Abstract
In the course of a Bacillus subtilis functional genomics project which involved screening for sporulation genes, we identified an open reading frame, yaaT, whose disruptant exhibits a sporulation defect. Twenty-four hours after the initiation of sporulation, most cells of the yaaT mutant exhibited stage 0 of sporulation, indicating that the yaaT mutation blocks sporulation at an early stage. Furthermore, the mutation in yaaT led to a significant decrease in transcription from a promoter controlled by Spo0A, a key response regulator required for the initiation of sporulation. However, neither the level of transcription of spo0A, the activity of σH, which transcribes spo0A, nor the amount of Spo0A protein was severely affected by the mutation in yaaT. Bypassing the phosphorelay by introducing an spo0A mutation (sof-1) into the yaaT mutant suppressed the sporulation defect, suggesting that the yaaT mutation interferes with the phosphorelay process comprising Spo0F, Spo0B, and histidine kinases. We also observed that mutation of spo0E, which encodes the phosphatase that dephosphorylates Spo0A-P, suppressed the sporulation defect in the yaaT mutant. These results strongly suggest that yaaT plays a significant role in the transduction of signals to the phosphorelay for initiation of sporulation. Micrographs indicated that YaaT-green fluorescent protein localizes to the peripheral membrane, as well as to the septum, during sporulation.
Initiation of sporulation in Bacillus subtilis is regulated by a signal transduction pathway, the phosphorelay, which is a multicomponent phosphotransfer system that is switched on in response to environmental, cell cycle, and metabolic signals (3, 36). The processing and integration of these signals by the phosphorelay control the level of phosphorylation of the transcription factor, Spo0A. Environmental and cellular signals that favor sporulation activate autophosphorylation of the sensor kinases KinA, KinB, KinC, and KinD, leading to input of a phosphate group into the phosphorelay (1, 2, 12, 13, 18, 20, 33, 37, 46). In this relay, the phosphate group is subsequently transferred to a response regulator, Spo0F. The resulting molecule, Spo0F-P, serves as a substrate for the Spo0B protein, a phosphotransferase which finally activates Spo0A by transferring the phosphate to the Spo0A protein (2). Spo0A-P, the activated form of Spo0A, indirectly controls the transcription of a number of genes by regulating the level of other transcription regulators. Spo0A-P directly activates transcription of the genes for many regulatory proteins and sigma factors required for cell-type-specific gene expression. Spo0A-P is also known to stimulate axial filament formation and asymmetric polar septation, which give rise to two unequal cells, a larger mother cell and a forespore cell (21, 38).
Just after septation, gene expression is controlled by the RNA polymerase sigma factors, σF in the forespore and σE in the mother cell. Later in sporulation, when the forespore has become engulfed by the mother cell, σF and σE are replaced by σG and σK, respectively (reviewed in references 22, 38, and 45). The coordinated functions of this cascade of sigma factors eventually transform the cell into an environmentally resistant spore.
The phosphorelay, which is obviously a process that is indispensable for efficient sporulation in B. subtilis, is subject to a variety of complex controls involving the transfer of phosphate through its component proteins. Interestingly, although the pathway and regulating proteins have been identified and investigated, the signals and the effectors of the regulators remain unknown.
In this study, we identified a sporulation-deficient yaaT mutant obtained by screening disruptants with disruptions in all genes having unknown functions within the framework of the B. subtilis functional genomics project conducted by a Japanese consortium. Here we present evidence that yaaT plays a significant role in phosphorelay during initiation of sporulation in B. subtilis.
MATERIALS AND METHODS
Bacterial strains, plasmids, phages, and general methods.
The bacterial strains, plasmids, and phages used in this study are listed in Table 1. The oligonucleotide primers used are shown in Table 2. Transformation of B. subtilis was performed by using the method described by Dubnau and Davidoff-Abelson (7). The efficiency of sporulation was measured by growing B. subtilis cells in Difco sporulation medium (DSM) (42) at 37°C for 24 h. The number of spores (CFU) per milliliter of culture was determined by determining the number of heat-resistant (80°C, 10 min) colonies on tryptose blood agar base. Plasmid construction was performed by using Escherichia coli JM105.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype, phenotype, and/or relevant characteristics | Source, reference, or constructiona |
|---|---|---|
| E. coli JM105 | supE endA sbcB15 hsdR4 rpsL thi Δ(lac-proAB)F′ [traD36 proAB+lacIqlacZΔM15] | 50 |
| B. subtilis strains | ||
| 168 | trpC2 | Laboratory stock |
| YAATd | trpC2 yaaT::pMUTyaaT | 30 |
| YABAd | trpC2 yabA::pMUTyabA | 30 |
| YAATK | trpC2 yaaT::pJMyaaT | pJMyaaT → 168 |
| YAAT44 | trpC2 yaaT44 | pCAyaaT → 168 |
| YAATGFP | trpC2 yaaT::pJMyaaTgfp | pJMyaaTgfp → 168 |
| TtcGFP | trpC2 yaaT::pJMTtcgfp | pJMTtcgfp → 168 |
| RL1740 | spoIIE::spoIIE-lacZ cat | R. Losick |
| IIEZ | trpC2 spoIIE::spoIIE-lacZ cat | RL1740 → 168 |
| RL388 | trpC2 pheA1 SPβ::spoVG-lacZ cat | R. Losick |
| VGZ | trpC2 SPβ::spoVG-lacZ cat | RL388 → 168 |
