Abstract
DivIVA of Bacillus subtilis and FtsZ of Escherichia coli were used to target heterologous protein complexes to cell division sites of E. coli and Agrobacterium tumefaciens. DivIVA and FtsZ that were fused to the dimerizing leucine zipper (LZ) domain of the yeast transcription activator GCN4 directed the green fluorescent protein (GFP) that was fused to an LZ domain to E. coli division sites, resulting in fluorescence patterns identical to those observed with DivIVA::GFP and FtsZ::GFP. These cell division proteins also targeted the VirE1 chaperone and VirE2 secretion substrate complex to division sites of E. coli and A. tumefaciens. Coproduction of the native VirE1 or VirE2 proteins inhibited the dihybrid interaction in both species, as judged by loss of GFP targeting to division sites. The VirE1 chaperone bound independently to N- and C-terminal regions of VirE2, with a requirement for residues 84 to 147 and 331 to 405 for these interactions, as shown by dihybrid studies with VirE1::GFP and DivIVA fused to N- and C-terminal VirE2 fragments. DivIVA also targeted homo- and heterotypic complexes of VirB8 and VirB10, two bitopic inner membrane subunits of the A. tumefaciens T-DNA transfer system, in E. coli and homotypic complexes of VirB10 in A. tumefaciens. VirB10 self-association in bacteria was mediated by the C-terminal periplasmic domain, as shown by dihybrid studies with fusions to VirB10 truncation derivatives. Together, our findings establish a proof-of-concept for the use of cell-location-specific proteins for studies of interactions among cytosolic and membrane proteins in diverse bacterial species.
A first step in defining the architectures of supramolecular surface structures of bacteria often involves definition of subunit-subunit interactions among the surface components. Such an undertaking with the popular yeast dihybrid screens can be complicated by problems associated with folding or translocation of membrane proteins to the correct cellular compartment, lack of posttranslational modification of secreted proteins, or formation of nonproductive complexes in the heterologous host (22, 24, 31, 46, 48, 49). Some of these problems can be circumvented with dihybrid assays tailored for use with Escherichia coli, yet many multisubunit machines, e.g., the type IV T-DNA transfer system of Agrobacterium tumefaciens (10), assemble properly only in the native host grown under specialized environmental conditions. Recent work has established that productive assembly of the VirB subunits into a functional T-DNA transfer system requires growth of A. tumefaciens cells in a low-phosphate minimal medium, pH 5.5, at a low temperature. These environmental conditions, which are thought to mimic the environment of wounded plant tissue, coordinate the temporal and stoichiometric production of VirB proteins and also direct the assembly and spatial distribution of VirB protein complexes at the cell envelope (3, 4, 27, 28).
In this study, we sought to develop a versatile dihybrid screen in order to do the following: (i) characterize interactions among both soluble and membrane-associated components of the T-DNA transfer system and (ii) study these interactions in a biologically relevant context of the native A. tumefaciens host. Specifically, we tested whether the cell division proteins DivIVA of Bacillus subtilis or FtsZ of E. coli, when fused to a “bait” protein X, can target an interacting “prey” protein Y to division sites. As a readout for the X-Y interaction, the prey protein is fused to the green fluorescent protein (GFP) for detection of DivIVA- or FtsZ-dependent targeting of fluorescent foci.
DivIVA was selected for this assay because of its ability to localize and persist at cell poles. In its native organism, B. subtilis, DivIVA first localizes to midcell, as detected by immunofluorescence and by localization of a DivIVA::GFP fusion protein (16). Unlike other known cell division proteins, DivIVA is then retained at the mature cell poles after division has been completed (38). E. coli and other gram-negative bacteria lack a DivIVA homolog, yet DivIVA::GFP efficiently localizes to division sites and poles in E. coli (17). The capacity of DivIVA to localize in cells that divide independently of DivIVA function is potentially valuable for development of a generalizable targeting system. DivIVA also localizes to division sites in Schizosaccharomyces pombe (17), possibly extending the application of such a system to eukaryotic cells.
FtsZ was of interest as a potential targeting protein because of the novel ring structure—the Z ring—it forms along the cell circumference during cell division. This ring remains associated with the rest of the division machinery throughout septum formation (8, 32, 33, 35, 37, 41). When expressed at low levels, FtsZ::GFP forms fluorescent bands at the midpoints of dividing E. coli cells (44). When expressed at higher levels, FtsZ::GFP assembles into aggregates that mark the sites of incomplete septation in filamentous cells or spirals along the lengths of cells (33). Untagged FtsZ also can form these structures under certain conditions, showing that the GFP tag is not the sole cause of this behavior (1, 34, 40). Targeting of other GFP-tagged cell division proteins that appear to bind FtsZ directly, such as FtsA and ZipA, to the Z ring has been demonstrated cytologically (20, 21, 33, 34). Finally, FtsZ is broadly conserved among most bacteria, many archaea, plant chloroplasts, and protist mitochondria (6, 37, 51); thus, FtsZ also might be useful for protein targeting in diverse cell types.
The efficiency of this two-hybrid screen was investigated for E. coli and A. tumefaciens. Our studies show that both DivIVA and FtsZ can target GFP to division sites of both species via fusions to the following: (i) the dimerizing leucine zipper from the yeast transcription factor GCN4 (25, 43), (ii) the VirE1 secretion chaperone and two independent domains of the VirE2 effector proteins of A. tumefaciens (10, 55), and (iii) the bitopic membrane proteins VirB8 and VirB10 of the T-DNA transfer machine.
MATERIALS AND METHODS
Enzymes, chemicals, and reagents.
Restriction endonucleases were purchased from Promega (Madison, Wis.), New England Biolabs (Beverly, Mass.), or Gibco-BRL (Grand Island, N.Y.). Klenow fragment of E. coli DNA polymerase I and T4 DNA ligase were from Promega (Madison, Wisc.). Iso-propyl-thio-β-d-galactopyranoside (IPTG), l-arabinose, chloramphenicol, and kanamycin were from Sigma Chemical Co. (St. Louis, Mo.), and carbenicillin was from Gemini Bio-Products Inc. (Calabasas, Calif.).
Bacterial strains, growth conditions, and fractionation procedures.
E. coli strain DH5α and A. tumefaciens strain A348 or isogenic mutants were used for cell localization studies. A. tumefaciens A348 is A136 with the tumor-inducing plasmid pTiA6NC. KE1 is A348 with a deletion of the virE operon (39). E. coli strains were maintained on Luria-Bertani medium, and A. tumefaciens strains were maintained on MG/L medium (55). Conditions for induction of the A. tumefaciens vir genes in ABIM medium (glucose-containing minimal medium [pH 5.5], 1 mM phosphate) with acetosyringone (AS) have been described previously (55). Medium was supplemented with antibiotics (concentrations given in micrograms per milliliter) as follows: chloramphenicol (20), carbenicillin (100), kanamycin (50), and tetracycline (5). Cells were examined by microscopy (see below) and biochemically for formation of insoluble inclusion bodies. Cells were lysed with a French press, cell extracts were solubilized by treatment with a solution containing 2% Triton X-100, 5 mM EDTA, 250 mM NaCl, and 50 mg of lysozyme (pH 8.0) for 1 h at 25°C. Following centrifugation at 14,000 × g for 15 min, the pelleted material was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie brilliant blue staining or immunostaining for the presence of insoluble inclusion bodies.
Recombinant DNA techniques.
DNA manipulations and DNA electrophoresis were performed as described previously (42). PCR amplification was performed with a Perkin-Elmer Cetus DNA thermocycler using Pwo DNA polymerase from Boehringer Mannheim (Mannheim, Germany). DNA sequencing was carried out at the DNA Core Facility of the Department of Microbiology and Molecular Genetics with an ABI 373A DNA sequencer (Perkin-Elmer, Applied Biosystems Division) using Taq polymerase in a thermal cycling reaction. Oligonucleotides were purchased from Sigma-Genosys (The Woodlands, Tex.).
Construction of DivIVA fusion proteins.
