Abstract
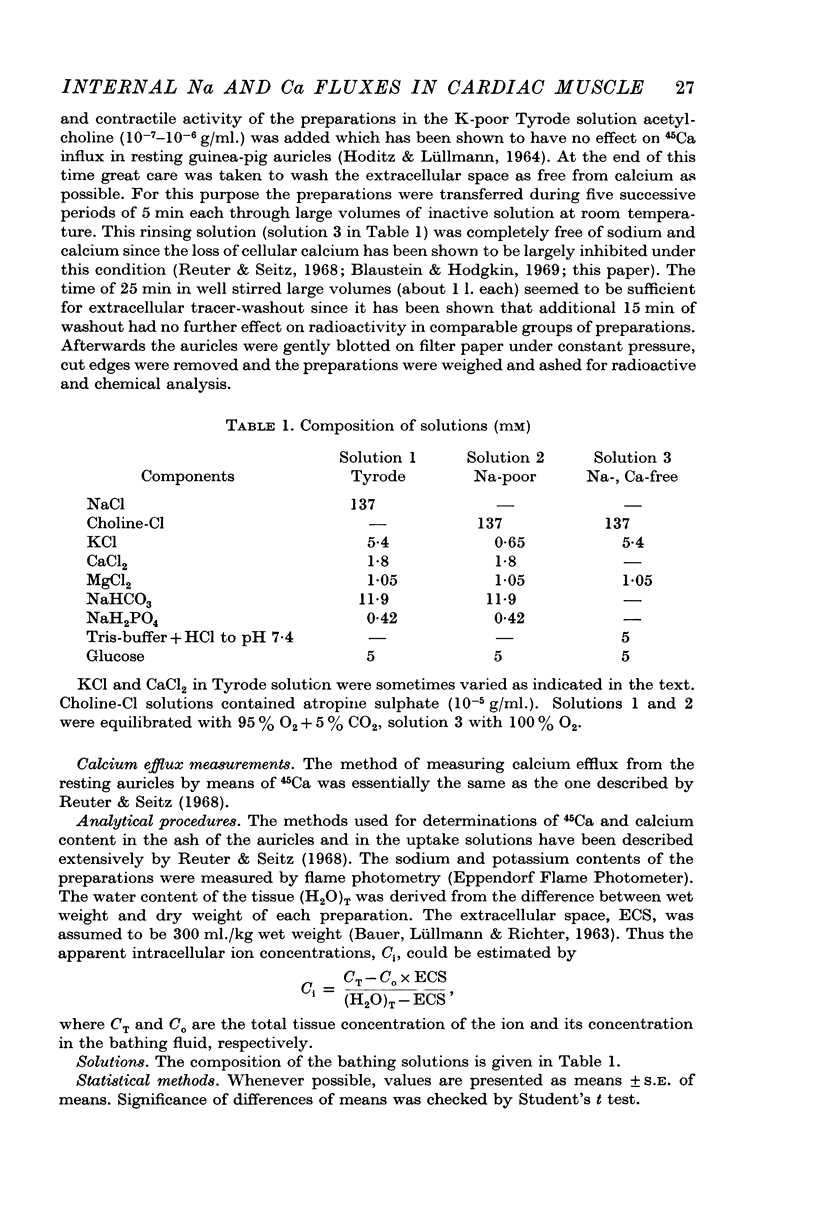
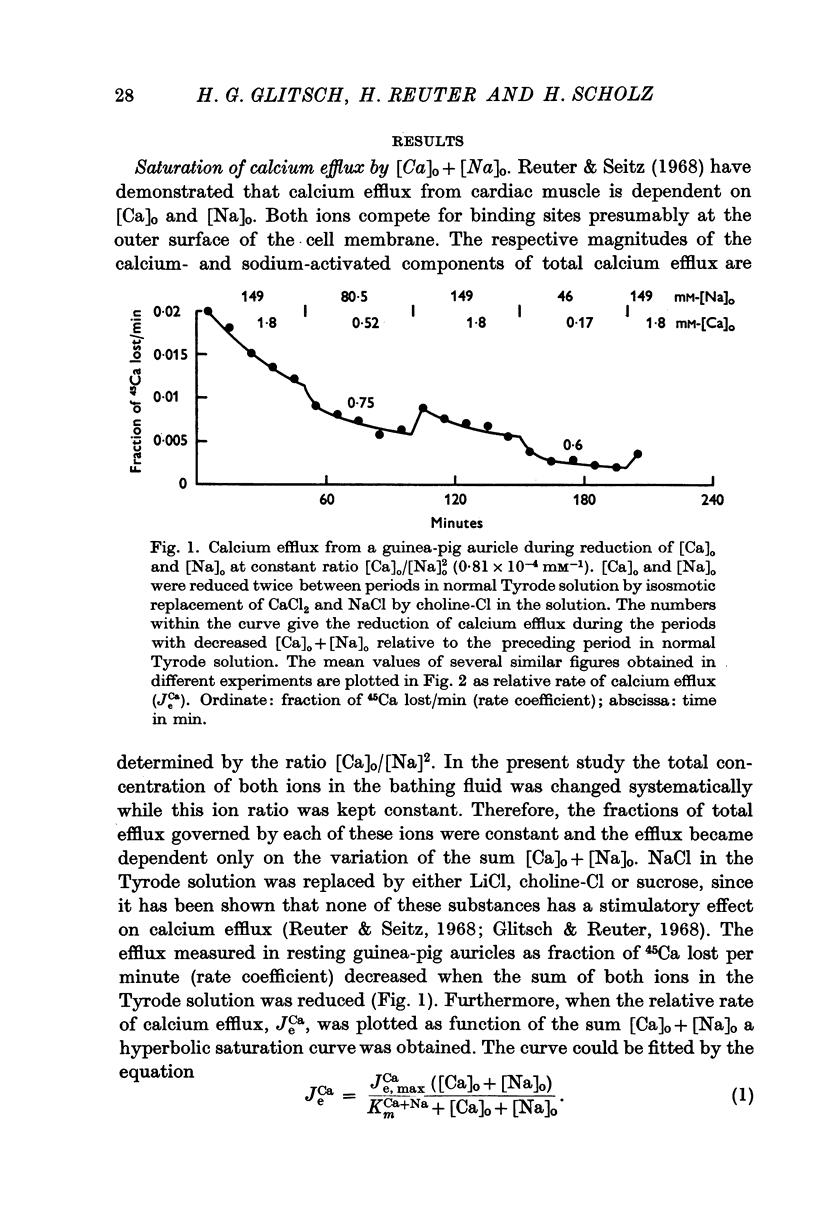
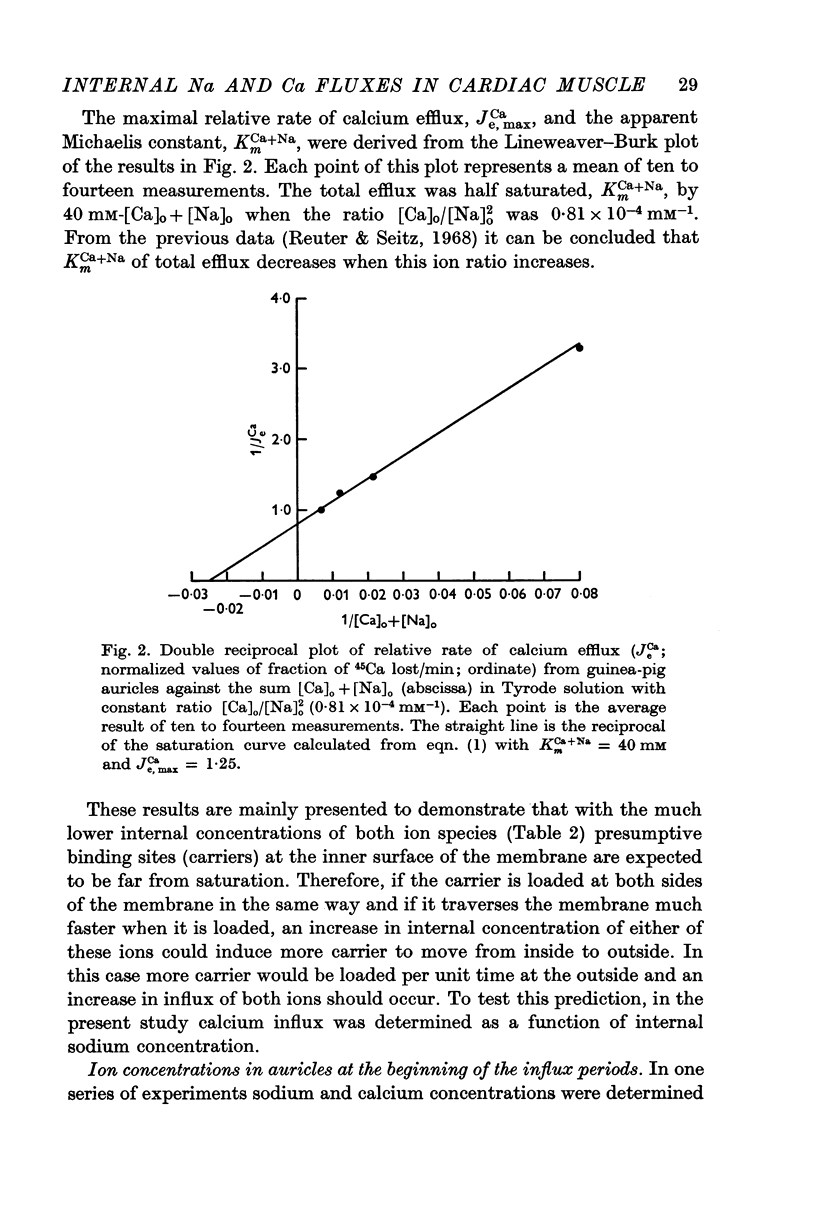
1. Calcium efflux from guinea-pig auricles followed saturation kinetics when [Ca]o and [Na]o were changed while the ratio [Ca]o/[Na]o2 was kept constant. The Michaelis constant, KmCa+Na = 40 mM, suggests that a hypothetical carrier system, responsible for sodium—calcium exchange, is far from saturation with the inside concentrations of these ions.
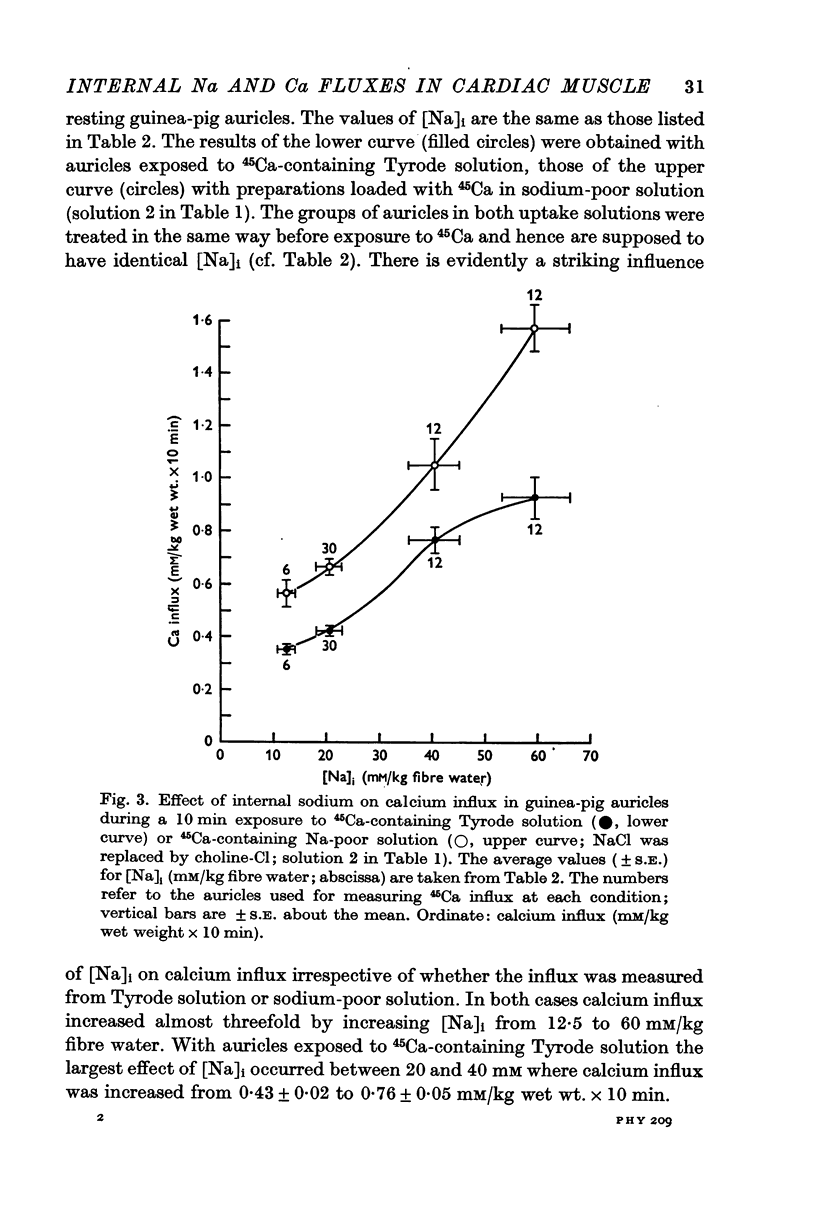
2. [Na]i was altered in the auricles between 12·5 and 60 mM/kg fibre water while total cellular calcium concentration ([Ca]t) at the beginning of the influx period was not significantly different in the various groups of preparations.
