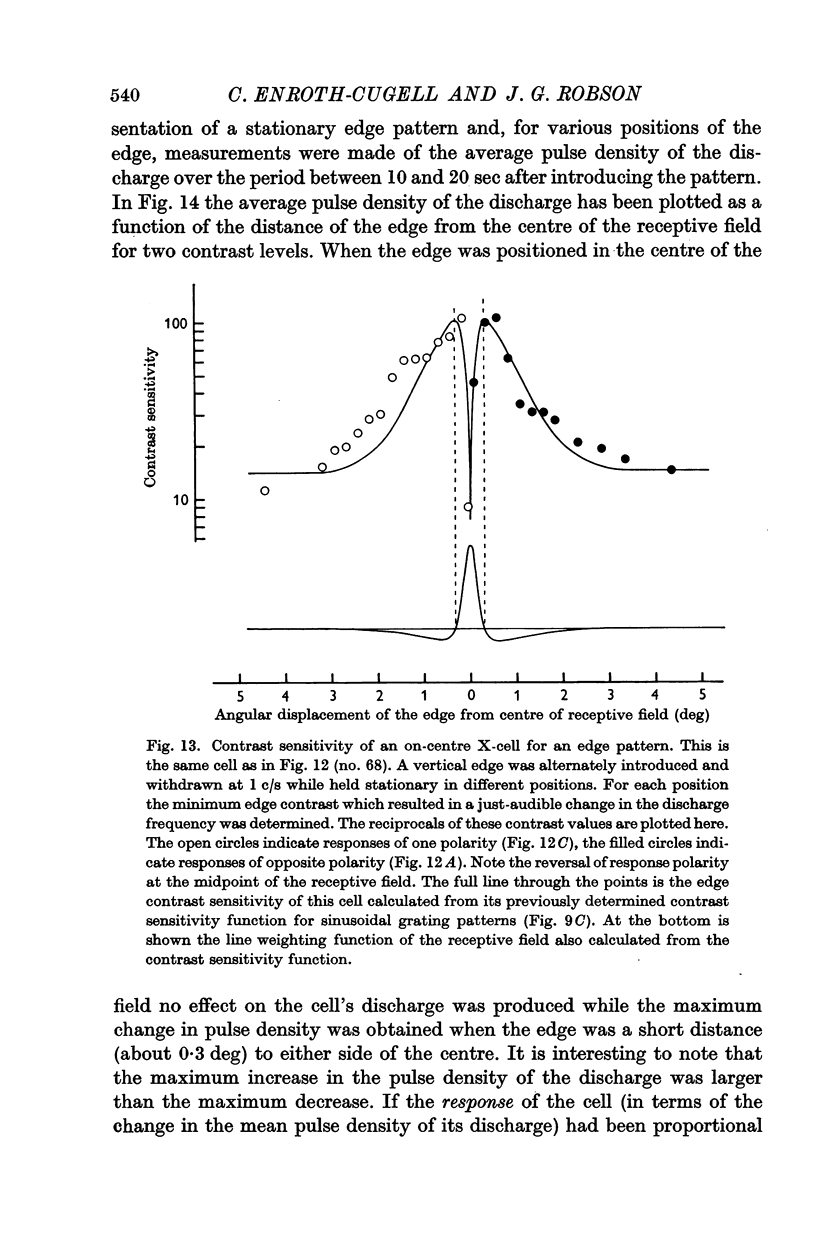
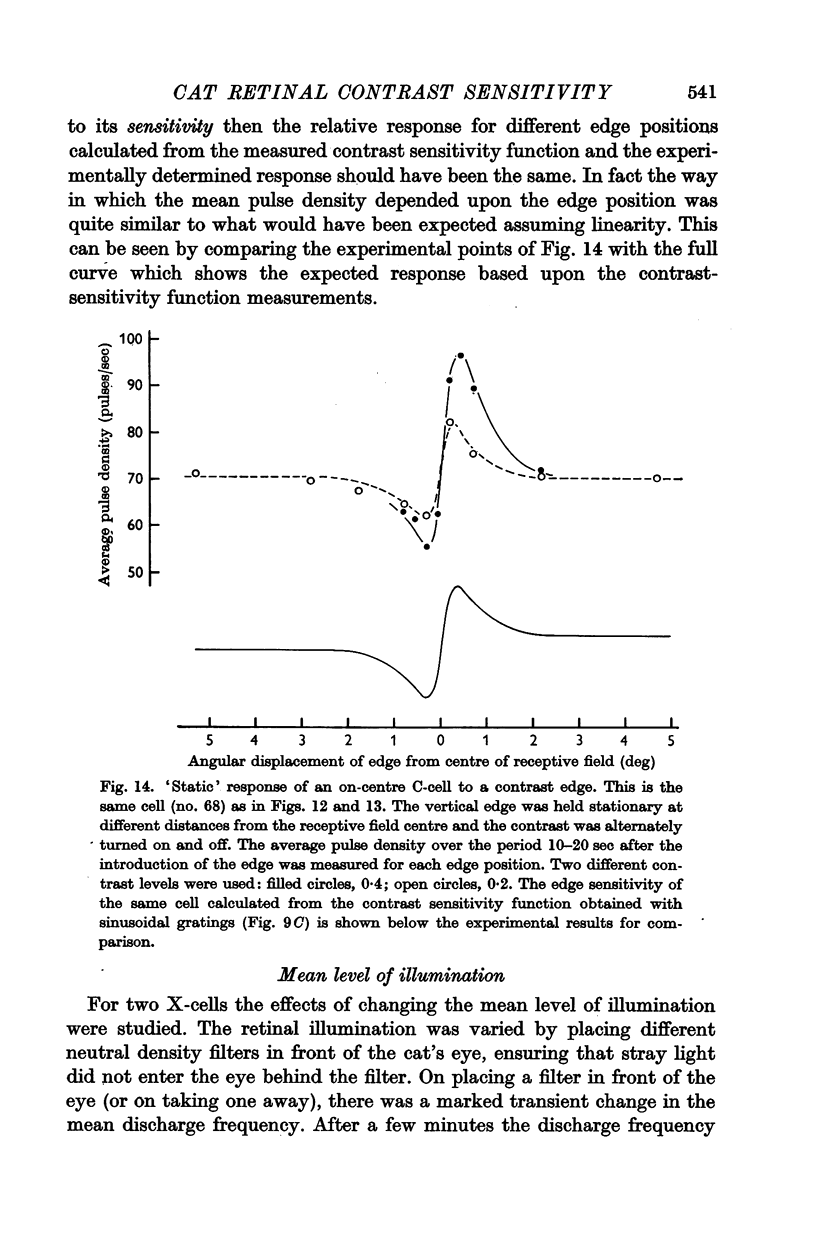
6. The way in which the sensitivity of an X-cell for a contrast-edge pattern varied with the distance of the edge from the receptive field centre was determined and found to be consistent with the cell's measured contrast sensitivity function.
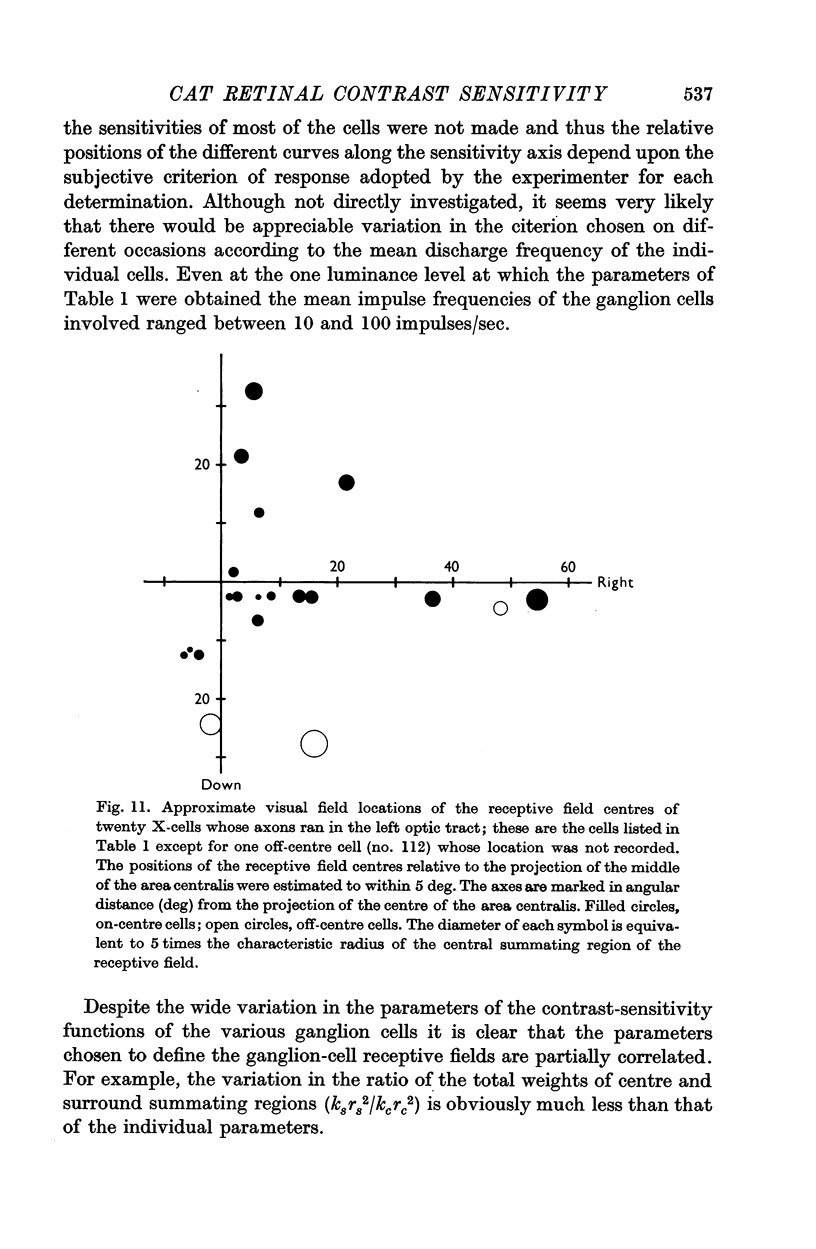
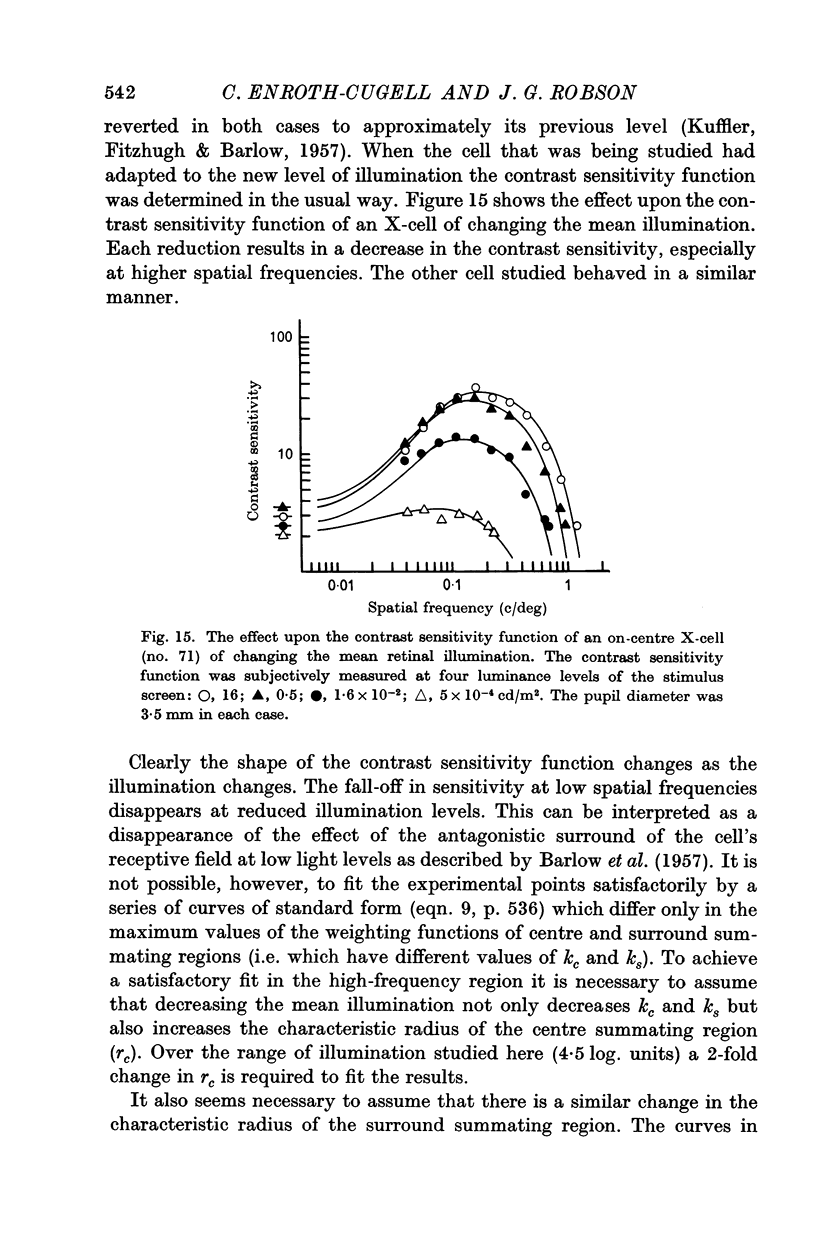
7. Reducing the retinal illumination produced changes in the contrast sensitivity function of an X-cell which suggested that the diameters of the summating regions of the receptive field increased while the surround region became relatively ineffective.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Temporal and spatial summation in human vision at different background intensities. J Physiol. 