Abstract
1. An examination was made of the mechanisms responsible for the inhibition of synaptic transmission through the lateral geniculate nucleus (LGN) of the rat following a single shock to the optic nerve.
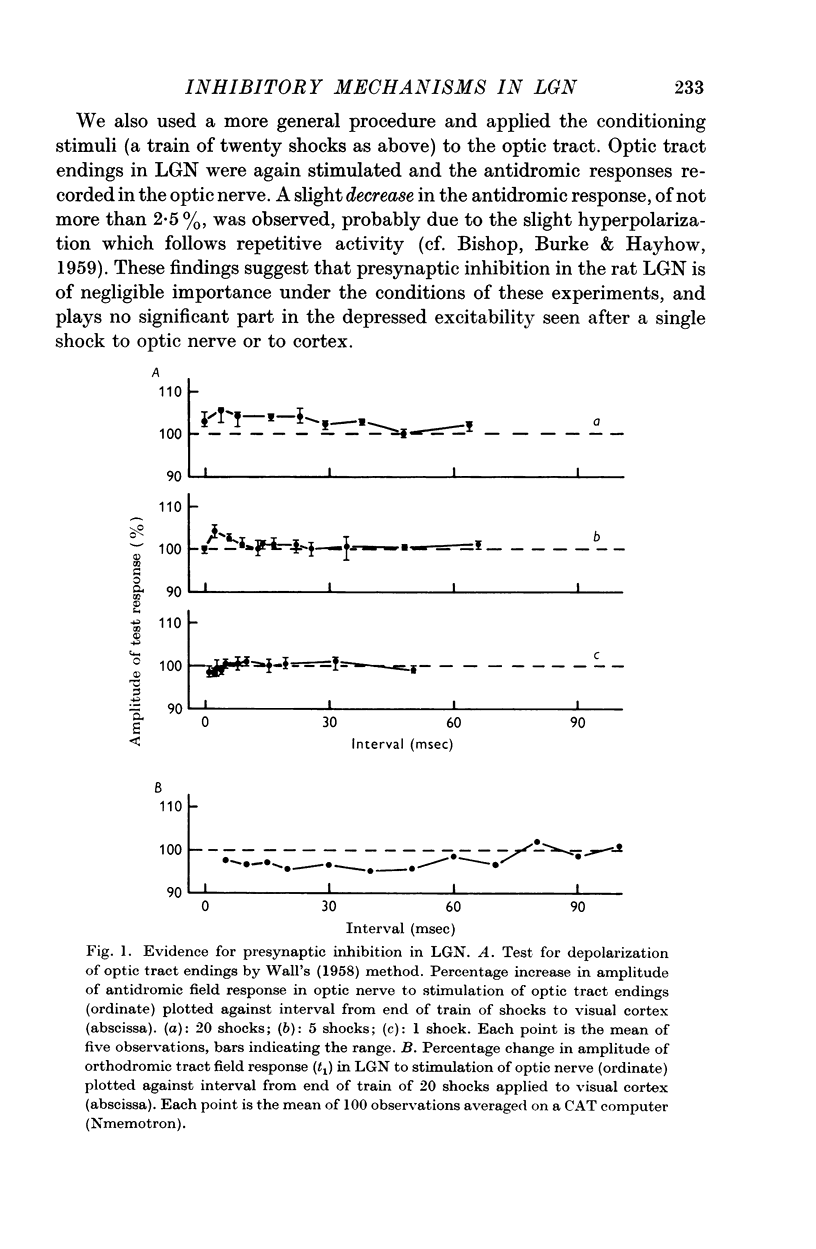
2. In the rat anaesthetized with paraldehyde we found no evidence that optic nerve stimulation produced any presynaptic inhibition in LGN. In agreement with other workers it was found that repetitive stimulation of visual cortex produced effects attributable to presynaptic inhibition. However, this was of small magnitude in the conditions of our experiments.
