Abstract
pMG1 (65.1 kbp) is a pheromone-independent Enterococcus faecium conjugative plasmid conferring gentamicin resistance. pMG1 is able to transfer to enterococci at a high frequency in broth mating experiments, and it does not respond to the sex pheromones which are involved in the high-frequency transfer system of some conjugative plasmids in Enterococcus faecalis. To analyze regulation of tra gene expression in pMG1, transcripts of pMG1 were examined during conjugation. RNA samples were prepared from mating mixtures 20, 40, 80, and 160 min after initiation of mating and were subsequently analyzed by Northern hybridization by using a variety of pMG1 DNA fragments as probes. One transcript of gene 71ORF2 increased to the maximal level 20 min after the start of mating. The level of this transcript decreased after 40 min of mating to the same level as the level in the control donor culture. The increase was not observed in cultures of the donor cells or recipient cells. These findings suggested that the increase was a conjugation-specific event. The 71ORF2 gene of pMG1 was disrupted by using the suicide vector pMG226 carrying an erythromycin resistance gene that is expressed in enterococci. The transfer frequencies of mutant plasmid pMG229, which had a disrupted 71ORF2 gene in pMG1, and the parent plasmid pMG1 were 1.6 × 10−7 and 1.1 × 10−3 per donor cell, respectively, in broth mating experiments, and the transfer frequencies of pMG229 and pMG1 were 2.7 × 10−3 and 8.5 × 10−2 per donor cell, respectively, in filter mating experiments. This indicated that the transfer frequency of plasmid pMG229 was reduced during broth mating and was not altered during filter mating. 71ORF2, which is designated traA, is a gene involved in the tra gene system for conjugation, and the product of this gene is associated with the formation or stabilization of mating aggregates during broth mating.
Conjugation consists of several steps to achieve plasmid transfer (1, 10, 18). In the case of gram-negative bacteria, conjugation begins with contact between the cell surface of the recipient cell and the tip of a sex pilus of a donor cell, followed by wall-to-wall contact between the donor and recipient cells. Unstable wall-to-wall contact changes to stable wall-to-wall contact. After formation of stable mating aggregates, plasmid DNA is transferred to the recipient cell. Recircularization of transferred plasmid DNA is followed by dissociation of the mating aggregates caused by surface exclusion. Conjugation requires expression of numerous tra genes encoded on a conjugative plasmid, and it is thought that there are mechanisms to initiate expression of the tra genes in response to conjugation (10). The presence of a so-called mating signal has been proposed, which would be generated at the beginning of conjugation and would initiate the process of DNA transfer (16, 20). Until now, in spite of the many studies of numerous conjugative plasmids, conjugation-specific expression of tra genes has not been observed, and no information on the regulatory system of the tra genes during the early stage of conjugation has been obtained (10).
The sex pheromone of Enterococcus faecalis is the best example of a signal in conjugation (4-6, 8, 9, 17). A donor cell harboring a sex pheromone-responsive plasmid responds to the pheromone specific for the plasmid, which generally consists of seven or eight amino acids and is secreted from a potential recipient cell (4-7, 9, 14). The sex pheromone signal induces synthesis of the surface aggregation substance that facilitates formation of the mating aggregate (13, 25, 27). The sex pheromone also activates the genes required for plasmid transfer (21). The pheromone (in a culture filtrate of a plasmid-free strain) induces self-aggregation of donor cells and makes donor cells ready for conjugation without mating with recipient cells. The process of conjugation after the formation of mating aggregates has not been clarified (6).
The gentamicin resistance plasmid pMG1 (65.1 kbp) was isolated from an Enterococcus faecium clinical isolate (15). pMG1 transfers at a high frequency to Enterococcus strains during liquid mating, does not respond to the E. faecalis pheromone, and does not show any homology in DNA-DNA hybridization experiments with the pheromone-responsive plasmids pAD1, pAM373, and pPD1 (15) or with other enterococcal conjugative plasmids (i.e., pAMβ1 and pIP501) (15). These facts imply that pMG1 conjugation is different from the pheromone-induced transfer system of the E. faecalis plasmid. In this study, we investigated a pMG1 gene which is induced during conjugation.
Analysis of transcript during conjugation.