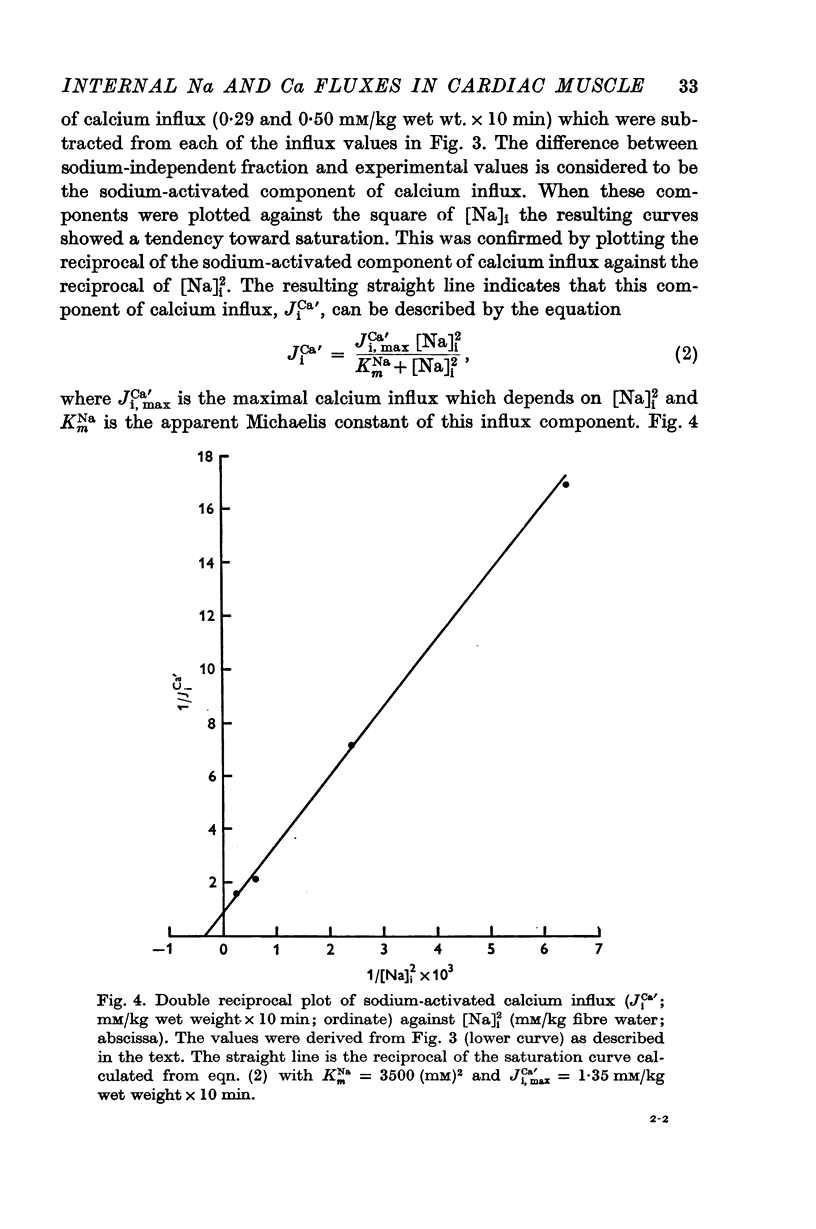
3. 45Ca influx increased appreciably with increasing [Na]i. 45Ca influx from sodium-poor solution corresponded to an almost equal increase in [Ca]t, while [Ca]t did not change much in preparations loaded with 45Ca in Tyrode solution. When the sodium-activated fraction of calcium influx was plotted against [Na]i2 the resulting curve indicated saturation with KmNa = 3500 (mM [Na]i)2 and maximal influx rate, Ji, maxCa' = 1·35 mM/kg wet weight × 10 min.