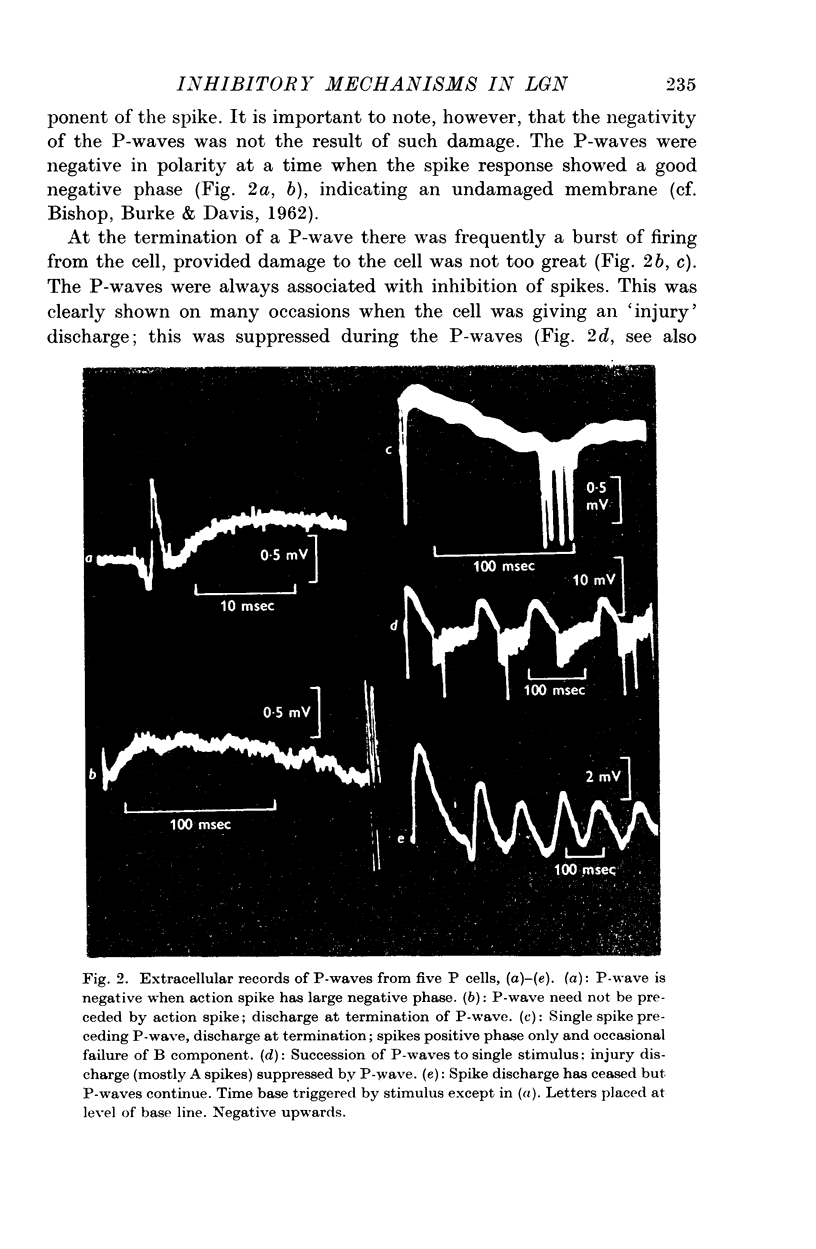
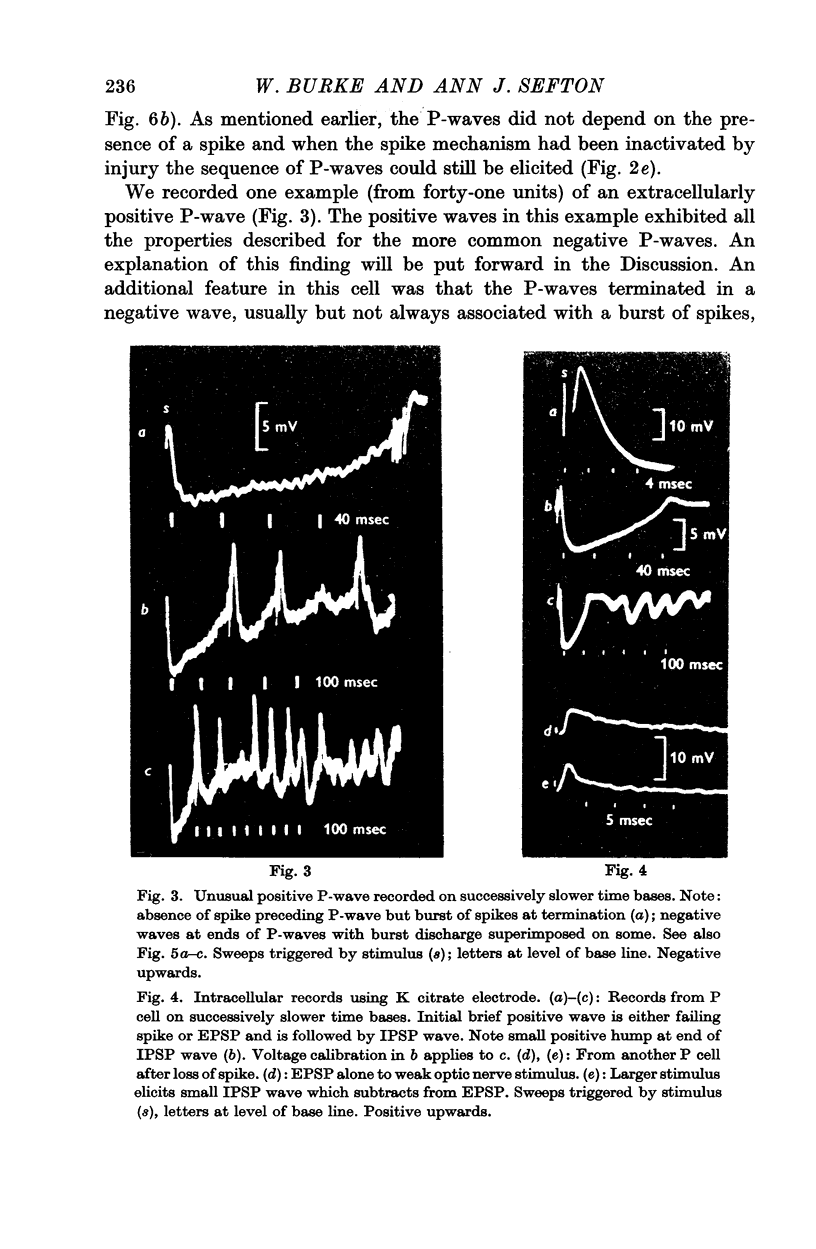
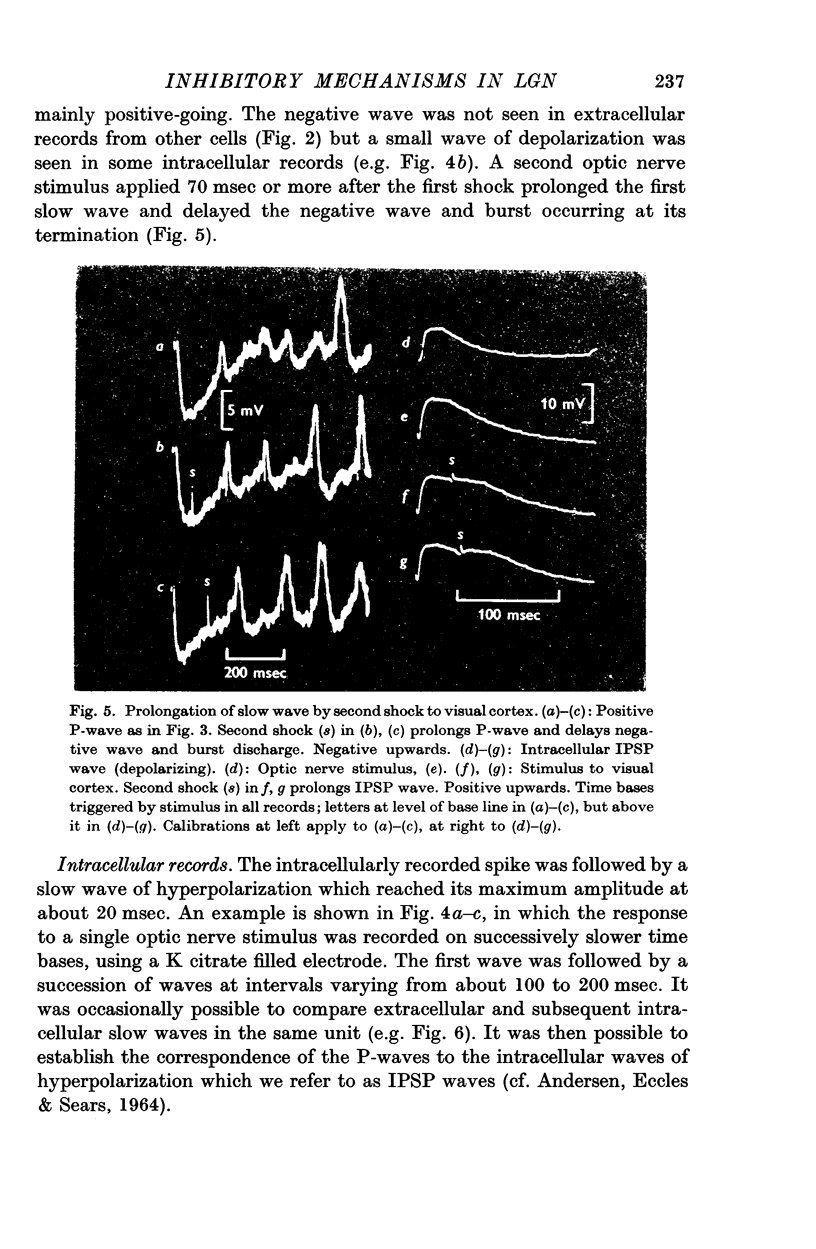
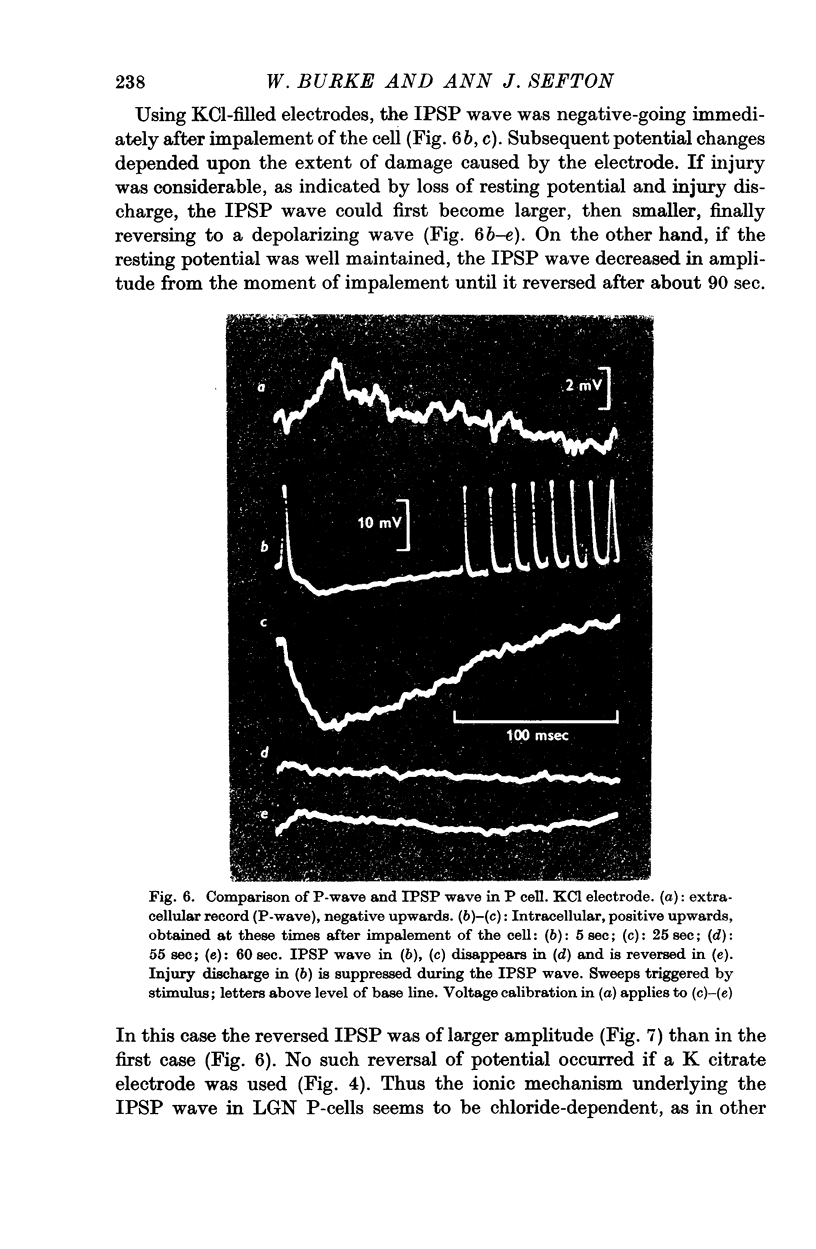
3. Stimulation of the optic nerve elicited an action potential in a P cell (principal cell) which was followed by a wave of hyperpolarization lasting about 150 msec (inhibitory post-synaptic potential, IPSP, wave).
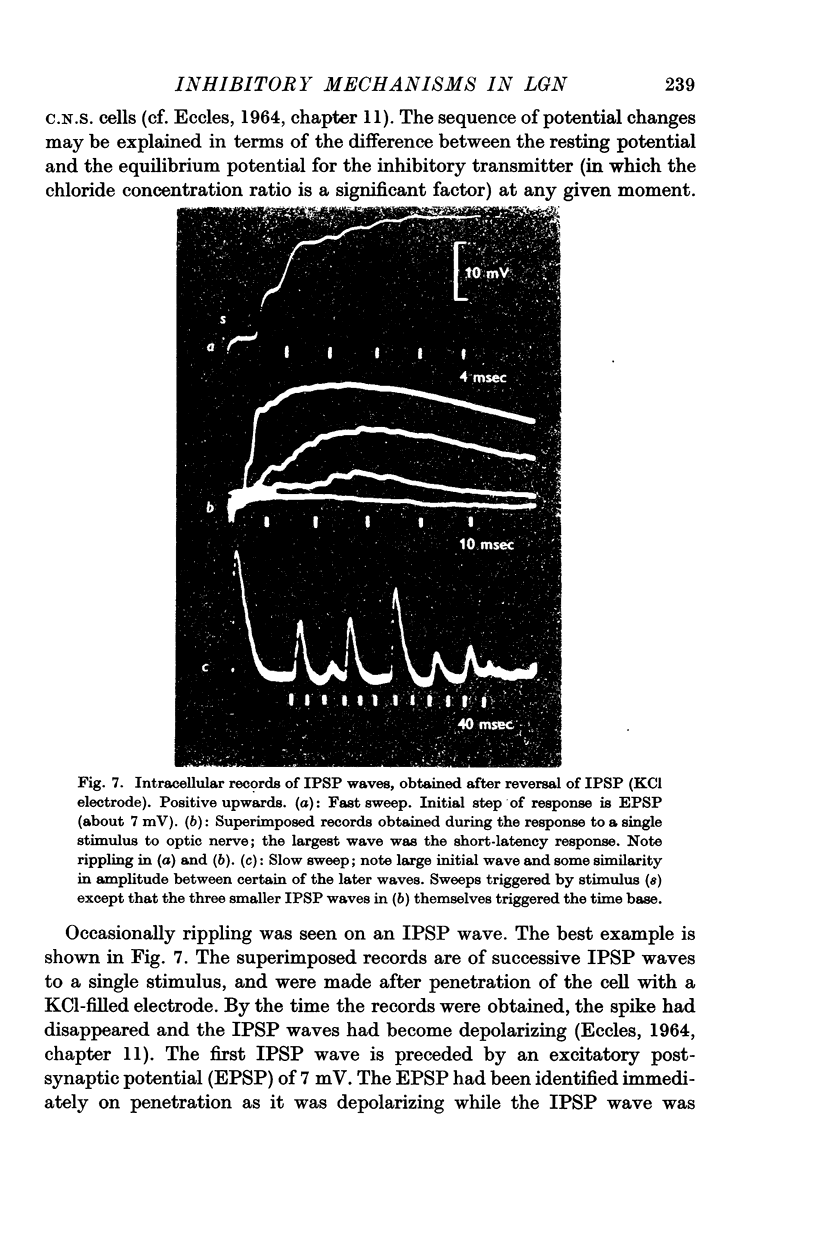
4. The IPSP wave was chloride-dependent and was associated with inhibition of the P cell discharge. Occasional rippling on the IPSP wave suggests that it was produced by the repetitive discharge of I cells (interneurones).
5. These observations support the model proposed previously wherein P cells are inhibited by I cells which in turn are excited by axon collaterals of P cells. There is evidence for diffuse interconnexions between P cells and I cells.
6. The observation that the extracellularly recorded wave of hyperpolarization (P-wave) is usually negative suggests that most of the inhibitory synapses are not on the soma of the P cell.