| UOT0531(φCAZ-1) | trpC2 (φCAZ-1) metB51 leuA8 nonB1 | 49 |
| 0AZ | trpC2 (φCAZ-1) | φCAZ-1—168 |
| 1L34 | trpC2 metB10 xin-1 (φ105dI:1t) SPβ(S) | BGSCb |
| 1L34(φEDTA) | trpC2 metB10 xin-1 (φEDTA) SPβ(S) | pEDTA → 1L34 |
| YAATGFP(φEDTA) | trpC2 yaaT::pJMyaaTgfp (φEDTA) | φEDTA—YAATGFP |
| CD301 | trpC2 (φCD301) | φCD301—168 |
| spoIIEΩpPE1 | spoIIEΩpPE1 (spoIIE′-gfp superglow) | 17 |
| JGFP | trpC2 spoIIIJ::pIIIJ-GFP | 25 |
| SOFc | trpC2 leuA8 metB51 nonB1 Δspo0F sof-1 | 16 |
| SOJ | trpC2 Δsoj-spo0J erm | Laboratory stock |
| SDA | trpC2 Δsda::spc | This study |
| 0ES | trpC2 spo0E::pUCS0E | This study |
| 0ESIIEZ | trpC2 spo0E::pUCS0E spoIIE::spoIIE-lacZ cat | RL1740 → 0ES |
| T44SOJ | trpC2 yaaT44 Δsoj-spo0J erm | SOJ → YAAT44 |
| T44SDA | trpC2 yaaT44 Δsda::spc | SDA → YAAT44 |
| T440ES | trpC2 yaaT44 spo0E::pUCS0E | 0ES → YAAT44 |
| T440ESIIEZ | trpC2 yaaT44 spo0E::pUCS0E spoIIE::spoIIE-lacZ cat | 0ESIIEZ → YAAT44 |
| T44IIEZ | trpC2 yaaT44 spoIIE::spoIIE-lacZ cat | RL1740 → YAAT44 |
| T44VGZ | trpC2 yaaT44 SPβ::spoVG-lacZ cat | RL388 → YAAT44 |
| T440AZ | trpC2 (φCAZ-1) yaaT44 | φCAZ-1—YAAT44 |
| TKSOF | trpC2 leuA8 metB51 nonB1 yaaT::pJMyaaT Δspo0F sof-1 | YAATK → SOF |
| Plasmids | ||
| pUC19 | bla | 50 |
| pCA191 | bla cat | 25 |
| pJM114 | bla kan | 35 |
| pBEST517A | bla spc | M. Itaya |
| pUCS192 | bla spc | This study |
| pCAyaaT | bla cat | This study |
| pJMyaaT | bla kan | This study |
| pJMyaaTgfp | bla kan | This study |
| pJMTtcgfp | bla kan | This study |
| pUCS0E | bla spc | This study |
| pED405 | bla erm | Laboratory stock |
| pEDTA | bla erm | This study |
The arrows indicate transformation from donor DNA to recipient strain. A dash indicates lysogenization of the host strain with phage.
BGSC, Bacillus Genetic Stock Center, Ohio State University.
The original designation was UOT0550.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′ to 3′)a | Description, location, and restriction siteb |
|---|---|---|
| yaaTF | AAAACTGCAGATGTAATTGGTGTCCGC | yaaT sense sequence, +24, PstI |
| yaaTR | CGCGGATCCCGACCTGGCCGTACTCA | yaaT antisense sequence, +117, BamHI |
| yaaTUF | CGCGGATCCAACAAAGACACAATGGCCG | yaaT sense sequence, −563, BamHI |
| yaaTUR | AAAACTGCAGACCTGGCCGTACTCAACGC | yaaT antisense sequence, +113, PstI |
| yaaTDF | AAAACTGCAGGTAATTGCAAATAAACAGGTGG | yaaT sense sequence, +154, PstI |
| yaaTDR | CCCAAGCTTTTCCAGAATATTTAGTCCG | yaaT antisense sequence, +708, HindIII |
| yabAR | CGCGGATCCTCATGTCCTCTGCCAGC | yabA antisense sequence, +457, BamHI |
| 0EF | CCCAAGCTTTGGGCGGTTCTTCTGAA | spo0E sense sequence, +18, HindIII |
| 0ER | CGCGGATCCACAATCCAGCTCCTGAC | spo0E antisense sequence, +116, BamHI |
| yaaTgfpF | CGCGGATCCCGAGTCGACTTTAGAGAGCTTG | yaaT sense sequence, +388, BamHI |
| yaaTgfpR | AAAACTGCAGAATCTGTGGTTTGTGCG | yaaT antisense sequence, +810, PstI |
| gfpsgF | GGATCCCCCGGGCTGCAGGAATTCGATTAG | gfp sense sequence, −6, PstI |
| gfpsgR | GCTCTAGATTTGTATAGTTCATCCATGCC | gfp antisense sequence, +694, XbaI |
| gfpsgtc | AAAACTGCAGAAGGTGGTGAACTACTATGAGTAAAGGAGAAGAACTTT | gfp sense sequence, +22, PstI |
| sdaUF | TGCCAACTAATAAGATAGGGTTTC | sda sense sequence, −1149 |
| sdaUR | ACATGTATTCACGAACGAAAATCGATGCATATAAGAACAATCGTTCTG | sda antisense sequence, −127 |
| sdaDF | ATTTTAGAAAACAATAAACCCTTGAGGGCCGGTTATTATGCTAACCAG | sda sense sequence, +310 |
| sdaDR | ATGAAAGCAGAATGATATTCACTG | sda antisense sequence, +1304 |
Additional sequences and restriction sites that do not correspond to the sequences of genes are indicated by italics and underlining, respectively. Sequences corresponding to the spectinomycin resistance gene are indicated by boldface type.
The locations are the 3′ end positions of the primers corresponding to the numbers of nucleotides from the initiation codons of the genes.
Plasmid, phage, and strain construction.
Plasmid pJMyaaT was constructed with the internal fragment of yaaT amplified with primers yaaTF and yaaTR. The PCR product and plasmid pJM114 (35) used for construction were completely digested with PstI and BamHI and then ligated. The resulting construct was used to transform E. coli JM105 and was selected on ampicillin-supplemented Luria-Bertani solid media.
pUCS192 was constructed by cloning a BamHI (blunted)-XbaI (blunted) fragment of pBEST517A containing the spc gene into the NdeI (blunted) site of pUC19 (50). To construct pUCS0E, a HindIII-BamHI fragment bearing an internal fragment of spo0E was generated by PCR amplification with primers 0EF and 0ER and subcloned into the HindIII-BamHI site of pUCS192.
Plasmid pJMyaaTgfp carrying the yaaT-gfp fusion gene was constructed with the oligonucleotide primers yaaTgfpF and yaaTgfpR to obtain a BamHI- and PstI-digestible PCR fragment. Next, PstI- and XbaI-digestible fragments were generated by using the chromosomal DNA of B. subtilis spoIIEΩpPE1 (17) as the template with primers gfpsgF and gfpsgR. The insert was then ligated into the BamHI-XbaI site of pJM114.
pEDTA was constructed with primers yaaTUF and yabAR by amplifying the yaaT-yabA coding region containing the putative promoter (as determined by Northern blot analysis as described at the BSORF website [http://bacillus.genome.ad.jp/]) by using chromosomal DNA of B. subtilis 168 as the template. The PCR products and pED405 (which contained the erm gene cassette in the PstI-SmaI site of a 3.0-kb HindIII-EcoRI fragment of φ105 DNA) that were used for construction were completely digested with BamHI and then ligated. pEDTA was cloned by the prophage transformation method in the temperate phage φ105dI:1t (9, 15). The recombinant phage was designated φEDTA.