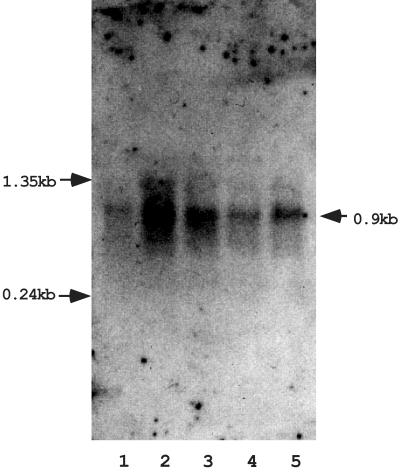
Generally, only a few phenotypic characteristics, such as formation of mating aggregates and the ability to transfer during broth mating or during filter mating, are available for genetic analysis of conjugation. These phenotypes are not sufficient to observe the regulation of tra gene expression. To identify a conjugation-specific transcript(s), which could result from expression of a gene(s) during conjugation, the transcripts of plasmid pMG1 were analyzed by Northern hybridization. A series of relational clones of pMG1 were constructed as described previously. (12). Plasmid pMG1 DNA was partially digested with HindIII, and the digested DNA was separated by agarose gel electrophoresis. Fragments larger than 6 kbp were eluted from the gel and cloned into pBluescript. The resulting clones were used as probes in Northern hybridization analyses. Total RNAs were prepared from mating mixtures of the donor OG1X(pMG1) and the recipient FA2-2 20, 40, 80, and 160 min after the initiation of mating and were analyzed by Northern hybridization. One probe, designated clone #71, was found to hybridize with a transcript that was about 900 bases long (Fig. 1). The transcript was detected in donor cells before conjugation, and the level increased to the maximum level by 20 min after initiation of conjugation and then gradually decreased. After 160 min of mating, the amount of transcript was the same as the amount detected in donor cells before mating. In the absence of recipient cells, the amount of the transcript did not increase in either the donor cells or the recipient cells after 20, 40, 80, or 160 min of incubation (data not shown). These results indicate that the increase in the transcript level resulted from induction of a gene(s) during mating. The amount of the transcript reached the maximum level after about 20 min of mating; thus, induction of the transcript occurred in the early stage of mating. In addition, no increase in the transcript level was observed after exposure of donor cells to the sex pheromones in culture filtrate of plasmid-free E. faecalis FA2-2 (data not shown). This result is consistent with the previously reported finding that transfer of pMG1 is not induced by exposure to a sex pheromone (15).
FIG. 1.
Northern blot analysis of the conjugation-regulating gene 71ORF2. Total RNAs were prepared from mating mixtures at various times during conjugation. Clone #71 DNA (6 kbp) was used as a probe. Matings carried out for the purpose of preparation of RNA were conducted as follows. After overnight growth, 5 ml of the donor strain was added to 25 ml of fresh THB in a 50-ml centrifugation tube and incubated on a roller at 37°C for 4 h. Concurrently, 5 ml of an overnight culture of the recipient strain was mixed with 25 ml of fresh THB in a 100-ml flask and incubated with gentle shaking at 37°C for 4 h. Then 2.5 ml of the recipient culture was added to the donor culture to initiate the mating. Mating was conducted at 37°C for the desired time and was then terminated by addition of chloramphenicol at a final concentration of 200 μg/ml. The mating mixture was then placed on ice for 15 min and harvested by centrifugation. The pellet was suspended in 600 μl of lysis buffer (50 mM glucose, 25 mM Tris-HCl; pH 8.0). Fifty microliters of a lysozyme solution (100 mg/ml) was added, and the cells were incubated at 37°C for 30 min. To lyse the cells, 800 μl of TRIzol (Invitrogen, Inc., Carlsbad, Calif.) and 80 μl of chloroform were added, and the mixture was vortexed for 20 s. The mixture was kept on ice for 5 min and spun for 15 min at 4°C. The aqueous phase was transferred to a new tube and extracted twice with phenol-chloroform-isoamyl alcohol. Nucleic acid was recovered by ethanol precipitation, and the pellet was suspended in 100 μl of DNase I buffer. DNA was digested by adding 30 U of RNase-free DNase I (10 U/μl; Roche Diagnostics GmbH, Mannheim, Germany) and incubating the preparation at 37°C for 2 h. The RNA was treated with phenol-chloroform, recovered by ethanol precipitation, and suspended in 50 μl of diethyl pyrocarbonate-treated distilled water. Northern blotting was performed by using standard protocols (2, 22, 24). RNA samples were electrophoresed on a 1% agarose gel with morpholinepropanesulfonic acid (MOPS)-formaldehyde buffer and were transferred to a nylon membrane (Roche Diagnostics GmbH) by the conventional capillary method. Hybridization and signal detection were performed by using a DIG DNA labeling and detection kit (Roche Diagnostics GmbH) according to the manufacturer's protocols. EasyHyb (Roche Diagnostics GmbH) was used as the prehybridization and hybridization solutions, and CSPD (Roche Diagnostics GmbH) was used as a substrate for chemical luminescence. Lane 1, OG1X(pMG1) donor cells before conjugation; lane 2, 20 min; lane 3, 40 min; lane 4, 80 min; lane 5, 160 min. The arrows on the left indicate the positions of the RNA molecular weight markers (Invitrogen, Inc.). The arrow on the right indicates the estimated position of the band.
