Abstract
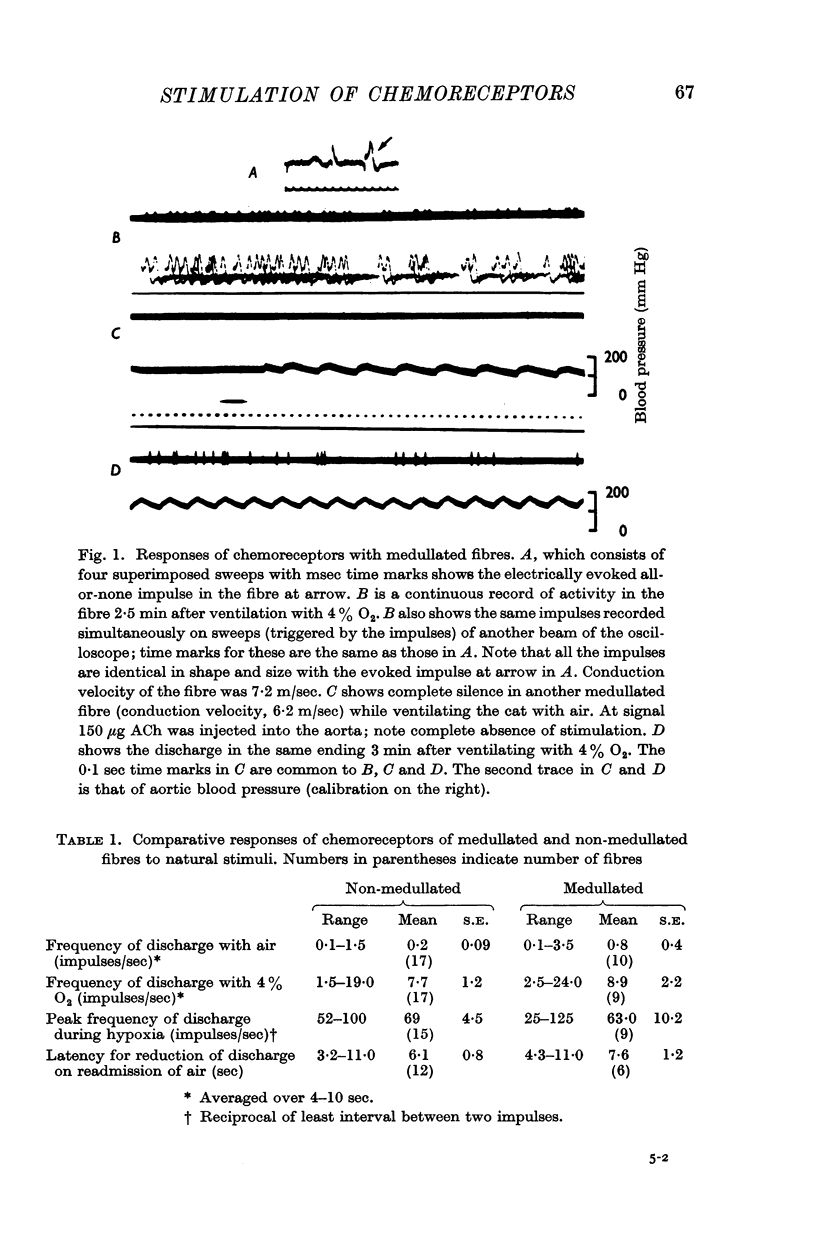
1. Impulses were recorded in single fibres of aortic chemoreceptors of cats anaesthetized with chloralose. There was no demonstrable difference between the responses of the endings of medullated and non-medullated fibres respectively to any of the natural stimuli, such as hypoxia, reduction in blood pressure, or reduction in O2 content. This indicates that the generator processes are qualitatively and quantitatively identical at the endings of both types of fibres.
2. Most of the endings were practically silent while ventilating the lungs with air. The maximum frequency of discharge averaged over 4-10 sec while ventilating the lungs with 4% O2 ranged from 1·5 to 24 impulses/sec; in most fibres (twenty-one out of twenty-six endings) it was less than 12 impulses/sec.
3. All the chemoreceptors tested were considerably stimulated following administration of 0·2 or 2% CO at a time when the O2 content was greater than 4 ml./100 ml.