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., BROOKS C. M., ECCLES J. C., SEARS T. A. THE VENTRO-BASAL NUCLEUS OF THE THALAMUS: POTENTIAL FIELDS, SYNAPTIC TRANSMISSION AND EXCITABILITY OF BOTH PRESYNAPTIC AND POST-SYNAPTIC COMPONENTS. J Physiol. 1964 Nov;174:348–369. doi: 10.1113/jphysiol.1964.sp007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SEARS T. A. THE VENTRO-BASAL COMPLEX OF THE THALAMUS: TYPES OF CELLS, THEIR RESPONSES AND THEIR FUNCTIONAL ORGANIZATION. J Physiol. 1964 Nov;174:370–399. doi: 10.1113/jphysiol.1964.sp007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSEN P., SEARS T. A. THE ROLE OF INHIBITION IN THE PHASING OF SPONTANEOUS THALAMO-CORTICAL DISCHARGE. J Physiol. 1964 Oct;173:459–480. doi: 10.1113/jphysiol.1964.sp007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARAKI T., ITO M., OSHIMA T. Potential changes produced by application of current steps in motoneurones. Nature. 1961 Sep 9;191:1104–1105. doi: 10.1038/1911104a0. [DOI] [PubMed] [Google Scholar]
- Adrian E. D. Afferent discharges to the cerebral cortex from peripheral sense organs. J Physiol. 1941 Sep 8;100(2):159–191. doi: 10.1113/jphysiol.1941.sp003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. The identification of single units in central visual pathways. J Physiol. 1962 Aug;162:409–431. doi: 10.1113/jphysiol.1962.sp006942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., HAYHOW W. R. Repetitive stimulation of optic nerve and lateral geniculate synapses. Exp Neurol. 1959 Dec;1:534–555. doi: 10.1016/0014-4886(59)90016-0. [DOI] [PubMed] [Google Scholar]
- BREMER F., BONNET V. Interprétation des réactions rythmiques prolongées des aires sensorielles de l'ecorce cérébrale. Electroencephalogr Clin Neurophysiol. 1950 Nov;2(4):389–400. doi: 10.1016/0013-4694(50)90076-9. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Davis R. Synaptic potentials, after-potentials, and slow rhythms of lateral geniculate neurones. J Physiol. 1960 Dec;154(3):514–546. doi: 10.1113/jphysiol.1960.sp006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Discharge patterns of principal cells and interneurones in lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):201–212. doi: 10.1113/jphysiol.1966.sp008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke W., Jervie Sefton A. Recovery of responsiveness of cells of lateral geniculate nucleus of rat. J Physiol. 