Table 1 lists relevant properties of plasmids used in these studies. pWM1396 is a pACYC184 derivative that expresses PBAD-divIVA::GFP. It was constructed by replacing the SacI-XbaI fragment containing ftsZ in pWM976 with B. subtilis divIVA lacking a stop codon. pKA11 expressing PBAD-divIVA was constructed by XbaI digestion and religation of pWM1396 to excise the GFP gene and insert a translational stop codon immediately downstream of the divIVA coding sequence. pZD1396 expressing PBAD-divIVA::LZ (leucine zipper domain of the yeast transcription activator GCN4; see below) was constructed by substituting the LZ coding sequence (see below) on a 0.3-kb XbaI-HindIII fragment from pZD2 for GFP of pWM1396. pZD1397 expressing PBAD-divIVA::virE2 was constructed by substituting virE2 on a 1.6-kb XbaI-XhoI (XhoI made blunt ended) fragment from pZZ10 (see below) for GFP of pWM1396. pZD1398 expressing PBAD-divIVA::virE1 was constructed by substituting virE1 on a 0.3-kb XbaI-HindIII fragment from pZZ7 for GFP of pWM1396. pZD1401 is pWM1396 with the Kanr cassette from pUC4K (Pharmacia) introduced as a 1.1-kb PstI fragment downstream of GFP. pZD6 expressing Ptac-divIVA::GFP was constructed by introducing a 2.5-kb SacI-SphI (both sites made blunt ended) fragment from pZD1401 into the unique HpaI site of the broad-host-range (BHR) IncQ plasmid pMMB22 (2). pZD1398 expressing Ptac-divIVA::virE2 was constructed by substituting virE2 on a 1.6-kb XbaI-XhoI fragment from pZZ10 for GFP of pZD6. Utilizing pZD7 as the starting vector, two plasmid series were constructed that produce DivIVA fused to N- or C-terminal fragments of VirE2 for identification of VirE1 interaction domains. Series pZD70x, where x is 1 to 9, express PBAD-divIVA fused to 3′ fragments of virE2. These 3′ fragments were isolated as BamHI (blunt ended)-XhoI fragments from the plasmids pXZ721 to pXZ729 (55). With plasmids pXZ721 to pXZ729, BamHI sites are located at various positions in virE2 as a result of the introduction of sequence encoding an i31 oligopeptide by TnlacIN transposition (36); the XhoI site is located downstream of virE2 (55). The 3′ virE2′ fragments were introduced into StuI-XhoI sites of pZD1398, placing the virE2′ fragments in-frame with DivIVA and the first 12 codons of virE2. Series pZD79x, where x is 1 to 9, express PBAD-divIVA fused to 5′ fragments of virE2 isolated as NdeI-BamHI (blunt-ended) fragments from the plasmids pXZ721 to pXZ729. These fragments were introduced into NdeI-XhoI (blunt-ended) sites substituting for full-length virE2 of pZD1398; a stop-codon resides immediately downstream of the virE2′ fragments. The plasmids pPC1308, pPC1309, and pPC1310 expressing PBAD-divIVA::virB8, PBAD-divIVA::virB9, and PBAD-divIVA::virB10 were constructed by substituting the corresponding virB genes as NdeI-XhoI fragments from plasmids pPC988, pPC998, and pPC9108 (7) for virE1 on pZD1398. pZD22 expressing Ptac-divIVA::virB10 from an IncQ BHR plasmid was constructed by substituting virB10 on a 1.2-kb XbaI-XhoI fragment from pPC1310 for GFP on pZD6. pZD37 with codons for the first 63 residues of VirB10 fused in-frame to signal-sequenceless PhoA (′phoA) was constructed by introducing a ∼200-bp EcoRI/HincII fragment from pKA1 into EcoRI/SmaI-digested pUI1156 (11). pZD39 expressing PBAD-divIVA::virB10.63::phoA was constructed by substituting virB10.63::phoA on a 2-kb NdeI-SacI (blunt-ended) fragment from pZD37 for virE1 by NdeI-HindIII (blunt-ended) digestion of pZD1398. pZD42 expressing PBAD-divIVA::virB10.63 was constructed by SpeI digestion, blunt ending, and religation of pZD39. ColE1 plasmids were introduced into A. tumefaciens by ligation to a BHR IncP plasmid; in each case, the cointegrate plasmid is given the ColE1 plasmid name plus a B to denote the BHR replicon.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| DivIVA constructs | ||
| pWM1396 | Camr, pACYC184-based replicon with PBAD-divIVA::GFP | This study |
| pZD6 | Crbr Kanr, pMMB22 (IncQ) with Ptac-divIVA::GFP | This study |
| pZD7 | Crbr, pMMB22 with Ptac-divIVA::E2 | This study |
| pZD22 | Crbr, pMMB22 with Ptac-divIVA::B10 | This study |
| pZD1396 | Camr, pACYC184 replicon with PBAD-divIVA::LZ | This study |
| pZD1397 | Camr, pACYC184 replicon with PBAD-divIVA::E2 | This study |
| pZD1398 | Camr, pACYC184 replicon with PBAD-divIVA::E1 | This study |
| pZD1401 | Camr, Kanr, pWM1396 with a Kan cassette | This study |
| pPC1308 | Camr, pACYC184 replicon with PBAD-divIVA::B8 | This study |
| pPC1309 | Camr, pACYC184 replicon with PBAD-divIVA::B9 | This study |
| pPC1310 | Camr, pACYC184 replicon with PBAD-divIVA::B10 | This study |
| pKA11 | Camr, pACYC184 replicon with PBAD-divIVA | This study |
| pXZ9104 | Crbr, pBKS+.NdeI (ColE1 replicon) with PvirB-B10 | X.-R. Zhou |
| pXZ721-pXZ729 | Crbr, pBSK+.NdeI with Plac-virE2.i31 insertion alleles | 55 |
| pZD701-pZD709 | Camr, pACYC184 replicon with PBAD-divIVA fused in-frame to 3′ fragments of virE2 | This study |
| pZD791-pZD799 | Camr, pACYC184 replicon with PBAD-divIVA fused in-frame to 5′ fragments of virE2 | This study |
| pZD37 | Crbr, pBSK+ with Plac-virB10.63::phoA | This study |
| pZD39 | Camr, pACYC184 replicon with PBAD-divIVA::virB10.63::phoA | This study |
| pZD42 | Camr, pACYC184 replicon with PBAD-divIVA::virB10.63 | This study |
| FtsZ constructs | ||
| pWM976 | Camr, pACYC184 replicon with PBAD-ftsZ::GFP | 33 |
| pZD2 | Camr, pACYC184 replicon with PBAD-ftsZ::LZ | This study |
| pZZ7 | Camr, pACYC184 replicon with PBAD-ftsZ::E1 | This study |
| pZZ10 | Camr, pACYC184 replicon with PBAD-ftsZ::E2 | This study |
| GFP constructs | ||
| pXZ63 | Crbr, pBSK+.NdeI with Plac-GFP | 55 |
| pXZ66 | Kanr, pBSK+.NdeI with Plac-E2::GFP | 55 |
| pXZ168 | Crbr, pBSIIKS+.NdeI with Plac-E1::GFP | 55 |
| pZD1 | Crbr, pBSK+.NdeI with Plac-GFP::LZ | This study |
| pZD9 | Crbr, pBKS+.NdeI with PvirB-E1::GFP | This study |
| pZD12 | Kanr, pBKS+.NdeI with PvirB-E1::GFP | This study |
| pZD20 | Kanr, pBR322 replicon, with Ptrc-GFP::B10 | This study |
| pZD23 | Crbr, pBR322 replicon, with Ptrc-GFP::B10::phoA | This study |
| pZD40 | Crbr, pBSK+ with Ptrc-GFP::virB10.63::phoA | This study |
| pZD43 | Crbr, pBSK+ with Ptrc-GFP::virB10.63 | This study |
| pZD45 | Crbr, pBR322 with Ptrc-GFP::phoA | This study |
| pZD169 | Crbr, pBSIIKS+.NdeI with Plac-E1::GFP and Plac-E2 | This study |
| pZD170 | Crbr, pBSIIKS+.NdeI with Plac-E1::GFP and Plac-E1 | This study |
| pZZ9 | Crbr, pBKS+.NdeI with PvirB-E1 | 54 |
| pDSW207 | Crbr, pBR322 (ColE1 replicon) with Ptrc-GFP | 9 |
| pKA1 | Crbr, pBR322 with Ptrc-GFP::B10 | This study |
| pKA2 | Crbr, pBR322 with Ptrc-GFP::B8 | This study |
| pKA4 | Crbr, pBR322 with Ptrc-GFP::B9 | This study |
Drug resistance phenotype abbreviations: Cam, chloramphenicol; Crb, carbenicillin; Kan, kanamycin.