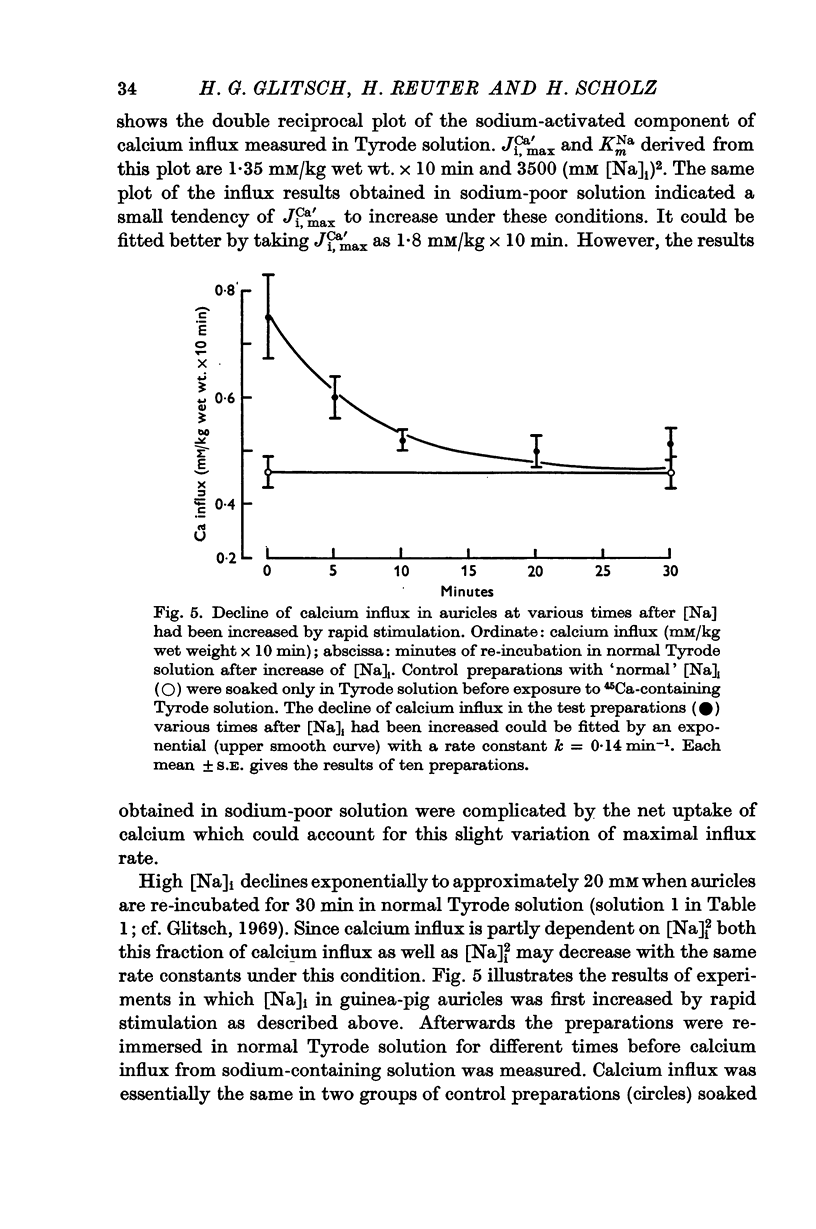
4. When the preparations were re-equilibrated for various times in normal Tyrode solution after [Na]i had been increased, both the sodium-activated component of calcium influx and [Na]i2 decreased with approximately the same rate constants.