Plasmid pJMTtcgfp carrying the PyaaT-gfp transcriptional fusion gene was constructed with oligonucleotide primers yaaTUF and yaaTUR to obtain a BamHI- and PstI-digestible PCR fragment. Next, PstI- and XbaI-digestible fragments were generated by using chromosomal DNA of B. subtilis spoIIEΩpPE1 (17) as the template with primers gfpsgtc and gfpsgR. The insert was then ligated into the BamHI-XbaI site of pJM114.
pCAyaaT was constructed by cloning the BamHI-PstI-digested PCR fragment amplified with oligonucleotide primers yaaTUF and yaaTUR and the PstI-HindIII-digested fragment amplified with primers yaaTDF and yaaTDR into BamHI-HindIII-digested pCA191 (25). The resulting plasmid, pCAyaaT, contained a frameshift mutation in the yaaT gene at the PstI site inserted at amino acid 44 (total number of amino acids, 275). The yaaT44 frameshift mutation was constructed by the gene replacement method of Stahl and Ferrari (43) by using pCAyaaT.
An sda deletion mutant was created by using the long-flanking homology PCR strategy (47). The specific primers used for construction were primers sdaUF and sdaUR and primers sdaDF and sdaDR (Table 2). The resulting mutant contained a spectinomycin cassette between positions −127 and +310 of the sda gene.
β-Galactosidase assay.
B. subtilis cells grown in hydrolyzed casein growth medium at 37°C were induced to sporulate by the resuspension method of Sterlini and Mandelstam (44), as specified by Nicholson and Setlow (28) and Partridge and Errington (32). The β-galactosidase activity was determined as previously described by the method of Miller (24) by using o-nitrophenyl-β-d-galactopyranoside as the substrate. The enzyme specific activity was expressed in nanomoles of substrate (o-nitrophenyl-β-d-galactopyranoside) hydrolyzed per milligram per minute.
Fluorescence microscopy.
Cells were grown and sporulated at 37°C in DSM containing FM4-64 (final concentration, 0.5 μg/ml; Molecular Probes) for staining of the cell membrane (39). Five hundred microliters of the culture was centrifuged, and 400 μl of the supernatant was aspirated off. The cells were then resuspended in the remaining 100 μl. Portions (2 μl) of each sample were mounted on glass slides treated with 0.1% (wt/vol) poly-l-lysine (Sigma). Microscopy was performed with an Olympus BX50 phase-contrast and fluorescence microscope with a 100× UplanApo objective. Images were captured by using a SenSys charge-coupled device camera (Photometrics) and Metamorph 4.5 software (Universal Image). FM4-64 and green fluorescent protein (GFP) were visualized by using a WIG filter set (Olympus) and an FITC filter set (Olympus), respectively. Image processing was done with Adobe Photoshop 4.0.1J.
Protoplasting, protein fraction, and Western immunoblot analysis.
In order to detect the Spo0A protein by Western immunoblotting, B. subtilis cells were grown in hydrolyzed casein growth medium at 37°C and induced to sporulate by the resuspension method. Samples were taken at different times and centrifuged to collect cells. Cell pellets were protoplasted as described by Wu and Errington (48). Protein concentrations of samples were determined by the Bio-Rad protein assay (Bio-Rad), and 30-μg samples of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western immunoblotting with anti-Spo0A antibody (26). For Western immunoblot analysis of YaaT-GFP protein, B. subtilis cells were grown and sporulated in DSM at 37°C. Samples were taken at different times and centrifuged to collect cells. The cell pellets were protoplasted and fractionated as described by Wu and Errington (48). Then samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western immunoblotting with anti-GFP antibody (Molecular Probes). Protein concentrations of samples were determined by the Bio-Rad protein assay before fractionation.
RESULTS
Identification of the new sporulation gene yaaT.
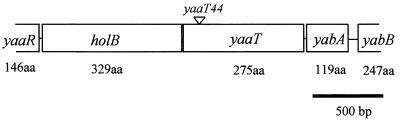
A yaaT plasmid insertion mutant, YAATd, was screened as a sporulation-defective phenotype from disruptant collections of genes having unknown functions in B. subtilis (30). Upstream and downstream of yaaT are the holB gene, which encodes a δ-subunit of DNA polymerase III, and the yabA gene, which encodes a protein that acts during initiation control on DNA replication. Both of these genes occur with the same direction of transcription on the chromosome DNA (19) (Fig. 1). YAATd exhibited significantly decreased production of heat-resistant spores (Table 3) in addition to a slow-growth phenotype (data not shown). We speculated that the slow growth of YAATd might be due to a polar effect on the downstream gene yabA. In order to test this possibility, we examined the phenotype of a yabA mutant, YABAdd. As expected, cells of the yabA mutant showed slow growth, but the yabA mutation had no significant effect on sporulation efficiency. Furthermore, in strain YAATd, in which yaaT was disrupted with the pMUTin1 vector, expression of the yaaT downstream gene yabA was ensured by an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter, and the slow-growth phenotype was completely restored by addition of IPTG. Moreover, Noirot-Gros et al. (29) have recently found a similar deficiency in a yabA mutant. We also constructed a frameshift yaaT mutant, YAAT44, whose mutation is not expected to affect the expression of yabA. This mutation caused a significant decrease in the production of heat-resistant spores (Table 3) but had no influence on cell growth. These results indicated that yaaT is involved in sporulation but is not required for cell growth.
FIG. 1.
Genetic organization of the yaaT region. The location of the yaaT44 mutation is indicated. aa, amino acids.
TABLE 3.
Sporulation frequencies of the yaaT mutants
| Strain | CFU/ml
|
Frequencyb | |
|---|---|---|---|
| Viable cellsa | Spores | ||
| 168 | 4.0 × 108 | 3.8 × 108 | 0.95 |
| YAATd | 2.8 × 108 | 6.0 × 103 | 2.1 × 10−5 |
| YABAd | 6.7 × 108 | 4.6 × 108 | 0.69 |
| YAAT44 | 3.1 × 108 | 1.3 × 104 | 4.2 × 10−5 |
Cells were grown in DSM.
The frequency is the ratio of the number of spores to the number of viable cells for each strain.
YaaT is required for the early stage of sporulation.