Construction of FtsZ fusion proteins.
The plasmid pWM976 is a pACYC184 derivative that expresses PBAD- ftsZ::GFP (33). pZD977 is pWM976 with a Kanr cassette from pUC4K (Pharmacia) introduced as a 1.1-kb PstI fragment downstream of GFP. pZZ7 expressing PBAD- ftsZ::virE1 was constructed by substituting virE1 as a ∼350-bp XbaI-HindIII fragment derived by PCR amplification for GFP in pWM976. The oligonucleotide primers 5′-AAATCTAGACATATGGCCATCATC-3′ (XbaI and NdeI sites underlined) and 5′-AAAAAGCTTCTCGAGCGCCATTCGTCATC-3′ (HindIII and XhoI sites underlined) were used for amplification of virE1 from the template plasmid pPC731 (54). pZZ10 expressing PBAD-ftsZ::virE2 was constructed by substituting full-length virE2 as a 1.6-kb NdeI-XhoI fragment from pPC730 (54) for virE1 in pZZ7 (51). pZD2 expressing PBAD-ftsZ::LZ was constructed by substitution of the leucine zipper (LZ) domain of GCN4 as a ∼300-bp NdeI-SalI PCR fragment for virE1 in pZZ7. The LZ sequence was amplified with oligonucleotide primers 5′-AGCTGCAGGACACATATG-3′ (PstI and NdeI sites underlined) and 5′-AAAGTCGACCTGTCGGAATGGAC-3′ (SalI site underlined) with the plasmid pJH370 as a template (24).
Construction of GFP fusion proteins.
pXZ63 is pBSIISK+.NdeI expressing Plac-GFP (M2 variant) (55). pXZ168 and pXZ66 express Plac-virE1::GFP and Plac-virE2::GFP, respectively (55). The plasmid pZD1 expressing Plac-GFP::LZ was constructed by introducing the LZ sequence on a 0.3-kb PstI-SalI PCR fragment downstream of GFP into pXZ63. pZD169 expressing both Plac-virE2 and Plac-virE1::GFP was constructed by introducing Plac-virE2 from pXZ46 as a partially digested PvuII fragment (55) into the unique NaeI site of pXZ168. Similarly, pZD170 expressing both Plac-virE1 and Plac-virE1::GFP was constructed by introducing Plac-virE1 from pZZ5 as a 1.0-kb PvuII fragment into the unique NaeI site of pXZ168. Plasmids pKA2, pKA4, and pKA1 expressing Ptrc-GFP::virB8, Ptrc-GFP::virB9, and Ptrc-GFP::virB10, respectively, were constructed by introducing the XbaI-HindIII fragments encoding the corresponding virB genes from plasmids pPC1308, pPC1309, and pPC1310 into similarly digested pDSW107. The plasmid pZD20 expressing Ptrc-GFP::virB10 is a Kanr derivative of pKA1 constructed by introducing a 1.1-kb HincII fragment from pUC4K into the ScaI site of pKA1. The plasmid pZD23 expressing Ptrc-GFP::virB10.258::phoA was constructed by introducing ′phoA on a 1.4-kb SalI-SacI (blunt-ended) fragment from pUI1158 (18) into SalI and HindIII (blunt-ended) digested pKA1. pZD40 expressing Ptrc-GFP::virB10.63::phoA was constructed by introducing virB10.63::phoA on an ∼1.6-kb XbaI (partial digest)-SacI (blunt-ended) fragment from pZD37 into XbaI- and HindIII (blunt-ended)-digested pKA1. pZD43 expressing Ptrc-GFP::virB10.63 was constructed by SpeI digestion, blunt ending, and religation of pZD40. pZD45 expressing Ptrc-GFP::phoA was constructed by introducing ′phoA on a 1.4-kb XbaI-SacI (blunt-ended) fragment from pZD37 into XbaI- and HindIII (blunt-ended)-digested pKA1.
Microscopy and image analysis.
Newly transformed cells were used for fluorescence analyses. Overnight cultures of E. coli were diluted 1:100 and incubated for 2 to 3 h to an optical density at 600 nm of 0.5 at 37°C. IPTG (final concentration, 25 μM) and l arabinose (0.2% wt/vol) were added to induce gene expression from the Plac or Ptrc and PBAD promoters, respectively, and samples were removed at various times. For A. tumefaciens, newly transformed cells were similarly induced in MG/L media or in ABIM with AS (100 μm) for growth also under vir-inducing conditions (54, 55). Cells were examined for GFP localization by fluorescence microscopy and for inclusion body formation by phase contrast and Nomarski microscopy. We routinely examined at least 1,000 cells per experiment, and each experiment was replicated at least three times. Results are presented for a representative experiment, whereby the number of cells showing a discernible fluorescence pattern is expressed as a percentage of the total number of cells examined. Images of cells were acquired with an Olympus BX60 microscope equipped with a ×100 oil immersion phase-contrast objective, a filter set for GFP, and either an Optronics Engineering DEI-750 24-bit color video camera or a Photometrics Coolsnap fx charge-coupled device camera. Images from the video camera were captured and digitized with a Scion CG-7 framegrabber, and images from the charge-coupled device camera were captured with QED image analysis software. Image files were then analyzed and optimized with Adobe Photoshop. Care was always taken to minimize exposure of the bacteria to the blue excitation light to minimize photobleaching.
RESULTS
DivIVA targets soluble protein complexes in E. coli.
DH5α(pWM1396) synthesizing DivIVA::GFP displays a bipolar pattern of fluorescence (Fig. 1A), as observed previously (17). Induction for 2 h with 0.2% l-arabinose yielded bipolar fluorescence in >95% of the cells, whereas a fluorescent band at midcell also was evident in ∼10% of these cells. By contrast, DH5α(pXZ63) cells synthesizing only GFP were uniformly fluorescent (Fig. 1A). In ≤1% of these cells, small foci lacking a specific intracellular localization were detected. These were inclusion bodies as shown by phase contrast microscopy and examination of the insoluble fraction following Triton X-100 treatment of cell extracts (see Materials and Methods). These and additional control experiments (see below) were routinely used to distinguish true GFP localization from inclusion body formation.
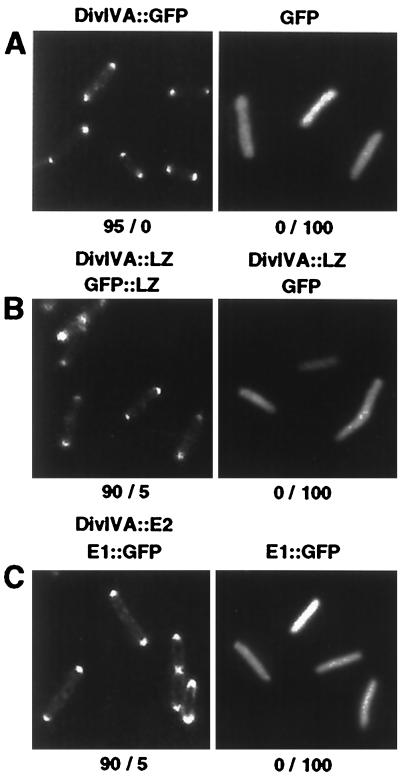
FIG. 1.
DivIVA targets protein complexes to division sites in E. coli. DH5α cells producing the proteins indicated above each panel were examined 2 h after induction with l-arabinose and/or IPTG by fluorescence microscopy. (A) DH5α(pWM1396) (left), DH5α(pXZ63) (right); (B) DH5α(pZD1396, pZD1), DH5α(pZD1396, pXZ63); (C) DH5α(pZD1397, pXZ168), DH5α(pXZ168). Numbers below each panel are the percentages of cells with bipolar (left number) versus homogeneous (right number) fluorescence.
