Abstract
1. The calcium-sensitive photoprotein aequorin has been used to follow the rapid changes in intracellular calcium concentration that occur during the contraction of single muscle fibres from the barnacle Balanus nubilus, Darwin.
2. The transient change in calcium-mediated light emission (calcium transient) and the changes in membrane potential and tension were recorded simultaneously, thus permitting an examination of the relationships between the chemical, electrical, and mechanical events of excitation—contraction coupling.
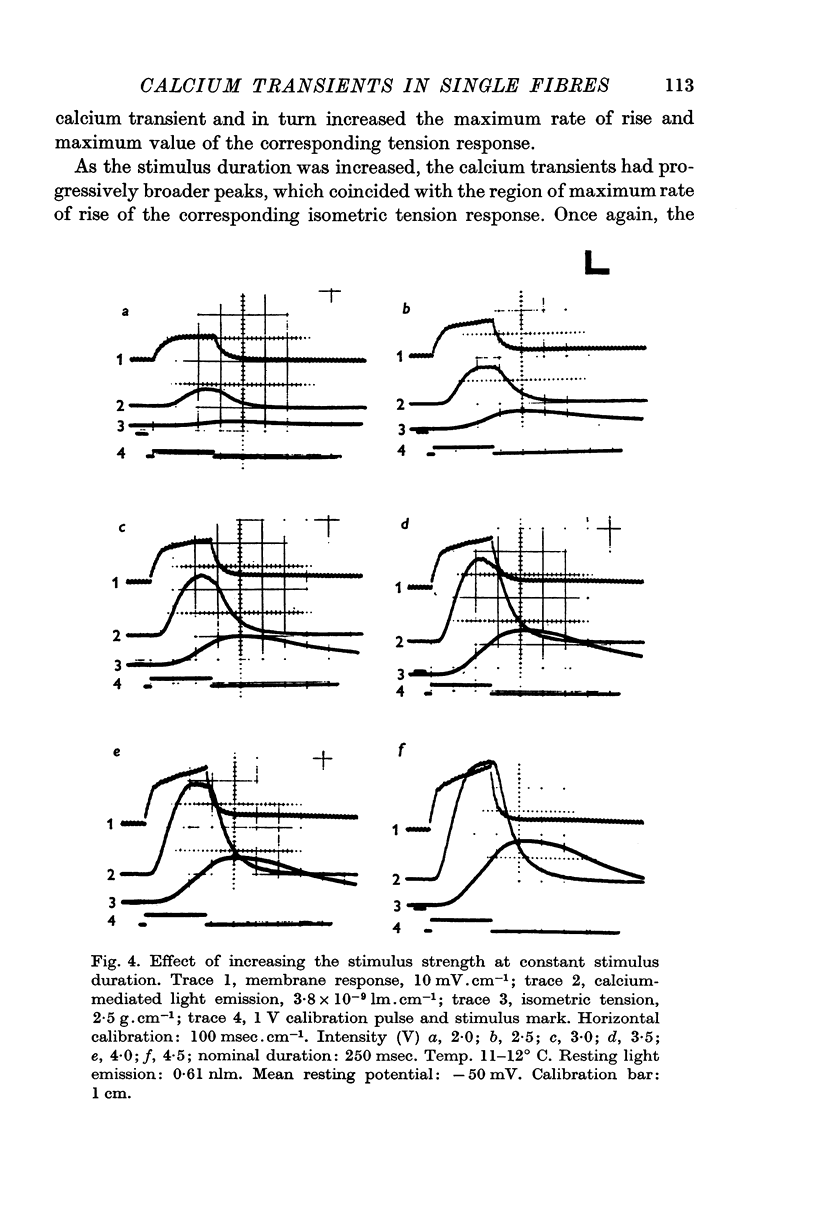
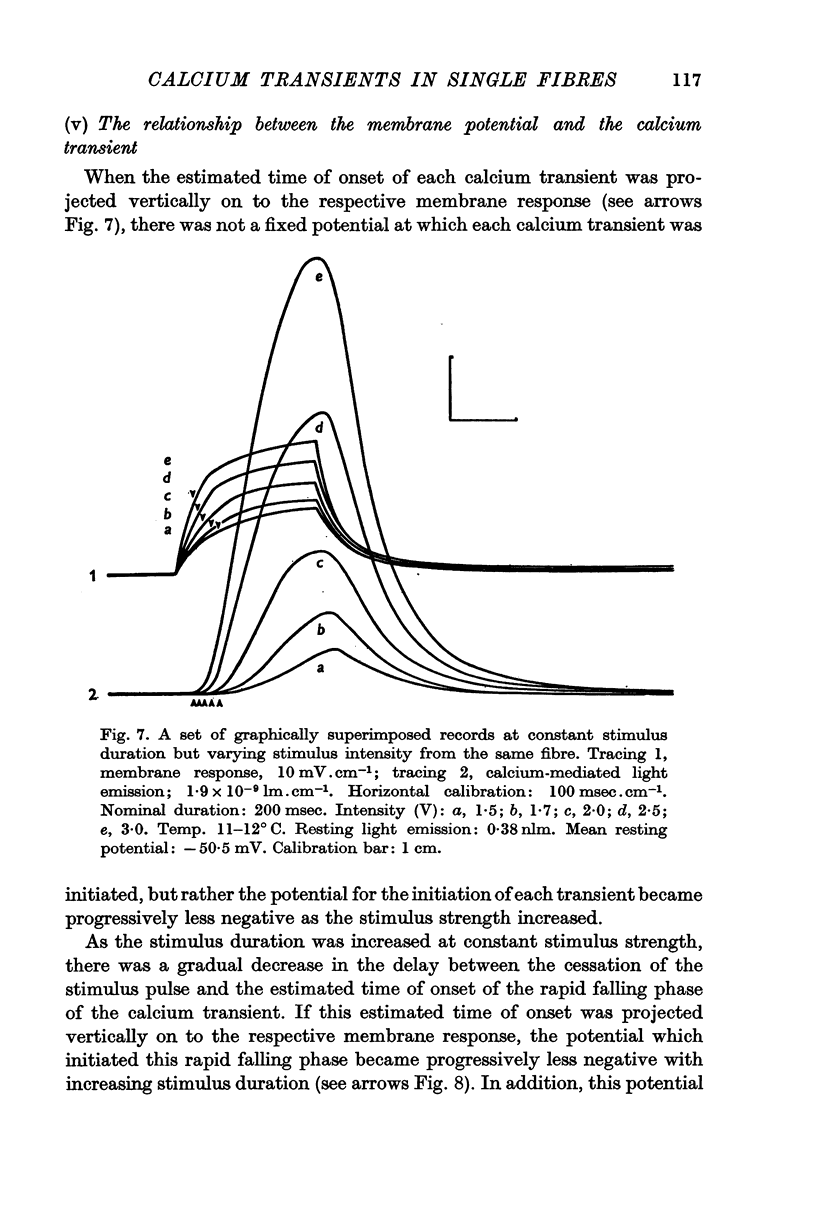
3. With short-duration stimuli (< 200 msec), the calcium transient shows an S-shaped rising phase reaching a maximum soon after the cessation of the stimulus pulse. During membrane repolarization the calcium transient begins an exponential falling phase which has a time constant of 50-80 msec at 11-12° C.