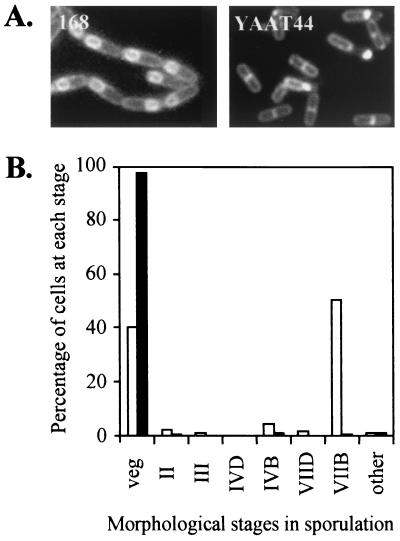
To determine the morphological stage at which the yaaT disruptant is blocked in the sporulation process, we observed the cells by phase-contrast and fluorescence microscopy by using the membrane-staining method and FM4-64 (39). Because FM4-64 is a membrane-impermeable fluorescent membrane stain, the culture was grown in DSM containing FM4-64 and sampled 6 h after the onset of sporulation (T6), at which point internal membrane structures derived from the cytoplasmic membrane were also stained. Figure 2A shows that the frameshift yaaT mutant (YAAT44) could not proceed to the late stage of sporulation. Furthermore, analysis of FM4-64-stained T24 cells by fluorescence microscopy showed that in approximately 98% of the yaaT mutant cells sporulation was arrested early, before asymmetric septum formation (Fig. 2B). Based on this observation, we classified yaaT as a stage 0 gene and concluded that the yaaT gene product is required for the early stage of sporulation.
FIG. 2.
Effect of yaaT mutation on sporulation. (A) Fluorescence microscopy of wild-type cells and YAAT44 cells stained with FM4-64 6 h after inoculation into DSM. (B) Quantification of morphological stages in the cell population of the yaaT mutant in DSM at T24. Open bars, wild type; solid bars, YAAT44. veg, vegetative cells and stage 0 cells (no asymmetric septa); II, stage II cells with asymmetric septa; III, stage III cells with spore protoplasts (forespores) within the rod-shaped mother cells; IVD and IVB, stage IV cells with forespores becoming phase dark and progressively phase bright, respectively; VIID and VIIB, spore bodies becoming phase dark and phase bright, respectively, with no surrounding rod-shaped mother cells. Altogether, 455 wild-type cells and 406 YAAT44 mutant cells were counted.
Effect of yaaT mutation on Spo0A activation.
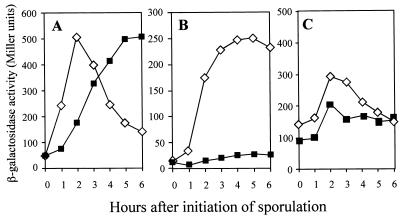
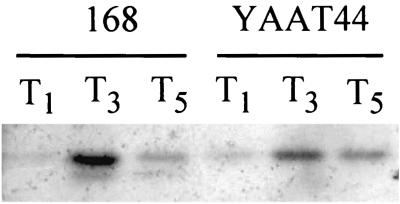
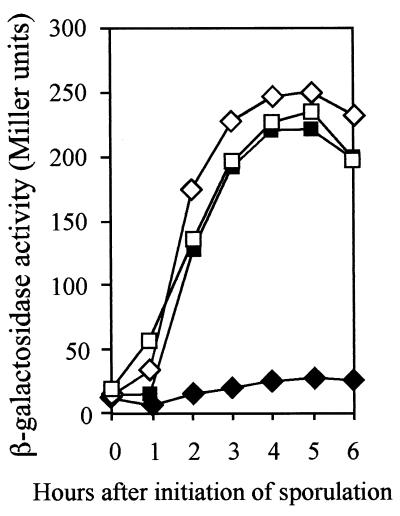
The initiation of sporulation is controlled by phosphorylation of a single transcription regulator, Spo0A, and stabilization of the early-stage-specific sigma factor σH (23). Since the morphological stage of blockage of the yaaT mutant is stage 0, the yaaT mutation was expected to interfere with the expression of σH- or Spo0A- and σA-dependent genes. To study the effect of the yaaT mutation on σH and Spo0A activities, a spoVG-lacZ transcriptional fusion, which originated from a σH-dependent promoter, and a spoIIE-lacZ transcriptional fusion, which originated from a σA-dependent promoter requiring Spo0A proteins, were used. In the wild-type cells, expression of spoVG-lacZ was dramatically elevated and reached the maximum level at T2 and then decreased. In the yaaT mutant, expression slowly increased and a reached maximum level at T5 with a 1-h delay compared to the wild type, but the maximum level of expression was almost the same as that of the wild type (Fig. 3A). On the other hand, expression of the spoIIE-lacZ fusion was induced shortly after the initiation of sporulation and reached the maximum level at T4 to T5 in wild-type cells. Conversely, the expression in the yaaT mutant was strongly inhibited (Fig. 3B). We also examined the levels of expression of spo0A in the yaaT mutant. spo0A is transcribed during vegetative growth from two promoters, a σA-dependent promoter (PV) that is turned off around T0 and a σH-dependent promoter that is substantially activated at the onset of the stationary phase (49). In the yaaT mutant, the level of expression of spo0A during sporulation was 70 to 80% of the wild-type level (Fig. 3C). Furthermore, we examined the amount of Spo0A protein during sporulation by performing a Western immunoblot analysis with anti-Spo0A antibody. In the wild type, a 29.5-kDa band of Spo0A protein was detected at T1 to T5, specifically at T3 (Fig. 4). On the other hand, in the yaaT mutant the intensity of the Spo0A protein signal was 50 to 60% of the wild-type intensity. This pattern of accumulation of the Spo0A protein closely correlated with expression of spo0A in the yaaT mutant (Fig. 3C). The point to be emphasized here is that the level of production of Spo0A was not substantially lower than the level of expression of Spo0A-P-dependent genes after introduction of the yaaT mutation, suggesting that the yaaT mutation interferes with the phosphorylation of Spo0A through a phosphorelay but does not interfere with the production of Spo0A.
FIG. 3.
Expression of the various early sporulation genes in the yaaT mutant. Various strains carrying the lacZ fusions were induced to sporulate, and the β-galactosidase activities were assayed. (A) spoVG-lacZ expression. Symbols: ⋄, VGZ (wild type); ▪, T44VGZ (yaaT). (B) spoIIE-lacZ expression. Symbols: ⋄, IIEZ (wild type); ▪, T44IIEZ (yaaT). (C) spo0A-lacZ expression. Symbols: ⋄, 0AZ (wild type); ▪, T440AZ (yaaT).