To assess whether DivIVA can target heterologous proteins to the midcell or cell poles corresponding to the old sites of cell division, we fused DivIVA and GFP to the dimerizing leucine zipper domain of the yeast transcription factor GCN4. DH5α(pZD1396, pZD1) coproducing DivIVA::LZ and GFP::LZ, respectively, showed bipolar fluorescence patterns identical to patterns observed with cells synthesizing DivIVA::GFP (Fig. 1B). More than 90% of these cells showed bipolar fluorescence, and a small percentage also showed a band of fluorescence at midcell. By contrast, DH5α(pZD1) producing GFP::LZ or DH5α(pKA11, pXZ63) coproducing DivIVA and GFP from separate promoters (data not shown) and DH5α(pZD1396, pXZ63) coproducing DivIVA::LZ and GFP (Fig. 1B) were uniformly fluorescent; less than 1% of these cells formed inclusion bodies. These findings suggest that DivIVA targets GFP to new and old sites of cell division via dimerization of the LZ domain.
The A. tumefaciens VirE1 chaperone interacts with its secretion substrate, the VirE2 single-stranded-DNA-binding protein. Chaperone binding stabilizes and maintains VirE2 in a translocation-competent state prior to export via a type IV secretion system (13, 45, 54, 55). Interestingly, >90% of DH5α(pZD1397, pXZ168) coproducing DivIVA::VirE2 and VirE1::GFP showed bipolar fluorescence (Fig. 1C), whereas ∼40% of DH5α(pZD1398, pXZ66) producing the reciprocal fusion proteins, DivIVA::VirE1 and VirE2::GFP, showed this pattern. By contrast, all cells producing VirE1::GFP alone (Fig. 1C) or together with native DivIVA (data not shown) were uniformly fluorescent. Cells exhibiting bipolar fluorescence as a result of the LZ-LZ or VirE1-VirE2 interactions often displayed an arc of fluorescence extending from the poles. In Fig. 1C, this arc pattern is most readily visualized among cells that cosynthesize DivIVA::VirE2 and VirE1::GFP. The dependence on production of DivIVA fusions for targeting of GFP to cell poles, detergent solubility of the presumptive protein complexes, and results of competition studies (see below) together support our conclusion that DivIVA directs GFP to cell poles via LZ-LZ or VirE1-VirE2 interactions.
FtsZ targets soluble protein complexes in E. coli.
DH5α(pWM976) cells synthesizing FtsZ::GFP often form long filaments (Fig. 2), a property attributed to dominant-negative effects of FtsZ::GFP on assembly of the division machinery (33). In filamentous cells induced with l-arabinose for 1 h, GFP fluorescence was evident primarily as bands, whereas in cells induced for 2 h, fluorescence appeared as spots at sites corresponding to undivided septa. Most cells induced for prolonged periods of ⩾18 h showed spiral-shaped fluorescence patterns (Fig. 2) (1, 33). As noted above, E. coli DH5α(pXZ63) cells producing native GFP fluoresce uniformly and rarely filament.
FIG. 2.
FtsZ targets protein complexes to division sites in E. coli. DH5α cells producing the proteins indicated at right were examined by fluorescence microscopy following 1, 2, and 18 h of IPTG and/or l-arabinose induction. Top panels show fluorescence patterns observed with DH5α(pWM976). Middle and bottom panels show patterns observed with DH5α(pZD2, pZD1) and DH5α(pZZ10, pXZ168), respectively. The fluorescence patterns of each strain were photographed at 1 (bands), 2 (spots), and 18 h (spirals) after induction. The histogram at the left shows the percentages of cells with bands or spots at division septa, or spirals; the black bar corresponds to the percentages of cells with homogeneous fluorescence.
DH5α(pZD2, pZD1), coproducing FtsZ::LZ and GFP::LZ, and DH5α(pZZ10, pXZ168), coproducing FtsZ::VirE2 and VirE1::GFP, displayed patterns of fluorescence reminiscent of cells producing FtsZ::GFP although the relative ratio of bands, spots, and spirals was altered (Fig. 2). Approximately 75 to 80% of cells of both strains formed spots of fluorescent foci at regular intervals, and 15 to 20% formed bands regardless of the duration of the induction period; approximately 5% formed spirals at 18 h following induction (Fig. 2). Targeting of GFP by FtsZ was dependent on fusion of both FtsZ and GFP to the LZ motif or the VirE1 and VirE2 partner proteins (data not shown). DH5α(pZZ7, pXZ66) producing the reciprocal fusion proteins, FtsZ::VirE1 and VirE2::GFP, also formed spots of fluorescence at regular intervals, although fewer cells, ∼30%, showed a clear pattern of GFP localization, perhaps because of steric hindrance of interactions associated with VirE2::GFP synthesis. Together, these data suggest that FtsZ targets GFP to division sites efficiently via the FtsZ::LZ-GFP::LZ and FtsZ::VirE2-VirE1::GFP interactions, and less efficiently via the FtsZ::VirE1-VirE2::GFP interaction.
Native protein production inhibits targeting.
Next, we examined the effect of native VirE protein synthesis on GFP targeting by cells producing DivIVA::VirE2 and VirE1::GFP. As noted above, nearly all DH5α(pZD1397, pXZ168) cells coproducing DivIVA::VirE2 and VirE1::GFP, exhibit bipolar fluorescence (Fig. 3A). In striking contrast, nearly all DH5α(pZD1397, pZD170) cells coproducing DivIVA::VirE2, VirE1::GFP, and the native VirE1 protein were uniformly fluorescent (Fig. 3A). More than half of DH5α(pZD1397, pZD169) cells coproducing DivIVA::VirE2, VirE1::GFP, and native VirE2 were uniformly fluorescent, and the remaining cells exhibited bipolar fluorescence (Fig. 3A).
FIG. 3.
Native VirE proteins interfere with dihybrid complex formation. DH5α cells producing the proteins indicated above each panel were examined by fluorescence microscopy. (A) DH5α(pZD1397, pXZ168) (left), DH5α(pZD1397, pZD170) (middle), DH5α(pZD1397, pXZ169) (right). (B) DH5α(pZZ10, pXZ168) (left), DH5α(pZZ10, pZD170) (middle), DH5α(pZZ10, pZD169) (right). Numbers below each panel are the percentages of cells with bipolar (DivIVA targeting) or a regular distribution of fluorescent foci (FtsZ targeting) versus homogeneous fluorescence.
Similar results were obtained with the FtsZ targeting system. In striking contrast to the regular distribution of bands or spots at midcell associated with coproduction of the partner hybrid proteins, cells producing the hybrid proteins and either native VirE1 or VirE2 exhibited a higher background of uniform fluorescence as well as abundant, irregularly distributed foci (Fig. 3B). There was a complete loss of a discernible pattern of fluorescence in these cells. In the competition studies shown in Fig. 3A and B, VirE1::GFP and native VirE1 or VirE2 were expressed from independent Plac promoters from the same ColE1 replicon. Western blot experiments confirmed that cells produced comparable levels of the expected fusion proteins and native VirE1 or VirE2 (data not shown). These findings suggest that native VirE1 effectively inhibits complex formation between VirE1::GFP and the DivIVA or FtsZ fusions to VirE2. Native VirE2 production inhibits these interactions but less effectively than VirE1.
Delineation of VirE1 interaction domains on VirE2.
A VirE1 interaction domain was identified within the C-terminal third of VirE2 by use of yeast dihybrid (Y2H) screens (Fig. 4B) (13, 45, 55). A second VirE1 interaction domain near the beginning of VirE2 was proposed on the basis of observed stabilizing effects of VirE1 on N-terminal fragments of VirE2 in A. tumefaciens (Fig. 4B) (54). This N-terminal domain could not be detected with the Y2H screen because the N terminus of VirE2 self-activates expression from the GAL4 and Lex promoters in yeast (45). We constructed two series of plasmids synthesizing DivIVA fused to either N- or C-terminal fragments of VirE2 (see Materials and Methods) and assayed for the capacity of the fusion proteins to target VirE1::GFP to new or old division sites.
FIG. 4.