Sequencing of the conjugation-regulated gene.
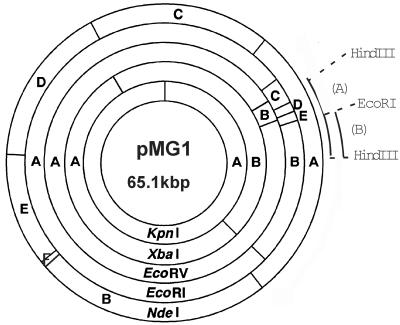
Clone #71 contains a 6-kbp HindIII fragment of pMG1 corresponding to EcoRI fragments D and E and part of fragments B and C (Fig. 2), indicating that clone#71 has four EcoRI sites (i.e., three sites that originated from plasmid pMG1 and one site that originated from the multicloning sites of vector plasmid pBluescript). Clone #71 was digested with EcoRI, and the digested DNAs were self-ligated and introduced into Escherichia coli DH5α by electroporation (11). One transformant was found to contain a plasmid, designated p71E, which represents a deletion derivative of clone #71 and retains 2.5 kbp of part of the EcoRI B fragment of pMG1. By using this plasmid as a probe, induction of the transcript was also detected in the early stage of mating with donor strain OG1X(pMG1) and recipient strain FA2-2 (data not shown). The 2.5-kbp fragment in p71E was sequenced. For sequencing, a series of nested deletion mutant plasmids were constructed from p71E with a nested deletion kit (Nippon Gene, Toyama, Japan). The DNA sequence was determined by using a dye primer cycle sequencing FS Ready Reaction kit (Perkin-Elmer, Wellesley, Mass.) with primers −21M13 and M13Rev (Perkin-Elmer) and an ABI Prism 377 sequencer (Perkin-Elmer). Open reading frame (ORF) analysis was performed by using MacVector, version 6.0 (Oxford Molecular Group, San Diego, Calif.), and a homology search with FASTA and BLAST was performed through the website of the DNA Data Bank of Japan (National Institute of Genetics, Mishima, Japan). Computer analysis of the sequence revealed the presence of two complete ORFs and one truncated ORF (Fig. 3a). One of the complete ORFs, which consists of 564 bp (187 amino acid residues), was designated 71ORF1. The ATG start codon is preceded by a putative ribosome binding site (AGAGG) located 7 bp upstream. The other complete ORF consists of 861 bp (286 amino acid residues) and was designated 71ORF2. The ATG start codon is preceded by a putative ribosome binding site (AGGGG) located 5 bp upstream. The deduced amino acid sequences encoded by the two ORFs do not show any significant homology to the sequences of other previously reported proteins. The size of the transcript which hybridized to clone #71 corresponds to the size of 71ORF2, suggesting that the transcript was produced by 71ORF2. Two pairs of PCR primers were designed to amplify the internal DNA sequences of 71ORF1 and 71ORF2. The sequences of the primers (primers 1F and 1B for 71ORF1 and primers 2F and 2B for 71ORF2) are as follows: 1F, CAGCCATTTCTTTCTCATAAAGTG; 1B, GCACTAAAAAATTCTAACGTATCAGAG; 2F, GCAAATTCATTTTCTGCCCCC; and 2B, TTAGGAAGTCTTGCTTTCAGCCTG. Northern hybridization was performed by using each of the amplified internal DNA sequences as a probe. The DNA fragments of the 71ORF2 internal sequence hybridized to a transcript that was the same size as the transcript which hybridized with clone #71. These results indicated that 71ORF2 was induced during conjugation.