FIG. 4.
Western blot analysis of Spo0A protein in the yaaT mutant. The cells were induced to sporulate and collected at different times. Western blots of whole-cell extracts were detected with an antibody that recognizes Spo0A.
Effect of sof-1 mutation on yaaT mutant.
In the wild-type cells, the phosphorylation pathway consists of at least four histidine kinases (KinA, KinB, KinC, and KinD), Spo0F, Spo0B, and Spo0A (reviewed in reference 3). A mutation in spo0A, sof-1, suppresses the sporulation defect caused by spo0F or spo0B mutations (11, 16). This suppression depends on kinC, indicating that Spo0Asof-1 can receive phosphate directly from KinC (18, 20). If a mutation in yaaT affects Spo0A-P production by inhibiting the phosphorelay, it is expected that the sporulation frequency in the yaaT mutant would be suppressed by introduction of the sof-1 mutation. We therefore introduced the sof-1 mutation into the yaaT mutant and checked whether the sof-1 mutation suppressed the sporulation defect in the yaaT mutant. As shown in Table 4, the sporulation defect in the yaaT mutant was completely suppressed by the sof-1 mutation, confirming that yaaT is involved in some step of the phosphorelay during activation Spo0A.
TABLE 4.
sof-1 mutation completely suppresses sporulation inefficiency in the yaaT mutant
| Strain | CFU/ml
|
Frequencyb | |
|---|---|---|---|
| Viable cellsa | Spores | ||
| 168 | 4.0 × 108 | 3.8 × 108 | 0.95 |
| YAATK | 2.9 × 108 | 5.0 × 103 | 1.7 × 10−5 |
| SOF | 1.5 × 108 | 1.5 × 108 | 1.00 |
| TKSOF | 1.2 × 108 | 1.2 × 108 | 1.00 |
Cells were grown in DSM.
The frequency is the ratio of the number of spores to the number of viable cells for each strain.
yaaT is probably involved in activation of Spo0A through spo0E.
It is known that several proteins that repress the phosphorelay and their specific repressors control the initiation of sporulation. This mechanism ensures that sporulation is not initiated unless conditions seem proper (reviewed in reference 3). It is known that the Soj, Sda, and Spo0E proteins are the negative factors of the phosphorelay (4, 5, 31, 34, 40). If the yaaT gene product affects the phosphorelay by inhibiting these negative factors, then the yaaT mutation is expected to suppress sporulation due to introduction of a mutation of these factors. To determine whether yaaT is related to the phosphorelay through soj, we introduced the soj mutation into the yaaT mutant and examined the sporulation frequency in the yaaT soj double mutant. As shown in Table 5, the sporulation deficiency in the yaaT mutant was not suppressed by the soj mutation, indicating that yaaT is not involved in the pathway through soj and spo0J. Likewise, to determine whether yaaT is involved in the DNA replication pathway through the sda gene, in which a mutation results in restoration of the sporulation deficiency of dna mutants (14), we introduced an sda mutation into the yaaT mutant and then examined the sporulation frequency in the yaaT sda double mutant. The sda mutation also did not suppress the sporulation deficiency in the yaaT mutant, implying that yaaT does not participate in a DNA replication pathway through sda (Table 5).
TABLE 5.
Effects of mutations in genes that negatively affect sporulation on yaaT mutants
| Strain | CFU/ml
|
Frequencyb | |
|---|---|---|---|
| Viable cellsa | Spores | ||
| 168 | 4.0 × 108 | 3.8 × 108 | 0.95 |
| YAAT44 | 3.1 × 108 | 1.3 × 104 | 4.2 × 10−5 |
| SOJ | 1.9 × 108 | 1.9 × 108 | 1.00 |
| SDA | 4.1 × 108 | 3.2 × 108 | 0.78 |
| 0ES | 4.5 × 108 | 4.1 × 108 | 0.85 |
| T44SOJ | 2.9 × 108 | 5.5 × 103 | 1.9 × 10−5 |
| T44SDA | 2.2 × 108 | 4.8 × 103 | 2.2 × 10−5 |
| T440ES | 2.8 × 108 | 1.4 × 108 | 0.50 |
Cells were grown in DSM.
The frequency is the ratio of the number of spores to the number of viable cells for each strain.
Finally, we examined the sporulation frequency and monitored expression of the spoIIE-lacZ activity in the yaaT spo0E double mutant. As shown in Table 5, the sporulation deficiency caused by the yaaT mutation was almost completely suppressed by the spo0E mutation. In addition, expression of spoIIE was completely restored in the double mutant (Fig. 5). These results suggest that YaaT plays an important role in the function of Spo0A-P by regulating the activity of Spo0E. It is thus possible that YaaT might increase the Spo0A-P level by controlling the Spo0E activity during the initiation of sporulation.
FIG. 5.
Expression of spoIIE-lacZ fusion in the yaaT spo0E double mutant. Various strains carrying the lacZ fusions were induced to sporulate, and the β-galactosidase activities were assayed. Symbols: ⋄, IIEZ (wild type); □, 0ESIIEZ (spo0E); ♦, T44IIEZ (yaaT); ▪, T440ESIIEZ (yaaT spo0E).
Localization of YaaT.