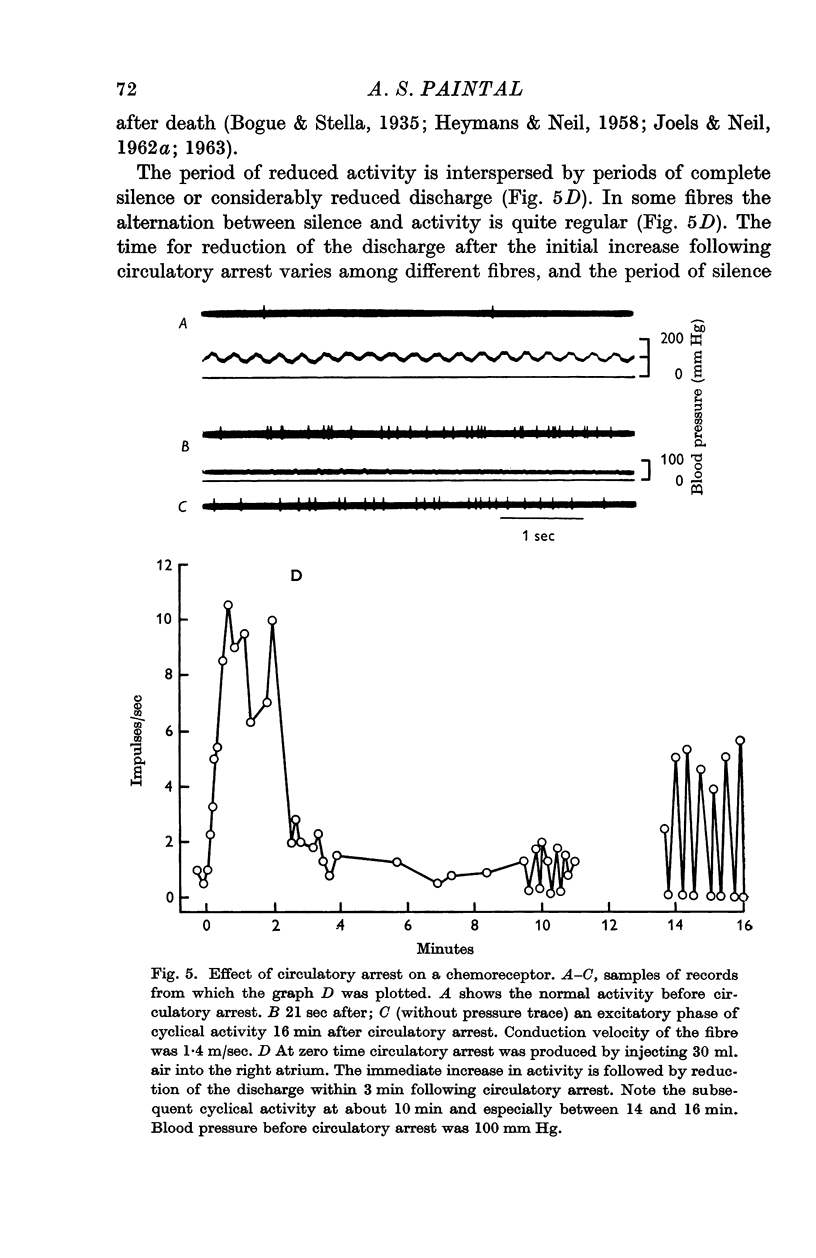
4. All the chemoreceptors were markedly and rapidly stimulated following circulatory arrest while the cat was ventilated with air. This stimulation fell considerably within 3 min of circulatory arrest. Very little or no excitation followed circulatory arrest while ventilating the cat with pure N2. These results suggest that excitation following circulatory arrest is not produced by a metabolite.
5. There was a remarkable difference between the sensitivities of endings of medullated and non-medullated fibres to drugs. The former were either unaffected by relatively large doses of ACh (100-200 μg) or phenyl diguanide, or if they were stimulated, the excitation so produced was much less than that produced in endings of non-medullated fibres. This supports the hypothesis that drugs produce their effects by an action at the regenerative regions of the endings, i.e. regions where the nerve impulse is initiated (Paintal, 1964). It also indicates that ACh is not likely to be a transmitter in the normal processes of excitation of chemoreceptors.