FIG. 2.
Physical map of pMG1. Fragments produced by restriction endonuclease digestion of pMG1 DNA are indicated by letters. Arcs A and B indicate the approximate regions contained in clone #71 and p71E, respectively.
FIG. 3.
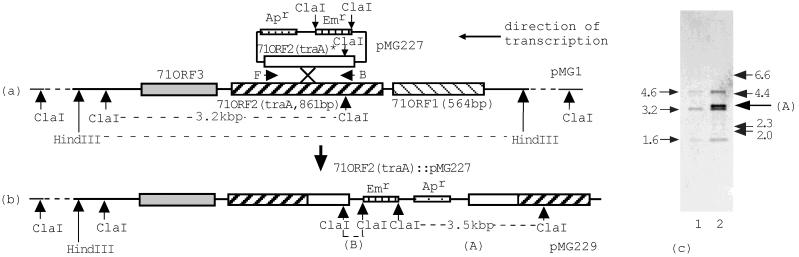
Gene disruption of 71ORF2 (traA). (a and b) Diagrams showing gene disruption of 71ORF2 (traA). The box labeled 71ORF2(traA)∗ represents the internal fragment of 71ORF2 (traA) which was amplified by PCR with primers having EcoRI recognition sequences at the 5′ ends and cloned into the suicide vector pMG226, creating pMG227. The approximate locations of the primers are indicated by horizontal arrows below the 71ORF2(traA)∗ box. The vertical arrows labeled ClaI and HindIII indicate the recognition sites of ClaI and HindIII, respectively. The dotted line between the two HindIII sites indicates the HindIII fragment contained in clone #71. ClaI fragments A and B are cointegrate plasmid specific and are expected to hybridize with the HindIII fragment contained in clone #71. (c) Southern hybridization of cointegrate plasmid pMG229. For pMG1 (lane 1) and pMG229 (lane 2), DNA was digested with ClaI. DNA fragments were separated on an agarose gel, and the gel was treated with alkali transfer buffer (0.4 N NaOH, 1.5 M NaCl) for 20 min; then the DNA fragments were transferred to a nylon membrane by capillary action with alkali transfer buffer (2, 22). After transfer, the membrane was neutralized with 0.5 M Tris-HCl (pH 7.0) for 5 min and rinsed with 2× SSC for 2 min (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The membrane was air dried and baked at 80°C for 2 h. Hybridization and signal detection were performed with a DIG DNA labeling and detection kit (Roche Diagnostics GmbH). The insert of clone #71 was used as a probe. The arrows on the left indicate the positions of the fragments which hybridized with the probe in each lane. The numbers indicate the sizes of the fragments (in kilobase pairs) estimated on the basis of mobility. The arrows on the right indicate the positions of the molecular size markers, a λ HindIII digest; the numbers indicate their sizes (in kilobase pairs). Arrow A indicates the position of the cointegrate plasmid-specific ClaI fragment shown in panel b.
The DNA sequence revealed a putative promoter sequence in front of 71ORF1 but not in front of 71ORF2. Both ORFs were therefore expected to be transcribed as a single RNA molecule. However, Northern hybridization experiments with the PCR-amplified internal fragments of both 71ORF1 and 71ORF2 did not detect a large transcript of the size expected for 71ORF2 and 71ORF1 together (data not shown).
The beginning of another ORF, designated 71ORF3, is located about 400 bp downstream of 71ORF2. Although the function of 71ORF3 was not examined, at the amino acid level its product has similarity to the dihydrofolate reductases of Staphylococcus aureus, Listeria monocytogenes, and Bacillus subtilis.
Construction of a new suicide vector and plasmid for gene disruption of 71ORF2.