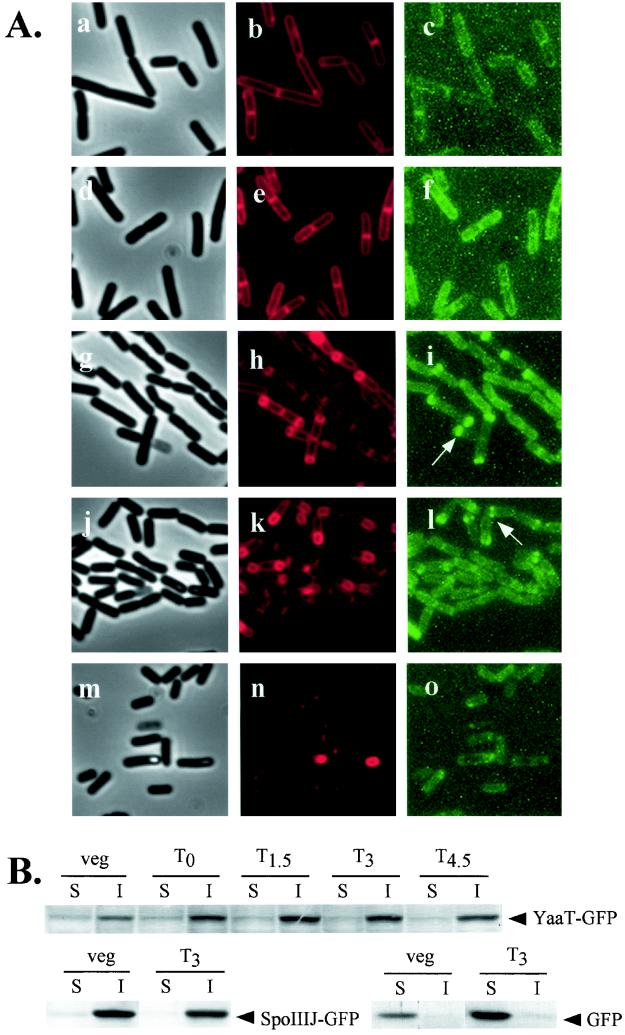
The yaaT product was predicted to be a cytoplasmic protein based on its amino acid sequence, as analyzed by the SOSUI prediction system. In order to determine the localization of YaaT, we constructed a translational fusion of yaaT to the gfp (GFP) gene. To avoid inhibition of cell growth due to prevention of expression of yabA caused by integration of pJMyaaTgfp, we introduced φEDTA, which contains an intact copy of the yabA gene, into YAATGFP. The activity of YaaT was not inhibited by fusion to GFP, since this strain was able to sporulate as efficiently as the wild type (data not shown). A culture of YAATGFP(φEDTA) in DSM was grown in the presence of the vital membrane stain FM4-64, and samples were taken at the vegetative and sporulation phases. We then observed the membrane morphology and location of the YaaT-GFP fusion protein in B. subtilis by fluorescence microscopy (Fig. 6A ). In the vegetative phase, YaaT-GFP localized throughout the periphery of the cell and the division septum. In the sporulation stages, fluorescence of the YaaT-GFP was observed throughout the periphery of the cell; however, in 80% of the cells two fluorescent dots were observed at the sides of an asymmetric septum and at the edges of the forespore (T1.5 to T3). The fluorescence diminished at the late stages of sporulation (T4.5).
FIG.6.
Localization of YaaT-GFP fusion protein. (A) Typical phase-contrast (panels a, d, g, j, and m), membrane-stained (FM4-64) (panels b, e, h, k, and n), and GFP fluorescence (panels c, f, i, l, and o) micrographs. Strains carrying YaaT-GFP were observed in the vegetative stage (panels a, b, and c) and at T0 (panels d, e, and f), T1.5 (panels g, h, and i), T3 (panels j, k, and l), and T4.5 (panels m, n, and o). (B) Western blot analysis of fractionated YaaT-GFP, SpoIIIJ-GFP, and GFP. YAATGFP(φEDTA), JGFP, and TtcGFP cells were grown in DSM at 37°C and collected at different times. Each cell extract was fractionated into soluble and insoluble fractions and examined with an antibody that recognizes GFP. veg, vegetative cells; S, soluble fraction, I, insoluble fraction.
We analyzed cellular distribution of the YaaT-GFP fusion protein with SpoIIIJ-GFP (a membrane protein [25]) and the native GFP (a typical soluble protein), which were used as controls in Western immunoblotting experiments performed with antibody against GFP (Fig. 6B). The localization of these proteins was analyzed by the cell fractionation method (48). As expected, YaaT-GFP and SpoIIIJ-GFP were detected mainly in the insoluble fractions, whereas GFP was detected in the soluble fraction, suggesting that YaaT is membrane associated, as predicted.
DISCUSSION
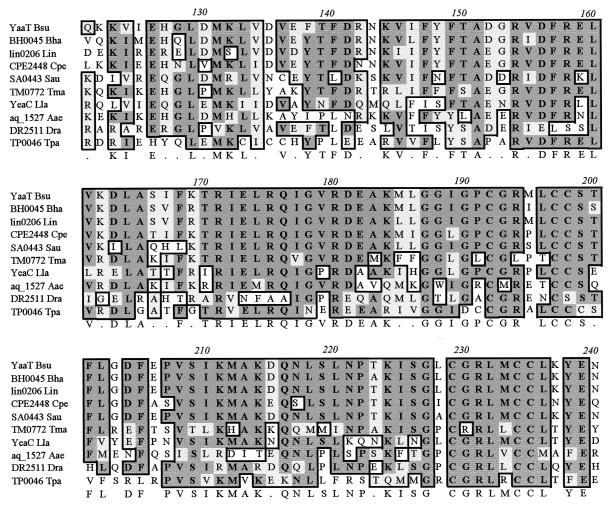
Initiation of sporulation in B. subtilis is governed by a complex phosphorylation mechanism. Identification of a new gene involved in phosphorylation is essential for deciphering the phosphorelay process. We describe here identification of the new sporulation gene yaaT, which is essential for the phosphorelay during initiation of sporulation. The sequence of the yaaT gene is widely conserved in prokaryotes (some gram-positive bacteria and archaea), but the functions of the gene are unknown (Fig. 7). Interestingly, no yaaT homologue exists in gram-negative bacteria typically, implying that the function of YaaT in sporulation has developed through an evolutionary process within the gram-positive bacteria. The yaaT gene is located downstream of holB, which encodes the δ-subunit of DNA polymerase III, and upstream of yabA, which encodes a negative regulator of initiation of DNA replication (29). Considering the information on the adjacent open reading frames, it is possible that the yaaT gene product is also related to DNA replication. Burkholder et al. (4) proposed that the sda gene is involved in the phosphorylation pathway, which receives a signal from the DNA replication cycle. However, we have shown that the sda mutation does not suppress the yaaT mutation, suggesting that there is no relationship between sda and yaaT. Therefore, yaaT is not likely to be involved in DNA replication, at least not through the pathway involving sda.
FIG. 7.
Comparison of the amino acid sequences of the YaaT homologues from B. subtilis (YaaT Bsu), Bacillus halodurans (BH0045 Bha), Listeria innocua (lin0206 Lin), Clostridium perfringens (CPE2448 Cpe), Staphylococcus aureus (SA0443 Sau), Thermotoga maritima (TM0772 Tma), Lactococcus lactis (YeaC Lla), Aquifex aeolicus (aq_1527 Aae), Deinococcus radiodurans (DR2511 Dra), and Treponema pallidum (TP0046 Tpa).