Two regions of VirE2 are required for interaction with VirE1. (A) Hatched bars are schematic representations of VirE2 N-terminal fragments of the number of residues indicated fused to DivIVA. Plasmids producing the DivIVA::VirE2′ fusion proteins are listed at the left (79x series). The numbers in the vertical columns adjacent to the bars are the percentages of E. coli cells with bipolar fluorescent foci upon cosynthesis of the corresponding DivIVA::VirE2′ fusion protein and VirE1::GFP. The stabilities of the DivIVA::VirE2′ fusion proteins are shown by immunostaining with anti-VirE2 antiserum. A ∼55-kDa, cross-reactive species is present in all lanes and occludes detection of DivIVA::VirE2.1-331. Sizes in kilodaltons of molecular mass markers are indicated at the left of each immunoblot. (B) Full-length VirE2 (black bar) with the locations of the NLSs (white boxes). C2H boxes identify regions of VirE2 required for interaction with VirE1 as shown by the cytology-based two-hybrid screen. STAB box identifies a region shown previously (55) to be important for VirE1-mediated stabilization of VirE2. Y2H boxes correspond to regions shown to interact with VirE1 by the yeast two-hybrid screen (15, 45, 55). (C) A corresponding analysis of VirE2 C-terminal fragments fused at the residue indicated to DivIVA. The DivIVA::VirE2.529-533 fusion protein was not immunoreactive.
As summarized in Fig. 4A, DivIVA that was fused to the first 147 residues or to larger N-terminal fragments targeted VirE1::GFP to the cell poles, whereas fusions to residues 1 to 39 or 1 to 84 did not target VirE1::GFP. All of these fusion proteins were detectable with anti-VirE2 antiserum, and most proteins accumulated to abundant levels within 2 h following l-arabinose induction. The fusion proteins composed of DivIVA joined to fragments 1 to 405, 1 to 437, and 1 to 529 were less abundant than other fusion proteins, yet cells producing these fusion proteins still efficiently targeted VirE1::GFP to the cell poles (Fig. 4A). DivIVA fused to residues 331 to 533, or larger C-terminal fragments, targeted VirE1::GFP to the cell poles in an appreciable percentage of cells (Fig. 4C). By contrast, DivIVA fusions to residues 405 to 533 or 437 to 533 did not target the GFP fusion protein. All these fusion proteins except for DivIVA::VirE2.147-533 accumulated at abundant levels (Fig. 4C).
Although strains producing the different DivIVA::VirE2′ constructs exhibited a fairly wide range with respect to the percentages of cells showing GFP targeting, it is important to note that cells producing VirE1::GFP only were uniformly fluorescent and completely devoid of any discernible pattern of fluorescence. The results of these studies therefore support a proposal that residues 84 to 147 and 331 to 405 of VirE2 independently contribute to complex formation with VirE1. These regions are necessary for complex formation with VirE1, but the question of whether they are sufficient for binding requires further study. The identification of these domains and their general localization with the cytological screen is in complete agreement with results of interaction studies using yeast two-hybrid screens and the demonstration that the VirE2 N terminus is necessary for stabilization by VirE1 in A. tumefaciens (Fig. 4B) (13, 45, 54, 55).
Interestingly, ∼60 to 80% of cells producing DivIVA that was fused to nearly full-length fragments of VirE2 cells exhibited a bipolar fluorescence pattern, whereas ≤40% of cells producing fusions to smaller fragments—those predicted to carry only one VirE1 interaction domain—showed this pattern (Fig. 4A). Overall, there was no correlation between the steady-state levels of the DivIVA::VirE2′ fusion proteins and the percentage of cells exhibiting bipolar fluorescence (Fig. 4), suggesting that this variation is not due to differences in stabilities of the fusion proteins. It is conceivable the larger VirE2 fragments adopt a native structure that is conducive to formation of high-affinity contacts with VirE1, whereas the smaller fragments interact more weakly with VirE1. Even so, the findings support a proposal that VirE2 possesses two independent VirE1 interaction domains in its N- and C-terminal regions.
DivIVA targets soluble protein complexes in A. tumefaciens.
Next, we tested whether DivIVA can serve as a targeting protein in A. tumefaciens. To this end, we introduced constructs encoding DivIVA::VirE2 and VirE1::GFP into A. tumefaciens A348 and KE1, an isogenic A348 mutant with a deletion of the virE operon. DivIVA::GFP or DivIVA::VirE2 were synthesized from the IPTG-inducible Ptac promoter, and VirE1::GFP was synthesized from the AS-inducible PvirB promoter. Initial studies showed that nearly all A348(pZD6) and KE1(pZD6) cells producing DivIVA::GFP showed fluorescence exclusively at the cell poles, whereas A348(pZDB69) and KE1(pZDB69) cells producing GFP were uniformly fluorescent (Fig. 5A and data not shown). These patterns were observed with cells induced with IPTG in rich (MG/L) medium or cells induced with IPTG and AS in AB minimal medium (see Materials and Methods). No inclusion bodies were detected in cells induced for ≤18 h.
FIG. 5.
DivIVA targets protein complexes to division sites in A. tumefaciens. A348 and the ΔvirE operon mutant, KE1, producing the proteins indicated above each panel were examined by fluorescence microscopy. (A) A348(pZD6) (left) and A348(pZDB69) (right). (B) KE1(pZD7, pZDB12) (left) and KE1(pZDB12) (right). (C) A348(pZD7, pZDB12) (left) and A348(pZDB12) (right). All strains were induced for vir gene expression by growth in IM plus AS. Numbers below each panel are the percentages of cells with bipolar versus homogeneous fluorescence.
Approximately 85% of IPTG- and AS-induced KE1(pZD7, pZDB12) cells producing DivIVA::VirE2 and VirE1::GFP showed polar fluorescence, and the remaining cells were uniformly fluorescent (Fig. 5B). By contrast, similarly grown KE1(pZDB12) cells producing only VirE1::GFP were uniformly fluorescent (Fig. 5B). To further test whether GFP targeting is dependent on the DivIVA::VirE2-VirE1::GFP interaction, A348(pZD7, pZDB12) cells were induced with IPTG and AS for synthesis of DivIVA::VirE2, VirE1::GFP, and the native VirE1 and VirE2 proteins. Interestingly, these cells were uniformly fluorescent, suggesting that native protein production competitively inhibits the dihybrid interaction (Fig. 5C). Similarly grown A348(pZDB12) producing VirE1::GFP and the native VirE proteins also were uniformly fluorescent (Fig. 5C), as were A. tumefaciens strains engineered to synthesize DivIVA and VirE1::GFP with or without coproduction of the native VirE proteins (data not shown). We conclude that DivIVA targets GFP to A. tumefaciens cell poles when both proteins are fused to interacting partners.
DivIVA targets integral membrane protein complexes in E. coli.
The T-DNA transfer system of A. tumefaciens translocates substrates across the cell envelope and assembles a T pilus for establishing contacts with target cells (10). To examine the utility of the cytology-based dihybrid screen for characterizing interactions among the membrane protein components of this transfer system, we first assayed for interactions among the VirB8, VirB9, and VirB10 proteins in E. coli. Previous work has shown that VirB8 and VirB10 are bitopic inner membrane proteins with small (∼25 residues) N-terminal cytoplasmic domains, a transmembrane domain (TM), and large C-terminal periplasmic domains. VirB9 carries a characteristic signal sequence and is secreted across the inner membrane, where it associates predominantly or exclusively with the outer membrane. Soluble domains of VirB8, VirB9, and VirB10 have been shown to interact with Y2H screens, and complementary biochemical studies support the proposal that these proteins form a subcomplex(es) in A. tumefaciens (5, 12, 19).
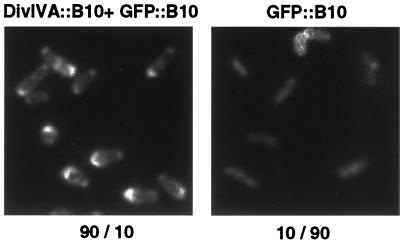
Figure 6 summarizes results of the cytological screen for VirB8, VirB9, and VirB10 pairwise interactions. Strains coproducing the following combinations of fusion proteins—DivIVA::VirB8 plus GFP::VirB8, DivIVA::VirB8 plus GFP::VirB10, DivIVA::VirB10 plus GFP::VirB8, and DivIVA::VirB10 plus GFP::VirB10—exhibited bipolar fluorescence. Pattern formation was detected most frequently (∼90% of cells examined) among cells producing DivIVA::VirB10 and GFP::VirB8, but still evident in a majority (∼60%) of cells producing the other combinations of fusion proteins. Most of the remaining cells displayed fluorescence around the cell periphery, suggestive of membrane localization, although a small fraction of cells producing the GFP::VirB10 fusion protein (≤5%) also formed inclusion bodies.
