Abstract
1. Mesopic increment threshold spectral sensitivities of sixty-six on-centre and twenty-five off-centre ganglion cells in the cat were determined by recording from single fibres of the left optic tract at a level posterior to the optic chiasma.
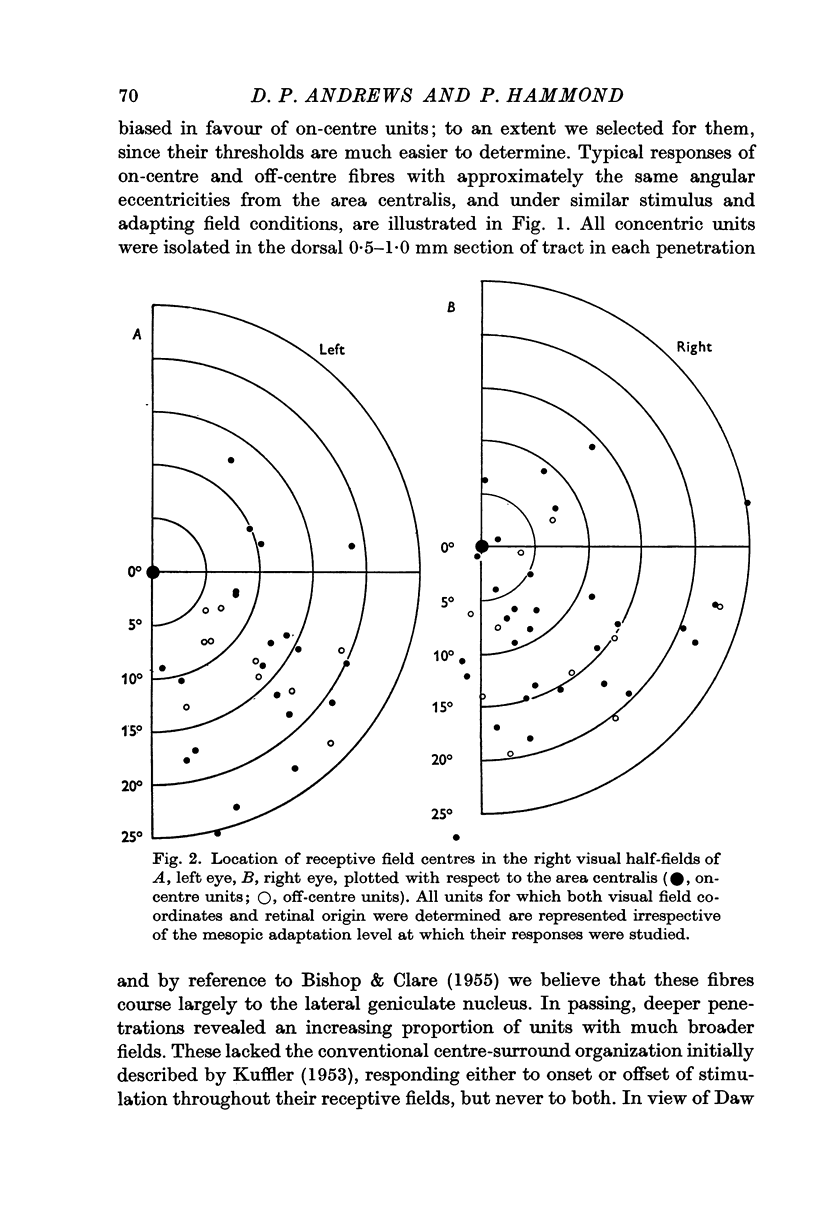
2. All units were monocularly driven; receptive fields were located almost exclusively in the right visual half-fields within 30° of the area centralis, but with slight overlap across each retinal mid line to the left half-fields. The extent of field spread to the right temporal hemi-retina was significantly larger than that to the left nasal hemi-retina. Field centre diameters ranged from less than 0·25° for central units to 2° for more peripheral units.
3. At high mesopic adaptation of 1 log cd/m2 all responsive units (forty-four fibres) received mixed cone—rod input. Threshold curves could always be fitted by the absorption spectra of visual pigment 556 together with varying contributions from visual pigment 507, each derived from the Dartnall nomogram.
4. Of forty-seven fibres analysed under low mesopic background (0 log cd/m2) 92% received similar cone—rod input, being fitted predominantly by visual pigment 507 with slight cone contamination. The remaining 8% received pure rod input and could be matched by visual pigment 507 alone.
5. In conclusion, the cat retina presumably contains a single class of cones with absorption maxima at 556 nm, and a single class of rods. Discrepancy between the presumed rod absorption maximum (502 nm) and the low-mesopic sensitivity maxima of tract fibres (507 nm) is considered in terms of tapetal reflectivity, and absorption by ocular media. Both mechanisms input to the great majority of retinal ganglion cells. At high mesopic levels the cone mechanism predominates. At low mesopic levels the rod mechanism predominates. A small proportion of ganglion cells within the central 30° of the retina receive input only from rods, and in these the rod mechanism saturates completely below 1 log cd/m2.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Suprathreshold spectral properties of single optic tract fibres in cat, under mesopic adaptation: cone-rod interaction. J Physiol. 1970 Jul;209(1):83–103. doi: 10.1113/jphysiol.1970.sp009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. J Physiol. 1957 Aug 6;137(3):327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP G. H., CLARE M. H. Organization and distribution of fibers in the optic tract of the cat. J Comp Neurol. 1955 Oct;103(2):269–304. doi: 10.1002/cne.901030204. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTER R. The spectral sensitivity of dark-adapted cats. J Physiol. 1952 Nov;118(3):395–404. doi: 10.1113/jphysiol.1952.sp004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTER R. The spectral sensitivity of light-adapted cats. J Physiol. 1954 Feb 26;123(2):409–415. doi: 10.1113/jphysiol.1954.sp005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Spectral properties of dark-adapted retinal ganglion cells in the plaice (Pleuronectes platessa, L.). J Physiol. 1968 Apr;195(3):535–556. doi: 10.1113/jphysiol.1968.sp008473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Lythgoe R. J. The absorption spectra of visual purple and of indicator yellow. J Physiol. 1937 Jun 3;89(4):331–358. doi: 10.1113/jphysiol.1937.sp003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLO N. K., PETERSON N. J. BEHAVIORAL EVIDENCE FOR COLOR DISCRIMINATION IN CAT. J Neurophysiol. 1964 May;27:323–333. doi: 10.1152/jn.1964.27.3.323. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- SECHZER J. A., BROWN J. L. COLOR DISCRIMINATION IN THE CAT. Science. 1964 Apr 24;144(3617):427–429. doi: 10.1126/science.144.3617.427. [DOI] [PubMed] [Google Scholar]
- Stone J. The naso-temporal division of the cat's retina. J Comp Neurol. 1966 Apr;126(4):585–600. [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O. THE SCHEMATIC EYE IN THE CAT. Vision Res. 1963 Nov;61:357–381. doi: 10.1016/0042-6989(63)90009-9. [DOI] [PubMed] [Google Scholar]
- WEALE R. A. Photochemical reactions in the living cat's retina. J Physiol. 1953 Nov 28;122(2):322–331. doi: 10.1113/jphysiol.1953.sp005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEALE R. A. The spectral reflectivity of the cat's tapetum measured in situ. J Physiol. 1953 Jan;119(1):30–42. doi: 10.1113/jphysiol.1953.sp004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]