Spo0E is a protein phosphatase that dephosphorylates active Spo0A-P to inactive Spo0A. The spo0E gene is transcribed by the σA form of RNA polymerase and is repressed by AbrB. The time of expression is from T0 to T3 (34). It has been predicted that Spo0E is involved in sensing some inhibitory signals unfavorable for sporulation. One of the protein Spo0E mutants, Spo0E11, which has a deletion at its C-terminal end, is a hyperactive phosphatase, and the spo0E11 mutant exhibits sporulation deficiency (31). Perhaps this mutant protein has lost a controlling site and is unable to respond to a signal that modulates Spo0E phosphatase activity (31). This region of the molecule might be responsible for signal interpretation that may take the form of a discrete signal or may delineate a region that interacts with another compartment of the phosphorelay (31). In this work, we showed that the spo0E mutation suppressed the sporulation deficiency and restored the transcriptional level of spoIIE-lacZ in the yaaT mutant. Although it is not clear if YaaT and Spo0E interact directly, it is possible that YaaT controls the Spo0A-P level through Spo0E activity during sporulation. In this light, yaaT may inhibit the activity of Spo0E, which dephosphorylates the Spo0A-P protein and then supports accumulation of Spo0A-P during the early stage of sporulation. However, it has been shown that a null mutation of spo0E does not lead to a significant increase in the activity of Spo0A-P compared with the activity in the wild type, meaning that Spo0E can dephosphorylate Spo0A-P only at a low level (34, 41) (Fig. 4). Alternatively, some unknown inhibitory signals may inactivate YaaT or the interaction between YaaT and Spo0E at a later stage of sporulation, making it possible for Spo0E to perhaps inhibit excess accumulation of Spo0A-P. Interestingly, Nanamiya et al. (27) reported that ClpP also controlled Spo0A-P activity by negatively regulating the Spo0E function.
Two components of the phosphorelay, KinB and KinC, are both predicted to be integral membrane proteins, and they localize to the cell membrane and sense signals for initiation of sporulation. In our study we observed that YaaT-GFP localized throughout the periphery of the cell and the division septum. In the sporulation stages, the fluorescence of YaaT-GFP was observed as two dots at the side or edge of the engulfing asymmetric septum and the forespore. The yaaT product was predicted to be a cytoplasmic protein based on its amino acid sequence. We therefore speculate that YaaT is associated with the cell membrane along with another unknown protein that localizes to the cell membrane. We also speculate that YaaT directly or indirectly senses the signals for sporulation at the surface of the cell. However, it is still not known why YaaT-GFP localizes as two dots at the side or edge of the engulfing asymmetric septum and the forespore. Eichenberger et al. (8) observed localization of the SpoIIM-GFP fusion protein as a spherical focus at the forespore and as two dots at the distal polar division site of the cell, suggesting that the dots represent in cross section a ring that encircles the inside surface of the cell. The division proteins DivIB, DivIC, and PBP2B localize as a two-dot pattern before septum formation (6, 10). Harry and Wake (10) proposed that the two-dot pattern of DivIB represents an encircling ring of molecules attached to the cell membrane, that the two dots were located on the edge of the cell, and that the top and bottom regions of the ring could not be visualized by changing the focus. Therefore, it is possible that YaaT-GFP also forms a ring-like structure. However, most probably YaaT acts at stage 0 of sporulation, since inhibition of sporulation by the yaaT mutation was almost completely suppressed by the sof-1 mutation and the spo0E mutation. The two-dot pattern of localization of YaaT-GFP after asymmetric septation might have just a minor role or no role in sporulation.
Identification of the new sporulation gene yaaT, mutation of which results in significant inhibition of phosphorylation and sporulation of cells, provides another major piece of information concerning the complex phosphorylation mechanism during sporulation. Further work on YaaT might lead to greater understanding of sporulation in B. subtilis.
Acknowledgments
We thank Richard Losick for providing B. subtilis strains, Masaya Fujita for providing Spo0A antibody, and Samuel Amiteye for critically reading the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (C) (Genome Biology) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Antoniewski, C., B. Savelli, and P. Stragier. 1990. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J. Bacteriol. 172:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burbulys, D., K. A. Trach, and J. A. Hoch. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545-552. [DOI] [PubMed] [Google Scholar]
- 3.Burkholder, W. F., and A. D. Grossman. 2000. Regulation of the initiation of endospore formation in Bacillus subtilis, p. 151-166. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 4.Burkholder, W. F., I. Kurtser, and A. D. Grossman. 2001. Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell 104:269-279. [DOI] [PubMed] [Google Scholar]
- 5.Cervin, M. A., G. B. Spiegelman, B. Raether, K. Ohlsen, M. Perego, and J. A. Hoch. 1998. A negative regulator linking chromosome segregation to developmental transcription in Bacillus subtilis. Mol. Microbiol. 29:85-95. [DOI] [PubMed] [Google Scholar]
- 6.Daniel, R. A., E. J. Harry, and J. Errington. 2000. Role of penicillin-binding protein PBP 2B in assembly and functioning of the division machinery of Bacillus subtilis. Mol. Microbiol. 35:299-311. [DOI] [PubMed] [Google Scholar]
- 7.Dubnau, D., and R. Davidoff-Abelson. 1971. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of the donor-recipient complex. J. Mol. Biol. 56:209-221. [DOI] [PubMed] [Google Scholar]
- 8.Eichenberger, P., P. Fawcett, and R. Losick. 2001. A three-protein inhibitor of polar septation during sporulation in Bacillus subtilis. Mol. Microbiol. 42:1147-1162. [DOI] [PubMed] [Google Scholar]
- 9.Flock, J. I. 1977. Deletion mutants of temperate Bacillus subtilis bacteriophage phi105. Mol. Gen. Genet. 155:241-247. [DOI] [PubMed] [Google Scholar]
- 10.Harry, E. J., and R. G. Wake. 1997. The membrane-bound cell division protein DivIB is localized to the division site in Bacillus subtilis. Mol. Microbiol. 25:275-283. [DOI] [PubMed] [Google Scholar]
- 11.Hoch, J. A., K. Trach, F. Kawamura, and H. Saito. 1985. Identification of the transcriptional suppressor sof-1 as an alteration in the Spo0A protein. J. Bacteriol. 161:552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, M., Y. L. Tzeng, V. A. Feher, M. Perego, and J. A. Hoch. 1999. Alanine mutants of the Spo0F response regulator modifying specificity for sensor kinases in sporulation initiation. Mol. Microbiol. 33:389-395. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, M., W. Shao, M. Perego, and J. A. Hoch. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 38:535-542. [DOI] [PubMed] [Google Scholar]