FIG. 6.
DivIVA targets bitopic membrane protein complexes to division sites in E. coli. DH5α cells producing the proteins indicated above each panel were examined by fluorescence microscopy. (A) DH5α(pPC1308, pKA2) (left), DH5α(pPC1310, pKA2) (middle), and DH5α(pKA2) (right). (B) DH5α(pPC1308, pKA1) (left), DH5α(pPC1310, pKA1) (middle) and DH5α(pKA1) (right). (C) DH5α(pPC1308, pKA4) (left), DH5α(pPC1310, pKA4) (middle), and DH5α(pKA4) (right). Numbers below each panel are the percentages of cells with bipolar versus homogeneous fluorescence. Approximately 5% of cells producing the GFP::VirB10 fusion proteins produced inclusion bodies.
In contrast, nearly all cells of strains producing only GFP::VirB8 or GFP::VirB10 in the absence or presence of native DivIVA exhibited a distinct pattern of fluorescence around the cell periphery characteristic of a membrane localization (Fig. 6). Western blot analyses confirmed that induced cells produced the VirB8 and VirB10 fusion proteins, although species the sizes of native VirB8 or VirB10 and GFP were detectable at comparatively low levels by immunostaining, suggesting that a fraction of these proteins were proteolytically cleaved at their fusion junctions (data not shown).
We next examined whether the addition of DivIVA and GFP to the N terminus affects the capacity of VirB10 to insert into the membrane. Two GFP::VirB10′::PhoA fusion proteins were constructed by addition of signal-sequenceless PhoA at residue 63 or 258 of VirB10, resulting in substitution of PhoA for VirB10 C-terminal residues. DivIVA::VirB10′::PhoA was constructed by addition of the PhoA moiety at residue 63 of VirB10. DH5α(pZD23), DH5α(pZD40), and DHα(pZD42) synthesized the GFP::VirB10.258::PhoA, GFP::VirB10.63::PhoA, and DivIVA::VirB10.63.PhoA fusion proteins of the expected sizes and reactivities with the GFP and/or VirB10 antibodies (data not shown). Further, DH5α(pZD23) and DH5α(pZD40) were uniformly fluorescent and exhibited high levels of AP activity, indicating that both the GFP::VirB10′::PhoA fusion proteins are composed of cytoplasmically localized GFP, a TM domain of VirB10, and periplasmically localized PhoA. The cytoplasmic location of GFP is inferred from available data showing that the general secretory pathway used for insertion of integral inner membrane proteins cannot export an active form of GFP. By contrast, DH5α(pZD45) producing GFP that was fused in-frame directly to signal-sequenceless PhoA exhibited uniform fluorescence but was devoid of AP activity, establishing the importance of the TM domain of VirB10 for ′PhoA export. Finally, we tested whether PhoA dimerization could substitute for dimerization of the periplasmic domain of VirB10. DH5α(pZD39, pZD40) coproducing DivIVA:VirB10.63::PhoA and GFP::VirB10.63::PhoA exhibited high levels of PhoA activity and bipolar GFP fluorescence. Conversely, DH5α(pZD43, pZD42) cells coproducing DivIVA::VirB10Δ63-295 and GFP::VirB10Δ63-295 were uniformly fluorescent, establishing that the first 63 residues of VirB10 including its cytoplasmic domain and TM domain cannot mediate self-association (data not shown). PhoA dimerization in the periplasm, as evidenced by alkaline phosphatase activity, can therefore mediate DivIVA targeting of GFP to the cell poles.
Cells producing DivIVA::VirB9 or GFP::VirB9 failed to show any discernible pattern formation (Fig. 6). In contrast to the VirB8 and VirB10 bitopic inner membrane proteins, VirB9 is secreted across the inner membrane via an N-terminal secretion signal that is then cleaved by signal peptidase. In Western blots, we failed to detect either of the VirB9 fusion proteins. Instead, distinct protein species reactive with GFP or VirB9 antibodies comigrated with the native forms of these proteins, respectively (data not shown). The VirB9 fusion proteins probably fail to accumulate and show interactions because of proteolytic cleavage at the fusion junctions.
DivIVA targets integral membrane protein complexes in A. tumefaciens.
Finally, we screened for self-association of VirB10 in A. tumefaciens. Approximately 90% of A348(pZDB20) cells producing GFP::VirB10 showed a uniform fluorescence pattern when grown in rich (MG/L) medium (Fig. 7) or vir-inducing (AS-ABIM) (data not shown) medium. Among A348(pZDB20, pZD22) synthesizing GFP::VirB10 and DivIVA::VirB10 grown in rich medium (no vir gene induction), ∼90% showed arc-like patterns of polar fluorescence, and many of these cells also possessed fluorescent bands at midcell. A348(pZDB20, pZD22) grown in ABIM with AS for induction of the vir genes showed a reduction in the percentage of cells displaying polar fluorescence to ∼20%, indicating that native VirB10 or another Vir protein interferes with complex formation between the fusion proteins (data not shown). DivIVA therefore targets a bitopic inner membrane protein complex to old sites of cell division in A. tumefaciens.
FIG. 7.
DivIVA targets bitopic VirB10 homomultimers to division sites in A. tumefaciens. Proteins produced by A348(pZDB20, pZD22) (left) and A348(pZDB20) (right) are indicated above each panel. Strains were grown in rich (MG/L) medium; no Vir proteins are synthesized under these conditions. Numbers below each panel are the percentages of cells with polar versus homogeneous fluorescence.
DISCUSSION
We developed a novel dihybrid screen for studies of protein-protein interactions in bacteria, and we used this screen to refine our understanding of the requirements for complex formation among components of a type IV secretion system. In the case of DivIVA-dependent targeting, dihybrid interactions established by contacts between soluble (e.g., LZ-LZ and VirE1-VirE2) as well as integral membrane (VirB8-VirB10 and VirB10-VirB10) proteins had no obvious effects on cell division in either E. coli or A. tumefaciens. In these species, therefore, the dihybrid complexes resembled DivIVA::GFP by localizing predominantly at the poles—the sites of previous rounds of cell division—without disrupting new rounds of cell division. However, the use of FtsZ met with variable success. In E. coli, targeting of soluble dihybrid complexes resulted in division defects reminiscent of those observed with FtsZ::GFP. In addition, the GFP fluorescence patterns displayed by filamentous cells—bands and spots at regular intervals and spirals along the cell lengths—closely resembled patterns observed in cells producing FtsZ::GFP (33, 37). However, in A. tumefaciens, both FtsZ::GFP and corresponding soluble dihybrid complexes localized at division sites, yet targeting was evident in a relatively low (∼20 to 30%) number of cells. Furthermore, in both species, FtsZ fusions to integral membrane proteins VirB8 or VirB10 generally resulted in severe dominant-negative effects, such as reduced growth rates and loss of cell viability (data not shown). The insertion of large amounts of membrane proteins by the highly abundant FtsZ protein might directly interfere with the dynamic process of cell division or induce nonspecific toxic effects. In view of these findings, we have favored the use of DivIVA as the targeting protein for this dihybrid assay.
The cytology-based screen is an appealing choice for protein interaction studies, most importantly because it is well-suited for characterization of complex formation among both soluble and integral membrane proteins. Currently, there are only a few other dihybrid assays adapted for studies of membrane protein interactions, and most of these are restricted to use in E. coli hosts. This laboratory (11) and other groups (14, 15, 29, 30) have adapted the λ cI repressor fusion system (25), originally developed for studies of soluble, dimeric proteins, to characterize multimerization of peripheral or integral membrane proteins. An assay based on reconstitution of Bordetella pertussis adenylate cyclase activity also is being widely used for studies of membrane protein interactions in E. coli (26). Two other systems based on fluorescence (FRET [47]) and bioluminescence (BRET [53]) resonance energy transfer in principle are well suited for in vivo studies of both soluble and membrane proteins. FRET and BRET are appealing options for use in any bacterium but require extensive spectroscopic analysis and are constrained by a requirement for appropriate positioning and close juxtaposition of GFP (or luciferase) molecules for energy transfer. Another recently described system, termed bimolecular fluorescence complementation, is based on reconstitution of yellow fluorescence protein (YFP) fluorescence by fusion of its two halves to two interacting partner proteins (23). This system also is appealing because of its potential for general use for interaction studies of both soluble and membrane protein in any cell type, though YFP reconstitution has not yet been reported for bacteria. Like FRET and BRET, bimolecular fluorescence complementation is subject to positional constraints; in this case, the two halves of YFP must be able to productively associate when tethered to the interacting partner proteins (23).