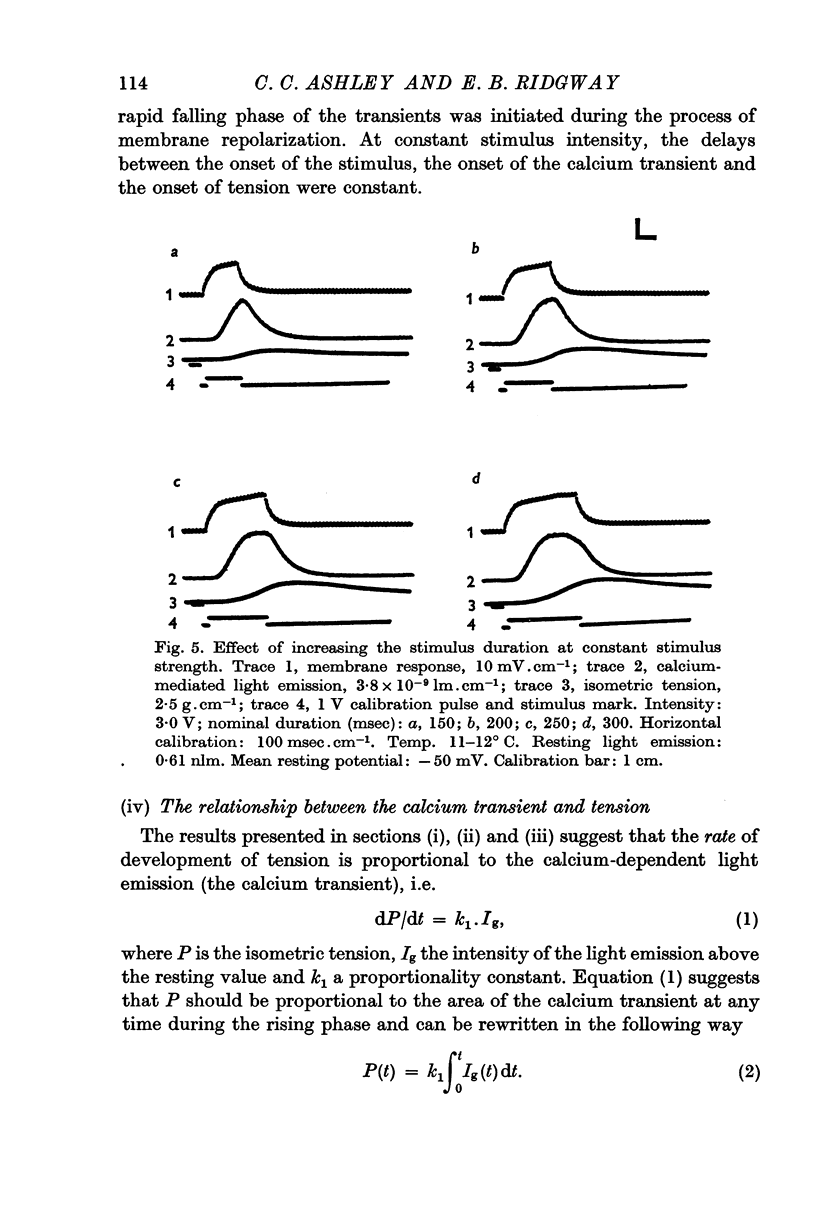
4. The shape of the calcium transient resembles the first derivative of the rising phase of the isometric tension response, thus suggesting that calcium controls the rate of tension development.
5. There is no detectable increase of the light emission above resting values, during the falling phase of isometric tension.
6. A plot of the calcium transient area (lumen × sec) versus peak isometric force (g. cm-2) is linear over, at least, a range of forces from ca. 50-400 g. cm-2.