1958 Apr 30;141(2):337–350. doi: 10.1113/jphysiol.1958.sp005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYNTON R. M. Spatial vision. Annu Rev Psychol. 1962;13:171–200. doi: 10.1146/annurev.ps.13.020162.001131. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Major D. Cat retinal ganglion cell dendritic fields. Exp Neurol. 1966 May;15(1):70–78. doi: 10.1016/0014-4886(66)90035-5. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODT E., ENROTH C. Retinal flicker response in cat. Acta Physiol Scand. 1954 May 15;30(4):375–390. doi: 10.1111/j.1748-1716.1954.tb01076.x. [DOI] [PubMed] [Google Scholar]
- FITZHUGH R. The statistical detection of threshold signals in the retina. J Gen Physiol. 1957 Jul 20;40(6):925–948. doi: 10.1085/jgp.40.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUESSER O. J., REIDEMEISTER C. [Flicker light studies on the cat retina. II. Off-neurons and discussion of the results]. Z Biol. 1959 Dec;111:254–270. [PubMed] [Google Scholar]
- Glezer V. D. The receptive fields of the retina. Vision Res. 1965 Oct;5(9):497–525. doi: 10.1016/0042-6989(65)90084-2. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in striate cortex of unrestrained cats. J Physiol. 1959 Sep 2;147:226–238. doi: 10.1113/jphysiol.1959.sp006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960 Dec;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- KOZAK W., RODIECK R. W., BISHOP P. O. RESPONSES OF SINGLE UNITS IN LATERAL GENICULATE NUCLEUS OF CAT TO MOVING VISUAL PATTERNS. J Neurophysiol. 1965 Jan;28:19–47. doi: 10.1152/jn.1965.28.1.19. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., FITZHUGH R., BARLOW H. B. Maintained activity in the cat's retina in light and darkness. J Gen Physiol. 1957 May 20;40(5):683–702. doi: 10.1085/jgp.40.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb Symp Quant Biol. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- MORRIS V. B., MARRIOTT F. H. The distribution of light in an image formed in the cat's eye. Nature. 1961 Apr 8;190:176–177. doi: 10.1038/190176a0. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Response of cat retinal ganglion cells to moving visual patterns. J Neurophysiol. 1965 Sep;28(5):819–832. doi: 10.1152/jn.1965.28.5.819. [DOI] [PubMed] [Google Scholar]
- SCHADE O. H., Sr Optical and photoelectric analog of the eye. J Opt Soc Am. 1956 Sep;46(9):721–739. doi: 10.1364/josa.46.000721. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]
- WESTHEIMER G. VISUAL ACUITY. Annu Rev Psychol. 1965;16:359–380. doi: 10.1146/annurev.ps.16.020165.002043. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]