A new suicide vector was constructed to disrupt 71ORF2. Plasmid pAM307 (63 kbp, Emr) is a derivative of pAD1 (58 kbp) with Tn917 (5 kbp, Emr) inserted into EcoRI fragment H (1.2 kbp) (3). EcoRI H::Tn917 of pAM307 has been cloned into pBR325, and the resulting plasmid was designated pAM225 (23). The erythromycin resistance determinant of Tn917 is present on the ClaI fragment (1.4 kb) of Tn917 (23). The ClaI fragment of pAM225 was eluted from an agarose gel after agarose gel electrophoresis of ClaI-digested pAM225. The eluted ClaI fragment was ligated with dephosphorylated ClaI-digested pBluescript. The cloned fragment was confirmed to contain an erythromycin resistance gene by DNA sequencing. The resulting plasmid, a new suicide vector, was designated pMG226. An internal region of 71ORF2, the gene disrupted in this study, was amplified by PCR with a pair of primers. These primers had the EcoRI recognition sequence at their 5′ ends and their sequences are as follows: primer F, CGGAATTCTGTCGTACAGGCTCTCCTGAAG; and primer B, CGGAATTCGGTCTGGCTATTCATTGCTAGAG. The PCR product was cloned into the EcoRI site in pMG226, and the resulting plasmid was designated pMG227. pMG227 contained the erythromycin resistance gene and the internal region of 71ORF2 in a Bluescript plasmid.
Disruption of the 71ORF2 gene.
To disrupt 71ORF2 of pMG1, pMG227 was constructed as described above. OG1X(pMG1) was transformed with pMG227 by electroporation (11). Transformants were selected on a Todd-Hewitt broth (THB) agar plate containing erythromycin. After purification of the transformants on drug-free plates, the transconjugants still expressed erythromycin resistance. To examine whether plasmid pM227 DNA cointegrated into pMG1, the plasmid DNAs of the transformants were examined by Southern hybridization analysis with the insert of clone #71 as the probe. Figure 3c shows the results of a Southern hybridization analysis of pMG1 and a plasmid, designated pMG229, which was typical of the plasmids isolated from the transformants. In the HindIII fragment (6 kbp) of pMG1, which was cloned into clone #71, there were two ClaI sites (Fig. 3a). When pMG1 DNA was digested with ClaI, the clone #71 probe hybridized to three ClaI fragments, which had molecular sizes of 4.6, 3.2, and 1.6 kbp, as estimated by their mobilities (Fig. 3c). The 3.2-kbp fragment corresponded to the internal 3.2-kbp ClaI fragment that was present in the 6-kbp HindIII fragment of pMG1. The other two fragments could be the fragments outside the internal 3.2-kbp fragment.
In a Southern hybridization analysis of pMG229, the clone #71 probe hybridized to two new ClaI fragments (fragments A [3.5 kbp] and B [94 bp]) in addition to three fragments of pMG1 (Fig. 3a and b). The molecular sizes of the A and B fragments contained in pMG227 were determined by DNA sequencing. The ClaI fragment containing the erythromycin resistance gene did not hybridize to the clone #71 probe. In Fig. 3c, lane 2, in addition to the three bands present in lane 1, a new band (band A) appeared just above the 3.2-kbp ClaI fragment of pMG1, and its size was estimated to be 3.5 kbp, which corresponded to the size determined by DNA sequencing. The smaller fragment (fragment B) (94 bp) was not identified by Southern hybridization analysis. This implied that this fragment was too small to identify under these electrophoresis conditions as it ran out of the gel. These results indicate that 71ORF2 was disrupted by integration of pMG227. The cointegrated plasmid, designated pMG229, had two imperfect 71ORF2 copies. One lacked the N-terminal part of 71ORF2, and the other lacked the C-terminal part.
Transferability of gene-disrupted mutant plasmid pMG229.
OG1X(pMG229) was examined to determine its ability to undergo conjugation. For broth mating, overnight cultures of both donor and recipient strains were diluted 50-fold in 5 ml of fresh THB and grown at 37°C for 4 h. Fifty microliters of the donor culture and 450 μl of the recipient culture were added to 4.5 ml of fresh THB, and the mating mixture was incubated at 37°C with gentle shaking for 3 h. After incubation, 100-μl samples were plated onto selective plates. For filter mating, bacterial cells in the mating mixture were collected on a membrane filter (pore size, 0.22 μm). The membrane was put on a THB agar plate and incubated at 37°C for 3 h. Cells were suspended in 1 ml of THB, and 100 μl was plated on a selective plate. Disruption of 71ORF2 resulted in a reduction in the transferability during liquid mating. pMG229 transferred during liquid mating at a frequency of 1.6 × 10−7 per donor cell. In addition to this result, the numbers and the sizes of the mating aggregates in liquid mating preparations were less than those observed during mating with OG1X(pMG1) (data not shown) On the other hand, during mating on the membrane filter, pMG229 transferred at a frequency of 2.7 × 10−3 per donor cell. pMG1 transferred at a frequency of 1.1 × 10−3 per donor cell during liquid mating and at a frequency of 8.5 × 10−2 per donor cell during filter mating. These results indicate that the mutant plasmid decreased the transfer frequency in broth mating but did not alter the transfer frequency in filter mating.