1966 Nov;187(1):213–229. doi: 10.1113/jphysiol.1966.sp008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The electrical constants of the motoneurone membrane. J Physiol. 1959 Mar 12;145(3):505–528. doi: 10.1113/jphysiol.1959.sp006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The electrical properties of the motoneurone membrane. J Physiol. 1955 Nov 28;130(2):291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALAMBOS R., ROSE J. E., BROMILEY R. B., HUGHES J. R. Microelectrode studies on medial geniculate body of cat. II. Response to clicks. J Neurophysiol. 1952 Sep;15(5):359–380. doi: 10.1152/jn.1952.15.5.359. [DOI] [PubMed] [Google Scholar]
- HSIANG-TUNG CHANG The repetitive discharges of corticothalamic reverberating circuit. J Neurophysiol. 1950 May;13(3):235–257. doi: 10.1152/jn.1950.13.3.235. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961 May;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- KIMURA D. Multiple response of visual cortex of the rat to photic stimulation. Electroencephalogr Clin Neurophysiol. 1962 Feb;14:115–122. doi: 10.1016/0013-4694(62)90013-5. [DOI] [PubMed] [Google Scholar]
- MORRIS N. R., GLASER G. H. Effects of a pyrimidine analog, 6-azauracil, on rat electroencephalogram and maze running ability. Electroencephalogr Clin Neurophysiol. 1959 Feb;11(1):146–150. doi: 10.1016/0013-4694(59)90016-1. [DOI] [PubMed] [Google Scholar]
- RATLIFF F., HARTLINE H. K. The responses of Limulus optic nerve fibers to patterns of illumination on the receptor mosaic. J Gen Physiol. 1959 Jul 20;42(6):1241–1255. doi: 10.1085/jgp.42.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEFANIS C., JASPER H. RECURRENT COLLATERAL INHIBITION IN PYRAMIDAL TRACT NEURONS. J Neurophysiol. 1964 Sep;27:855–877. doi: 10.1152/jn.1964.27.5.855. [DOI] [PubMed] [Google Scholar]
- Sefton A. J., Burke W. Mechanism of recurrent inhibition in the lateral geniculate nucleus of the rat. Nature. 