The cytology-based screen differs from most dihybrid screens by permitting an assessment of a given protein-protein interaction for each cell in a population. Minimally, this can facilitate efforts to optimize the assay, for example, by modulating cellular growth or gene expression conditions in order to maximize the percentage of cells showing GFP targeting. However, visual screens also offer the opportunity to monitor formation of protein-protein contacts in real time as a function of growth phase or developmental stage with synchronized cultures, or monitoring effects of environmental shifts, i.e., temperature or other culture conditions, on complex formation. Further, this type of assay permits studies of the dynamics of complex formation as influenced by chaperones, proteases, or other factors, as well as examination of interactions subject to competitive inhibition by other cellular proteins.
Additionally, the fact that FtsZ and DivIVA are so broadly conserved or at least display targeting behavior among both gram-negative and gram-positive bacteria suggests that a cytology-based dihybrid screen is well suited for use with phylogenetically diverse species. For example, this assay might be ideally suited for interaction studies in extremophiles, e.g., bacteria or even archaea living in high-pH or -salt environments or at high or low temperature. Such organisms have adapted diverse mechanisms at the molecular level for preserving protein contacts essential for survival, making it a real advantage to study complex formation in the native host under optimal growth conditions. As exemplified with the T-DNA transfer system, this screen should be generally applicable to studies of partner protein interactions whose formation or activities are dependent on growth in a natural environment such as a pathogen-host setting. Finally, we note that in principle, cytology-based dihybrid screens can be developed with any location-specific protein in the event that FtsZ or DivIVA does not localize in a bacterial, archaeal, or eukaryotic cell type of interest.
The following problems might be encountered with this assay. First, a comparatively low percentage of cells might display GFP targeting, possibly because the partner protein interaction under study is intrinsically weak or one or both fusion proteins sterically hinder complex formation. In such cases, both a demonstration that a GFP fusion protein itself shows no intrinsic localization and competitive inhibition are valuable criteria for assessing whether an interaction should be suspected. Second, the fusion proteins might form inclusion bodies. In our studies, this was more problematic when the partner proteins were expressed in E. coli as opposed to A. tumefaciens host cells. A combination of phase-contrast microscopy and detergent solubilization identifies the extent of the problem, and a number of genetic (e.g., promoter choice and plasmid copy number) and growth (e.g., temperature and media composition) parameters can be modulated to minimize protein aggregation, though admittedly some fusion proteins might aggregate under any conditions. Third, some fusion proteins might be unstable. This is a potential problem with all dihybrid screens and might be avoided by fusing the targeting or GFP proteins to opposite ends of the bait or prey proteins under study. Finally, this screen is suitable only for interaction studies of proteins that show no intrinsic localization to a discrete site(s) in the cell.
In addition to establishing a proof-of-concept for the cytology-based dihybrid screen, we have used this assay to refine our understanding of interactions among subunits of the T-DNA transfer system. We determined that the N-terminal region of VirE2 interacts with VirE1, confirming a prediction based on observed stabilizing effects of VirE1 on VirE2 truncation derivatives for A. tumefaciens (54). We supplied evidence for a second VirE1 interaction domain in the C-terminal region of VirE2, in agreement with previous findings (13, 45, 55). We confirmed predictions from Y2H and in vitro biochemical studies that VirB8 and VirB10 form both homo- and heterotypic interactions in bacteria (5, 12, 52). Finally, we determined that the periplasmic region of VirB10 is both necessary and sufficient for directing VirB10 self-association in bacteria. The finding that VirB10 self-associates in A. tumefaciens is especially notable because although a hydrophilic region of VirB10 was shown to self-associate with the Y2H screen (12), results of chemical cross-linking studies also led to the proposal that VirB10 oligomerization in A. tumefaciens is dependent on production of the VirB7-VirB9 heteromultimer (5, 52). The demonstration that VirB10 self-associates in the absence of other VirB proteins has important implications regarding the assembly pathway for this transfer system. Based on our findings, we propose that this pathway sequentially involves VirB10 synthesis, integration into the inner membrane, and formation of a homo-oligomer. Upon VirB10 oligomerization, contacts are established with the VirB7-VirB9 heteromultimer located at the outer membrane. This interaction must induce a conformational change in the VirB10 oligomer, as judged by results of chemical cross-linking studies (5, 52). We postulate that the conformational change accompanying VirB7-VirB9-VirB10 complex formation is required for establishment of further contacts between VirB10 and other subunits of the transfer system, e.g., VirB8, and the VirB4 and VirB11 traffic ATPases.
Acknowledgments
We acknowledge the intellectual contributions by Qin Sun and the technical help from Vidhya Krishmapoorthy and Brett Geissler on this project.
This work was supported by grants from the NIH to P.J.C. (GM48746) and to W.M. (GM61074) as well as a grant from the Texas Advanced Technology Program to W.M.
REFERENCES
- 1.Addinall, S. G., and J. Lutkenhaus. 1996. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol. Microbiol. 22:231-237. [DOI] [PubMed] [Google Scholar]
- 2.Bagdasarian, M., R. Lurz, B. Ruckert, F. C. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237-247. [DOI] [PubMed] [Google Scholar]
- 3.Banta, L. M., J. Bohne, S. D. Lovejoy, and K. Dostal. 1998. Stability of the Agrobacterium tumefaciens VirB10 protein is modulated by growth temperature and periplasmic osmoadaption. J. Bacteriol. 180:6597-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron, C., N. Domke, M. Beinhofer, and S. Hapfelmeier. 2001. Elevated temperature differentially affects virulence, VirB protein accumulation, and T-pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains. J. Bacteriol. 183:6852-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaupré, C. E., J. Bohne, E. M. Dale, and A. N. Binns. 1997. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J. Bacteriol. 179:78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beech, P. L., T. Nheu, T. Schultz, S. Herbert, T. Lithgow, P. R. Gilson, and G. I. McFadden. 2000. Mitochondrial FtsZ in a chromophyte alga. Science 287:1276-1279. [DOI] [PubMed] [Google Scholar]
- 7.Berger, B. R., and P. J. Christie. 1994. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J. Bacteriol. 176:3646-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi, E. F., and J. Lutkenhaus. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161-164. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J. C., D. S. Weiss, J. M. Ghigo, and J. Beckwith. 1999. Septal localization of FtsQ, an essential cell division protein in Escherichia coli. J. Bacteriol. 181:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christie, P. J. 2001. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol. 40:294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang, T. A. T., and P. J. Christie. 1997. The VirB4 ATPase of Agrobacterium tumefaciens is a cytoplasmic membrane protein exposed at the periplasmic surface. J. Bacteriol. 179:453-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das, A., and Y.-H. Xie. 2000. The Agrobacterium T-DNA transport pore proteins VirB8, VirB9, and VirB10 interact with one another. J. Bacteriol. 182:758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng, W., L. Chen, W.-T. Peng, X. Liang, S. Sekiguchi, M. P. Gordon, and E. W. Nester. 1999. VirE1 is a specific molecular chaperone for the exported single-stranded-DNA-binding protein VirE2 in Agrobacterium. Mol. Microbiol. 31:1795-1807. [DOI] [PubMed] [Google Scholar]