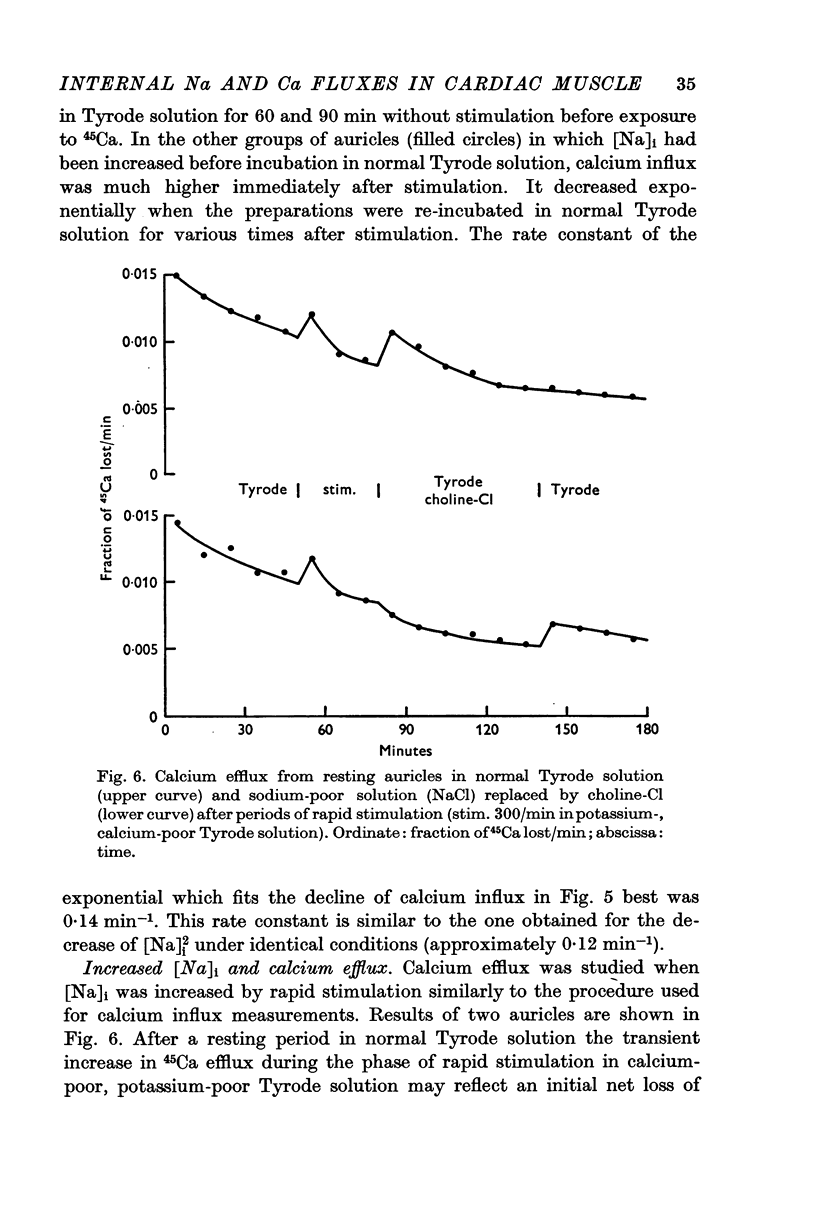
5. Calcium efflux from auricles with high [Na]i was increased when it was measured in Tyrode solution while the efflux in sodium-poor solution was inhibited.