1966 Sep 17;211(5055):1276–1278. doi: 10.1038/2111276a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kato E. Cortically induced presynaptic inhibition in cat's lateral geniculate body. Tohoku J Exp Med. 1965 Aug 25;86(3):277–289. doi: 10.1620/tjem.86.277. [DOI] [PubMed] [Google Scholar]
- VASTOLA E. F. After-positivity in lateral geniculate body. J Neurophysiol. 1959 May;22(3):258–272. doi: 10.1152/jn.1959.22.3.258. [DOI] [PubMed] [Google Scholar]
- VASTOLA E. F. Antidromic action potentials in lateral geniculate body. J Neurophysiol. 1957 Mar;20(2):167–185. doi: 10.1152/jn.1957.20.2.167. [DOI] [PubMed] [Google Scholar]
- VERZEANO M., CALMA I. Unit activity in spindle bursts. J Neurophysiol. 1954 Sep;17(5):417–428. doi: 10.1152/jn.1954.17.5.417. [DOI] [PubMed] [Google Scholar]
- VERZEANO M., NAQUET R., KING E. E. Action of barbiturates and convulsants on unit activity of diffusely projecting nuclei of thalamus. J Neurophysiol. 1955 Sep;18(5):502–512. doi: 10.1152/jn.1955.18.5.502. [DOI] [PubMed] [Google Scholar]
- VERZEANO M., NEGISHI K. Neuronal activity in cortical and thalamic networks. J Gen Physiol. 1960 Jul;43(6):177–195. doi: 10.1085/jgp.43.6.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]