- 14.Karamata, D., and J. D. Gross. 1970. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol. Gen. Genet. 108:277-287. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura, F., H. Saito, and Y. Ikeda. 1979. A method for construction of specialized transducing phage ρ11 of Bacillus subtilis. Gene 5:87-91. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura, F., and H. Saito. 1983. Isolation and mapping of a new suppressor mutation of an early sporulation gene spo0F mutation in Bacillus subtilis. Mol. Gen. Genet. 192:330-334. [DOI] [PubMed] [Google Scholar]
- 17.King, N., O. Dreesen, P. Stragier, K. Pogliano, and R. Losick. 1999. Septation, dephosphorylation, and the activation of σF during sporulation in Bacillus subtilis. Genes Dev. 13:1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi, K., K. Shoji, T. Shimizu, K. Nakano, T. Sato, and Y. Kobayashi. 1995. Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase. J. Bacteriol. 177:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunst, F., N. Ogasawara, I. Moszer, et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 20.LeDeaux, J. R., and A. D. Grossman. 1995. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J. Bacteriol. 177:166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin, P. A., and R. Losick. 2000. Asymmetric division and cell fate during sporulation in Bacillus subtilis, p. 167-189. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 22.Li, Z., and P. J. Piggot. 2001. Development of a two-part transcription probe to determine the completeness of temporal and spatial compartmentalization of gene expression during bacterial development. Proc. Natl. Acad. Sci. USA 98:12538-12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, J., W. M. Cosby, and P. Zuber. 1999. Role of Lon and ClpX in the post-translational regulation of a sigma subunit of RNA polymerase required for cellular differentiation in Bacillus subtilis. Mol. Microbiol. 33:415-428. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 25.Murakami, T., K. Haga, M. Takeuchi, and T. Sato. 2002. Analysis of the Bacillus subtilis spoIIIJ gene and its paralogue gene, yqjG. J. Bacteriol. 184:1998-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanamiya, H., Y. Ohashi, K. Asai, S. Moriya, N. Ogasawara, M. Fujita, Y. Sadaie, and F. Kawamura. 1998. ClpC regulates the fate of a sporulation initiation sigma factor, σH protein, in Bacillus subtilis at elevated temperatures. Mol. Microbiol. 29:505-513. [DOI] [PubMed] [Google Scholar]
- 27.Nanamiya, H., K. Takahashi, M. Fujita, and F. Kawamura. 2000. Deficiency of the initiation events of sporulation in Bacillus subtilis clpP mutant can be suppressed by a lack of the Spo0E protein phosphatase. Biochem. Biophys. Res. Commun. 279:229-233. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. Wiley, Chichester, United Kingdom.
- 29.Noirot-Gros, M.-F., E. Dervyn, L. J. Wu, P. Mervelet, J. Errington, S. D. Ehrlich, and P. Noirot. 2002. An expanded view of bacterial DNA replication. Proc. Natl. Acad. Sci. USA 99:8342-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogasawara, N. 2000. Systematic function analysis of Bacillus subtilis genes. Res. Microbiol. 151:129-134. [DOI] [PubMed] [Google Scholar]
- 31.Ohlsen, K. L., J. K. Grimsley, and J. A. Hoch. 1994. Deactivation of the sporulation transcription factor Spo0A by the Spo0E protein phosphatase. Proc. Natl. Acad. Sci. USA 91:1756-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Partridge, S. R., and J. Errington. 1993. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol. Microbiol. 8:945-955. [DOI] [PubMed] [Google Scholar]
- 33.Perego, M., S. P. Cole, D. Burbulys, K. Trach, and J. A. Hoch. 1989. Characterization of the genes for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J. Bacteriol. 171:6187-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perego, M., and J. A. Hoch. 1991. Negative regulation of Bacillus subtilis sporulation by the spo0E gene product. J. Bacteriol. 173:2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perego, M. 1993. Integrational vectors for genetic manipulation in Bacillus subtilis, p. 615-624. In A. L. Sonenshein, J. H. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington, D.C.
- 36.Perego, M., and J. A. Hoch. 1996. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:1549-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perego, M., and J. A. Hoch. 2002. Two-component systems, phosphorelays, and regulation of their activities by phosphatases, p. 473-481. In A. L. Sonenshein, J. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 38.Piggot, P. J., and R. Losick. 2002. Sporulation genes and intercompartmental regulation, p. 483-517. In A. L. Sonenshein, J. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 39.Pogliano, J., N. Osborne, M. D. Sharp, A. Abanes-De Mello, A. R. Perez, Y.-L. Sun, and K. Pogliano. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quisel, J. D., and A. D. Grossman. 2000. Control of sporulation gene expression in Bacillus subtilis by the chromosome partitioning proteins Soj (ParA) and Spo0J (ParB). J. Bacteriol. 182:3446-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quisel, J. D., W. F. Burkholder, and A. D. Grossman. 2001. In vivo effects of sporulation kinases on mutant Spo0A proteins in Bacillus subtilis. J. Bacteriol. 183:6573-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaeffer, P., J. Millet, and J. P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stahl, M. L., and E. Ferrari. 1984. Replacement of the Bacillus subtilis subtilisin structural gene with an in vitro-derived deletion mutation. J. Bacteriol. 158:411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterlini, J. M., and J. Mandelstam. 1969. Commitment to sporulation in Bacillus subtilis and its relationship to the development of actinomycin resistance. Biochem. J. 113:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stragier, P., and R. Losick. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30:297-341. [DOI] [PubMed] [Google Scholar]
- 46.Trach, K. A., and J. A. Hoch. 1993. Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway. Mol. Microbiol. 8:69-79. [DOI] [PubMed] [Google Scholar]
- 47.Wach, A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259-265. [DOI] [PubMed] [Google Scholar]
- 48.Wu, L. J., and J. Errington. 1997. Septal localization of the SpoIIIE chromosome partitioning protein in Bacillus subtilis. EMBO J. 16:2161-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamashita, S., H. Yoshikawa, F. Kawamura, H. Takahashi, T. Yamamoto, Y. Kobayashi, and H. Saito. 1986. The effect of spo0 mutations on the expression of spo0A- and spo0F-lacZ fusions. Mol. Gen. Genet. 205:28-33. [DOI] [PubMed] [Google Scholar]
- 50.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]