6. Auricles with increased [Na]i showed a positive inotropic contractile response.
7. The main conclusion reached by these experiments is that calcium influx is affected by [Na]i in a way which is compatible with a carrier-mediated sodium—calcium exchange system.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER H., LULLMANN H., RICHTER M. [The uptake of methylsulfate ions in the atrial musculature of guinea pigs]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;277:48–53. [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P. Sodium-dependent uptake of calcium by crab nerve. Biochim Biophys Acta. 1968 Jan 3;150(1):167–170. doi: 10.1016/0005-2736(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton H. G. Fluxes in passive, monovalent and polyvalent carrier systems. J Theor Biol. 1966 Jan;10(1):28–52. doi: 10.1016/0022-5193(66)90177-9. [DOI] [PubMed] [Google Scholar]
- Dransfeld H., Greeff K., Schorn A., Ting B. T. Calcium uptake in mitochondria and vesicles of heart and skeletal muscle in presence of potassium, sodium, k-strophanthin and pentobarbital. Biochem Pharmacol. 1969 Jun;18(6):1335–1345. doi: 10.1016/0006-2952(69)90246-9. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. Influence of intracellular sodium concentration on calcium influx in isolated guinea pig auricles. Naunyn Schmiedebergs Arch Pharmakol. 1969;264(3):236–237. doi: 10.1007/BF02431433. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H. Uber die Wirkung von Cholinchlorid und Strontiumchlorid auf den 45Ca-Efflux am Meerschweinchevorhof. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(2):121–122. [PubMed] [Google Scholar]
- Glitsch H. G. Uber das Membranpotential des Meerschweinchenvorhofes nach Hypothermie. Pflugers Arch. 1969;307(1):29–46. doi: 10.1007/BF00589457. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoditz H., Lüllmann H. Der Einfluss von Acetylcholin auf den Calciumumsatz ruhender und kontrahierender Vorhofmuskulatur in vitro. Experientia. 1964 May 15;20(5):279–280. doi: 10.1007/BF02151808. [DOI] [PubMed] [Google Scholar]
- KLAUS W. VERGLEICHENDE UNTERSUCHUNGEN UEBER DIE WIRKUNG VERSCHIEDENER DIGITOXIGENINDERIVATE AUF DIE KONTRAKTIONSKRAFT UND DEN CA-AUSTAUSCH ISOLIERTER MEERSCHWEINCHENVORHOEFE. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1963 Nov 30;246:226–238. [PubMed] [Google Scholar]
- Katz A. M., Repke D. I. Control of myocardial contraction: the sensitivity of cardiac actomyosin to calcium ion. Science. 1966 May 27;152(3726):1242–1243. doi: 10.1126/science.152.3726.1242. [DOI] [PubMed] [Google Scholar]
- LANGER G. A. KINETIC STUDIES OF CALCIUM DISTRIBUTION IN VENTRICULAR MUSCLE OF THE DOG. Circ Res. 1964 Nov;15:393–405. doi: 10.1161/01.res.15.5.393. [DOI] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R., HARRIS E. J. Accumulation of calcium (or strontium) under conditions of increasing contractility. Nature. 1957 May 25;179(4569):1068–1069. doi: 10.1038/1791068a0. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol. 1962 Nov;46:201–213. doi: 10.1085/jgp.46.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Palmer R. F., Posey V. A. Ion effects on calcium accumulation by cardiac sarcoplasmic reticulum. J Gen Physiol. 1967 Sep;50(8):2085–2095. doi: 10.1085/jgp.50.8.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Carafoli E. A study of the intracellular transport of calcium in rat heart. J Cell Physiol. 1968 Aug;72(1):29–37. doi: 10.1002/jcp.1040720106. [DOI] [PubMed] [Google Scholar]
- REPKE K. UBER DEN BIOCHEMISCHEN WIRKUNGSMODUS VON DIGITALIS. Klin Wochenschr. 1964 Feb 15;42:157–165. doi: 10.1007/BF01482616. [DOI] [PubMed] [Google Scholar]
- Reuter H., Beeler G. W., Jr Calcium current and activation of contraction in ventricular myocardial fibers. Science. 1969 Jan 24;163(3865):399–401. doi: 10.1126/science.163.3865.399. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Hidalgo C. Effect of temperature and metabolic inhibitors on 45Ca outflow from squid giant axons. Biochim Biophys Acta. 1968 Dec 10;163(4):550–556. doi: 10.1016/0005-2736(68)90084-9. [DOI] [PubMed] [Google Scholar]
- THOMAS L. J., Jr Increase of labeled calcium uptake in heart muscle during potassium lack contracture. J Gen Physiol. 1960 Jul;43:1193–1206. doi: 10.1085/jgp.43.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen D., Van Breemen C. Calcium exchange diffusion in a porous phospholipid ion-exchange membrane. Nature. 1969 Aug 30;223(5209):898–900. doi: 10.1038/223898a0. [DOI] [PubMed] [Google Scholar]
- von Hattingberg H. M., Klaus W., Lüllmann H., Zepf S. Fluorometrische Bestimmung von Mikromengen Calcium in Muskelgewebe. Experientia. 1966 Aug 15;22(8):553–555. doi: 10.1007/BF01898689. [DOI] [PubMed] [Google Scholar]