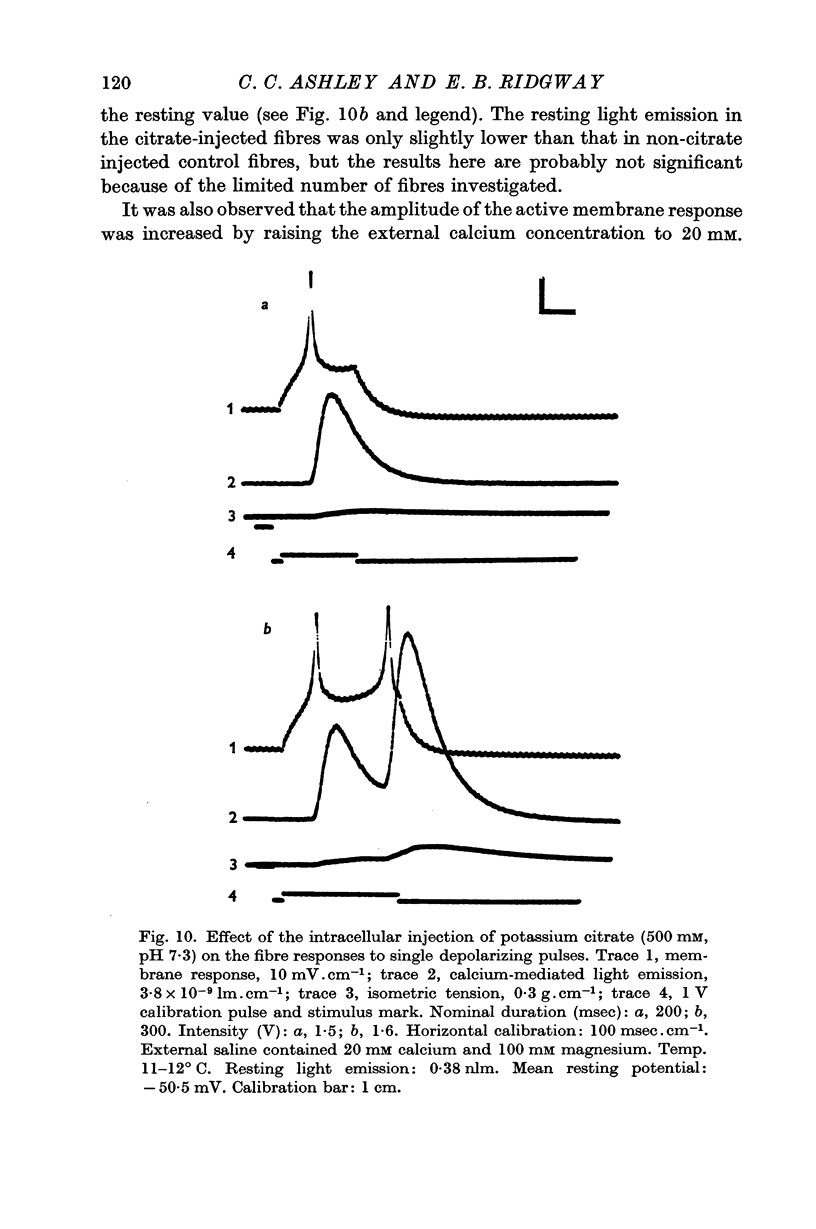
7. When the fibre is capable of producing an active membrane response following the intracellular injection of potassium citrate, the onset and cessation of the calcium transient follow closely the onset and cessation of the active membrane response. Tension responses under these conditions are much suppressed, suggesting that excitation—contraction coupling may be partially blocked between calcium release and the development of tension.
8. Hypertonic salines (1 M sucrose or 1 M glycerol) cause little change in the membrane response, but greatly suppress the calcium transient and completely abolish the tension responses. These effects are readily reversible when normal saline is reintroduced, suggesting that excitation—contraction coupling may be temporarily blocked between the membrane response and calcium release.