- 14.Dziejman, M., H. Kolmar, H. J. Fritz, and J. J. Mekalanos. 1999. ToxR co-operative interactions are not modulated by environmental conditions or periplasmic domain conformation. Mol. Microbiol. 31:305-317. [DOI] [PubMed] [Google Scholar]
- 15.Dziejman, M., and J. J. Mekalanos. 1994. Analysis of membrane protein interaction: ToxR can dimerize the amino terminus of phage lambda repressor. Mol. Microbiol. 13:485-494. [DOI] [PubMed] [Google Scholar]
- 16.Edwards, D. H., and J. Errington. 1997. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol. Microbiol. 24:905-915. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, D. H., H. B. Thomaides, and J. Errington. 2000. Promiscuous targeting of Bacillus subtilis cell division protein DivIVA to division sites in Escherichia coli and fission yeast. EMBO J. 19:2719-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez, D., T. A. T. Dang, G. M. Spudich, X.-R. Zhou, B. R. Berger, and P. J. Christie. 1996. The Agrobacterium tumefaciens virB7 gene product, a proposed component of the T-complex transport apparatus, is a membrane-associated lipoprotein exposed at the periplasmic surface. J. Bacteriol. 178:3156-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez, D., G. M. Spudich, X.-R. Zhou, and P. J. Christie. 1996. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J. Bacteriol. 178:3168-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hale, C. A., and P. A. de Boer. 1997. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 88:175-185. [DOI] [PubMed] [Google Scholar]
- 21.Hale, C. A., and P. A. de Boer. 1999. Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. J. Bacteriol. 181:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays, L. B., Y. S. Chen, and J. C. Hu. 2000. Two-hybrid system for characterization of protein-protein interactions in E. coli. BioTechniques 29:288-290, 292, 294. [DOI] [PubMed] [Google Scholar]
- 23.Hu, Chang-Deng, Y. Chinenov, and T. K. Kerppola. 2002. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9:789-798. [DOI] [PubMed] [Google Scholar]
- 24.Hu, J., M. Kornacker, and A. Hochschild. 2000. Escherichia coli one- and two-hybrid systems for the analysis and identification of protein-protein interactions. Methods 20:80-94. [DOI] [PubMed] [Google Scholar]
- 25.Hu, J. C., E. K. O'Shea, P. S. Kim, and R. T. Sauer. 1990. Sequence requirements for coiled-coils: analysis with lambda repressor-GCN4 leucine zipper fusions. Science 250:1814-1822. [DOI] [PubMed] [Google Scholar]
- 26.Karimova, G., A. Ullmann, and D. Ladant. 2000. A bacterial two-hybrid system that exploits a cAMP signaling cascade in Escherichia coli. Methods Enzymol. 328:59-73. [DOI] [PubMed] [Google Scholar]
- 27.Kumar, R. B., Y. H. Xie, and A. Das. 2000. Subcellular localization of the Agrobacterium tumefaciens T-DNA transport pore proteins: VirB8 is essential for the assembly of the transport pore. Mol. Microbiol. 36:608-617. [DOI] [PubMed] [Google Scholar]
- 28.Lai, E. M., O. Chesnokova, L. M. Banta, and C. I. Kado. 2000. Genetic and environmental factors affecting T-pilin export and T-pilus biogenesis in relation to flagellation of Agrobacterium tumefaciens. J. Bacteriol. 182:3705-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeds, J. A., and J. Beckwith. 1998. Lambda repressor N-terminal DNA-binding domain as an assay for protein transmembrane segment interactions in vivo. J. Mol. Biol. 280:799-810. [DOI] [PubMed] [Google Scholar]
- 30.Leeds, J. A., D. Boyd, D. R. Huber, G. K. Sonoda, H. T. Luu, D. M. Engelman, and J. Beckwith. 2001. Genetic selection for and molecular dynamic modeling of a protein transmembrane domain multimerization motif from a random Escherichia coli genomic library. J. Mol. Biol. 313:181-195. [DOI] [PubMed] [Google Scholar]
- 31.Legrain, P., J. Wojcik, and J. M. Gauthier. 2001. Protein-protein interaction maps: a lead towards cellular functions. Trends Genet. 17:346-352. [DOI] [PubMed] [Google Scholar]
- 32.Lutkenhaus, J., and S. G. Addinall. 1997. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66:93-116. [DOI] [PubMed] [Google Scholar]
- 33.Ma, X., D. W. Ehrhardt, and W. Margolin. 1996. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc. Natl. Acad. Sci. USA. 93:12998-13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma, X., and W. Margolin. 1999. Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J. Bacteriol. 181:7531-7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma, X., Q. Sun, R. Wang, G. Singh, E. L. Jonietz, and W. Margolin. 1997. Interactions between heterologous FtsA and FtsZ proteins at the FtsZ ring. J. Bacteriol. 179:6788-6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manoil, C., and J. Bailey. 1997. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J. Mol. Biol. 267:250-263. [DOI] [PubMed] [Google Scholar]
- 37.Margolin, W. 2000. Themes and variations in prokaryotic cell division. FEMS Microbiol. Rev. 24:531-548. [DOI] [PubMed] [Google Scholar]
- 38.Marston, A. L., H. B. Thomaides, D. H. Edwards, M. E. Sharpe, and J. Errington. 1998. Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev. 12:3419-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McBride, K. E., and V. C. Knauf. 1988. Genetic analysis of the virE operon of the Agrobacterium Ti plasmid pTiA6. J. Bacteriol. 170:1430-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mileykovskaya, E., Q. Sun, W. Margolin, and W. Dowhan. 1998. Localization and function of early cell division proteins in filamentous Escherichia coli cells lacking phosphatidylethanolamine. J. Bacteriol. 180:4252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothfield, L., S. Justice, and J. Garcia-Lara. 1999. Bacterial cell division. Annu. Rev. Genet. 33:423-448. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J. E., F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Struhl, K. 1989. Helix-turn-helix, zinc-finger, and leucine-zipper motifs for eukaryotic transcriptional regulatory proteins. Trends Biochem. Sci. 14:137-140. [DOI] [PubMed] [Google Scholar]
- 44.Sun, Q., and W. Margolin. 1998. FtsZ dynamics during the division cycle of live Escherichia coli cells. J. Bacteriol. 180:2050-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sundberg, C. D., and W. Ream. 1999. The Agrobacterium tumefaciens chaperone-like protein, VirE1, interacts with VirE2 at domains required for single-stranded DNA binding and cooperative interaction. J. Bacteriol. 181:6850-6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toby, G. G., and E. A. Golemis. 2001. Using the yeast interaction trap and other two-hybrid-based approaches to study protein-protein interactions. Methods 24:201-217. [DOI] [PubMed] [Google Scholar]
- 47.Truong, K., and M. Ikura. 2001. The use of FRET imaging microscopy to detect protein-protein interactions and protein conformational changes in vivo. Curr. Opin. Struct. Biol. 11:573-578. [DOI] [PubMed] [Google Scholar]
- 48.Tucker, C. L., J. F. Gera, and P. Uetz. 2001. Towards an understanding of complex protein networks. Trends Cell Biol. 11:102-106. [DOI] [PubMed] [Google Scholar]
- 49.Uetz, P., and R. E. Hughes. 2000. Systematic and large-scale two-hybrid screens. Curr. Opin. Microbiol. 3:303-308. [DOI] [PubMed] [Google Scholar]
- 50.van Heeckeren, W. J., J. W. Sellers, and K. Struhl. 1992. Role of the conserved leucines in the leucine zipper dimerization motif of yeast GCN4. Nucleic Acids Res. 20:3721-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vitha, S., R. S. McAndrew, and K. W. Osteryoung. 2001. FtsZ ring formation at the chloroplast division site in plants. J. Cell Biol. 153:111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward, J. E., Jr., E. M. Dale, E. W. Nester, and A. N. Binns. 1990. Identification of a VirB10 protein aggregate in the inner membrane of Agrobacterium tumefaciens. J. Bacteriol. 172:5200-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, Y., D. W. Piston, and C. H. Johnson. 1999. A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins. Proc. Natl. Acad. Sci. USA 96:151-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao, Z., E. Sagulenko, Z. Ding, and P. J. Christie. 2001. Activities of virE1 and the VirE1 secretion chaperone in export of the multifunctional VirE2 effector via an Agrobacterium type IV secretion pathway. J. Bacteriol. 183:3855-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou, X.-R., and P. J. Christie. 1999. Mutagenesis of Agrobacterium VirE2 single-stranded DNA-binding protein identifies regions required for self-association and interaction with VirE1 and a permissive site for hybrid protein construction. J. Bacteriol. 181:4342-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]