For complementation of disrupted 71ORF2 in pMG229, the 2.5-kbp EcoRI/HindIII fragment of plasmid p71E, which contained 71ORF1, 71ORF2, and part of 71ORF3 (Table 1 and Fig. 2) was cloned into shuttle vector pAM401 (26), and the cloned plasmid pAM401::EcoRI/HindIII (2.5 kbp) was introduced into OG1X(pMG229). In the resulting strain, pMG229 transferred at a frequency of 3.1 × 10−4 per donor cell during liquid mating, indicating that the disruption of 71ORF2 was complemented with wild-type 71ORF2 and that disruption of 71ORF2 did not have a polar effect.
TABLE 1.
Bacterial strains and plasmids used in this studya
| Strain or plasmid | Relevant genotype or phenotype | Comment(s) | Reference or sources |
|---|---|---|---|
| Bacterial strains | |||
| E. faecalis | |||
| FA2-2 | rif fus | Derivative of JH2 | 3 |
| OGIX | str | Protease-negative derivative of OG1-10 | 14 |
| E. coli DH5α | recA1 endA1 gyrA96 thi-1 relA1 hsdR17 supE44 ΔlacU169 φ80 lacZΔM15 | Invitrogen | |
| Plasmids | |||
| pMG1 | Gmr | 65.1-kb conjugative plasmid from E. faecium strain | 15 |
| pAM225 | tet | Tn917 was cloned in pBR325 with EcoRI fragment H of pAD1 | 23 |
| pBluescriptSK | amp | E. coli cloning vector | Stratagene |
| Clone #71 | amp | 6-kbp HindIII fragment of pMG1 was cloned in pBluescriptSK | This study |
| p71E | amp | EcoRI deletion derivative of clone #71 | This study |
| pMG226 | amp erm | 1.45-kbp ClaI fragment of Tn917 containing erm gene was cloned in pBluescript | This study |
| pMG227 | amp erm | Internal fragment of 71ORF2 gene was cloned in pMG226 | This study |
THB (Difco, Detroit, Mich.) was used for growth of E. faecalis and for broth mating. Luria-Bertani medium (19) was used for growth of E. coli. Agar plates were made by adding agar (1.5%) to the broth medium. Antibiotics were used at the following concentrations: erythromycin, 10 μg/ml; kanamycin, 500 μg/ml; gentamicin, 500 μg/ml; ampicillin, 100 μg/ml; fusidic acid, 25 μg/ml; and rifampin, 25 μg/ml.
These results implied that 71ORF2 was a gene involved in the tra gene system for conjugation, and the product of the gene was associated with formation or stabilization of the mating aggregate in the early stage of broth mating. Therefore, 71ORF2 was designated traA.
In a previous report (15), we showed that little transfer of pMG1 occurs in the first 15 to 30 min of mating but increased transfer occurs after around 60 min of mating. These results suggest that some factor necessary for plasmid transfer is induced during coincubation of the donor and recipient and that induction of this factor might require contact between the donor and the recipient. Together with these previous observations, the results of the present study suggested that the traA gene could be induced by a factor (a signal) which might also be produced by induction during coincubation of the donor and recipient in the early stage of mating. As the level of the traA transcript increased during mating, stable mating aggregates could be formed by the function of the traA gene product, and this could lead to efficient plasmid transfer.
Nucleotide sequence accession number.
The nucleotide sequence data reported here have been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number AB081477.
Acknowledgments
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology and by grants from the Japanese Ministry of Health, Labor, and Welfare.
We thank Elizabeth Kamei for helpful advice.
REFERENCES
- 1.Achtman, M., and R. Skurray. 1977. A redefinition of the mating phenomenon in bacteria, p. 233-279. In J. L. Reissig (ed.), Microbial interactions. Chapman and Hill, London, United Kingdom.