9. If the stimulus is prolonged (> 250-300 msec), the calcium transient falls slowly from its maximum value despite continued membrane depolarization, suggesting a time-dependent change in the ratio of the rate of release of calcium to the rate of calcium binding. The results from brief tetanic stimulation also support this suggestion.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C. Aequorin-monitored calcium transients in single Maia muscle fibres. J Physiol. 1969 Jul;203(1):32P–33P. [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. Aspects of the relationship between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1969 Jan;200(1):74P–76P. [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. Simultaneous recording of membrane potential, calcium transient and tension in single muscle fibers. Nature. 1968 Sep 14;219(5159):1168–1169. doi: 10.1038/2191168a0. [DOI] [PubMed] [Google Scholar]
- Ashley C. C. The role of cell calcium in the contraction of single cannulated muscle fibers. Am Zool. 1967 Aug;7(3):647–659. doi: 10.1093/icb/7.3.647. [DOI] [PubMed] [Google Scholar]
- Azzi A., Chance B. The "energized state" of mitochondria: lifetime and ATP equivalence. Biochim Biophys Acta. 1969 Oct 21;189(2):141–151. doi: 10.1016/0005-2728(69)90042-5. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C., WALSTER G. STUDIES ON THE MICRO-INJECTION OF VARIOUS SUBSTANCES INTO CRAB MUSCLE FIBRES. J Physiol. 1963 Nov;169:353–372. doi: 10.1113/jphysiol.1963.sp007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. C. Liquid junction potentials and their effect on potential measurements in biological systems. Int Rev Cytol. 1968;24:345–371. doi: 10.1016/s0074-7696(08)61402-3. [DOI] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., CHICHIBU S., HAGIWARA S. RELATION BETWEEN MEMBRANE POTENTIAL CHANGES AND TENSION IN BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Nov;48:225–234. doi: 10.1085/jgp.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJINO M., YAMAGUCHI T., SUZUKI K. 'Glycerol effect' and the mechanism linking excitation of the plasma membrane with contraction. Nature. 1961 Dec 23;192:1159–1161. doi: 10.1038/1921159a0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials without contraction in frog skeletal muscle fibers with disrupted transverse tubules. Science. 1967 Dec 29;158(3809):1702–1703. doi: 10.1126/science.158.3809.1702. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. Does heat production precede mechanical response in muscular contraction? Proc R Soc Lond B Biol Sci. 1950 Jul 24;137(887):268–273. doi: 10.1098/rspb.1950.0034. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G., SMYTH T., Jr NEUROMUSCULAR PHYSIOLOGY OF GIANT MUSCLE FIBERS OF A BARNACLE, BALANUS NUBILUS DARWIN. Comp Biochem Physiol. 1963 Dec;10:291–314. doi: 10.1016/0010-406x(63)90229-9. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Hayashi H., Takahashi K. Calcium and potassium currents of the membrane of a barnacle muscle fibre in relation to the calcium spike. J Physiol. 1969 Nov;205(1):115–129. doi: 10.1113/jphysiol.1969.sp008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K., Junge D. Excitation-contraction coupling in a barnacle muscle fiber as examined with voltage clamp technique. J Gen Physiol. 1968 Feb;51(2):157–175. doi: 10.1085/jgp.51.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbach W. Structural and enzymatic properties of the calcium transporting membranes of the sarcoplasmic reticulum. Ann N Y Acad Sci. 1966 Jul 14;137(2):1041–1048. doi: 10.1111/j.1749-6632.1966.tb50216.x. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Mitchell G., Mattingly P. H., Blinks J. R., Van Leeuwen M. Response of aequorin bioluminescence to rapid changes in calcium concentration. Nature. 1969 Jun 14;222(5198):1047–1050. doi: 10.1038/2221047a0. [DOI] [PubMed] [Google Scholar]
- Herz R., Weber A., Reiss I. The role of magnesium in the relaxation of myofibrils. Biochemistry. 1969 Jun;8(6):2266–2271. doi: 10.1021/bi00834a005. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Nature of the Excitatory Sarcoplasmic Reticular Junction. Science. 1965 Jul 2;149(3679):70–72. doi: 10.1126/science.149.3679.70-a. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Ridgway E. B., Ashley C. C. Calcium transients in single muscle fibers. Biochem Biophys Res Commun. 1967 Oct 26;29(2):229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. FURTHER DATA ON THE BIOLUMINESCENT PROTEIN, AEQUORIN. J Cell Physiol. 1963 Aug;62:1–8. doi: 10.1002/jcp.1030620102. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Properties of the bioluminescent protein aequorin. Biochemistry. 1969 Oct;8(10):3991–3997. doi: 10.1021/bi00838a015. [DOI] [PubMed] [Google Scholar]
- WEBER A., HERZ R., REISS I. THE REGULATION OF MYOFIBRILLAR ACTIVITY BY CALCIUM. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:489–501. doi: 10.1098/rspb.1964.0063. [DOI] [PubMed] [Google Scholar]