- 2.Ausubel, F. M., R. Brent, R. W. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Clewell, D. B., P. K. Tomich, M. C. Gawron-Burke, A. E. Franke, Y. Yagi, and F. Y. An. 1982. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J. Bacteriol. 152:1220-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clewell, D. B. 1993. Sex pheromones and the plasmid-encoded mating response in Enterococcus faecalis, p. 349-367. In D. B. Clewell (ed.), Bacterial conjugation. Plenum Press, New York, N.Y.
- 5.Clewell, D. B. 1993. Bacterial sex pheromone-induced plasmid transfer. Cell 73:9-12. [DOI] [PubMed] [Google Scholar]
- 6.Clewell, D. B. 1999. Sex pheromone systems in enterococci, p. 47-65. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 7.Dunny, G. M., B. L. Brown, and D. B. Clewell. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 75:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunny, G. M., R. A. Craig, R. L. Carron, and D. B. Clewell. 1979. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid 2:454-465. [DOI] [PubMed] [Google Scholar]
- 9.Dunny, G. M., B. A. B. Leonard, and P. J. Hedberg. 1995. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J. Bacteriol. 177:871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firth, N., K. Ippen-Ihler, and R. A. Skurray. 1996. Structure and function of the F factor and mechanism of conjugation, p. 2377-2401. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 11.Fujimoto, S., H. Hashimoto, and Y. Ike. 1991. Low cost device for electro-transformation and its application to the highly efficient transformation of Escherichia coli and Enterococcus faecalis. Plasmid 26:131-135. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto, S., H. Tomita, E. Wakamatsu, K. Tanimoto, and Y. Ike. 1995. Physical mapping of the conjugative bacteriocin plasmid pPD1 of Enterococcus faecalis and identification of the determinant related to the pheromone response. J. Bacteriol. 177:5574-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli, D., F. Lottspeich, and R. Wirth. 1990. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol. Microbiol. 4:895-904. [DOI] [PubMed] [Google Scholar]
- 14.Ike, Y., R. A. Craig, B. A. White, Y. Yagi, and D. B. Clewell. 1983. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc. Natl. Acad. Sci. USA 80:5369-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ike, Y., K. Tanimoto, H. Tomita, K. Takeuchi, and S. Fujimoto. 1998. Efficient transfer of the pheromone-independent Enterococcus faecium plasmid pMG1 (Gmr) (65.1 kilobases) to Enterococcus strains during broth mating. J. Bacteriol. 180:4886-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingsman, A., and N. Willetes. 1978. The requirements for conjugal DNA synthesis in the donor strain during Flac transfer. J. Mol. Biol. 122:287-300. [DOI] [PubMed] [Google Scholar]
- 17.Leonard, B. A., A. Podbielski, P. J. Hedberg, and G. M. Dunny. 1996. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc. Natl. Acad. Sci. USA 93:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning, P. A., and M. Achtman. 1979. Cell-cell interactions in conjugating Escherichia coli: the involvement of the cell envelope, p. 409-447. In M. Inouye (ed.), Bacterial outer membranes. John Wiley and Sons, New York, N.Y.
- 19.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Ou, J. T., and R. L. Reim. 1978. F− mating materials able to generate a mating signal in mating with HfrH dnaB(Ts) cells. J. Bacteriol. 133:442-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontius, L. T., and D. B. Clewell. 1992. Regulation of the pAD1-encoded sex pheromone response in Enterococcus faecalis: nucleotide sequence analysis of traA. J. Bacteriol. 174:1821-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Shaw, J. H., and D. B. Clewell. 1985. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J. Bacteriol. 164:782-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanimoto, K., and D. B. Clewell. 1993. Regulation of the pAD1-encoded sex pheromone response in Enterococcus faecalis: expression of the positive regulator TraE1. J. Bacteriol. 175:1008-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanner, G., H. Formanek, D. Galli, and R. Wirth. 1989. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones. An ultrastructural comparison using immunolabeling, transmission and high resolution scanning electron microscopic techniques. Arch. Microbiol. 151:491-497. [DOI] [PubMed] [Google Scholar]
- 26.Wirth, R., F. Y. An, and D. B. Clewell. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J. Bacteriol. 165:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagi, Y., R. E. Kessler, J. H. Shaw, D. E. Lopatin, F. Y. An, and D. B. Clewell. 1983. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J. Gen. Microbiol. 129:1207-1215. [DOI] [PubMed] [Google Scholar]