6. A mechanism of stimulation of chemoreceptors not involving metabolites is presented.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISCOE T. J., TAYLOR A. THE DISCHARGE PATTERN RECORDED IN CHEMORECEPTOR AFFERENT FIBRES FROM THE CAT CAROTID BODY WITH NORMAL CIRCULATION AND DURING PERFUSION. J Physiol. 1963 Sep;168:332–344. doi: 10.1113/jphysiol.1963.sp007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., PATHAK C. L. THE RESPONSE OF PERFUSED FROG HEARTS TO MINUTE QUANTITIES OF ACETYLCHOLINE, AND THE VARIATION IN SENSITIVITY WITH SEASON. J Physiol. 1965 Jan;176:191–204. doi: 10.1113/jphysiol.1965.sp007544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogue J. Y., Stella G. Afferent impulses in the carotid sinus nerve (nerve of hering) during asphyxia and anoxaemia. J Physiol. 1935 Mar 15;83(4):459–465. doi: 10.1113/jphysiol.1935.sp003243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C., WIDDICOMBE J. G. Chemoreceptor reflexes in the dog and the action of phenyl diguanide. Arch Int Pharmacodyn Ther. 1952;90(2-3):203–222. [PubMed] [Google Scholar]
- DE BURGH DALY M., LAMBERTSEN C. J., SCHWEITZER A. Observations on the volume of blood flow and oxygen utilization of the carotid body in the cat. J Physiol. 1954 Jul 28;125(1):67–89. doi: 10.1113/jphysiol.1954.sp005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CASTRO F. Sur la structure de la synapse dans les chemocepteurs; leur mécanisme d'excitation et rôle dans la circulation sanguine locale. Acta Physiol Scand. 1951 Feb 21;22(1):14–43. doi: 10.1111/j.1748-1716.1951.tb00747.x. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W. Is there chemical transmission at chemoreceptors? Pharmacol Rev. 1954 Mar;6(1):81–83. [PubMed] [Google Scholar]
- DUKE H. N., GREEN J. H., NEIL E. Carotid chemoceptor impulse activity during inhalation of carbon monoxide mixtures. J Physiol. 1952 Dec;118(4):520–527. doi: 10.1113/jphysiol.1952.sp004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., UCHIZONO K. Observations on the fibre content of nerves reaching the carotid body of the cat. J Physiol. 1961 Dec;159:268–281. doi: 10.1113/jphysiol.1961.sp006807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H. Effects of hypoxia, hypercapnia, and pH on the chemoreceptor activity of the carotid body in vitro. J Physiol. 1965 Jun;178(3):385–409. doi: 10.1113/jphysiol.1965.sp007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H. Effects of some pharmacological agents on chemoreceptor discharges. J Physiol. 1965 Jun;178(3):410–437. doi: 10.1113/jphysiol.1965.sp007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre C., Koyano H., Taylor J. R. Presence of acetylcholine and transmitter release from carotid body chemoreceptors. J Physiol. 1965 Jun;178(3):463–476. doi: 10.1113/jphysiol.1965.sp007637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARNER C. M., DUNCAN D. Observations on the fine structure of the carotid body. Anat Rec. 1958 Apr;130(4):691–709. doi: 10.1002/ar.1091300406. [DOI] [PubMed] [Google Scholar]
- GASSER H. S. Unmedullated fibers originating in dorsal root ganglia. J Gen Physiol. 1950 Jul 20;33(6):651–690. doi: 10.1085/jgp.33.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol. 1950 Oct 16;111(3-4):261–282. doi: 10.1113/jphysiol.1950.sp004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOCHE H., SCHMITT G. UBER CHEMO- UND PRESSORECEPTORENFELDER AM CORONARKREISLAUF. Z Zellforsch Mikrosk Anat. 1963 Dec 3;61:524–560. [PubMed] [Google Scholar]
- LANDGREN S., NEIL E. Chemoreceptor impulse activity following haemorrhage. Acta Physiol Scand. 1951 Aug 25;23(2-3):158–167. doi: 10.1111/j.1748-1716.1951.tb00805.x. [DOI] [PubMed] [Google Scholar]
- LEE K. D., MAYOU R. A., TORRANCE R. W. THE EFFECT OF BLOOD PRESSURE UPON CHEMORECEPTOR DISCHARGE TO HYPOXIA, AND THE MODIFICATION OF THIS EFFECT BY THE SYMPATHETIC-ADRENAL SYSTEM. Q J Exp Physiol Cogn Med Sci. 1964 Apr;49:171–183. doi: 10.1113/expphysiol.1964.sp001717. [DOI] [PubMed] [Google Scholar]
- LILJESTRAND G. Transmission at chemoreceptors. Pharmacol Rev. 1954 Mar;6(1):73–78. [PubMed] [Google Scholar]
- NEIL E. Chemoreceptor areas and chemoreceptor circulatory reflexes. Acta Physiol Scand. 1951 Feb 21;22(1):54–65. doi: 10.1111/j.1748-1716.1951.tb00750.x. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. EFFECTS OF DRUGS ON VERTEBRATE MECHANORECEPTORS. Pharmacol Rev. 1964 Dec;16:341–380. [PubMed] [Google Scholar]
- PAINTAL A. S. VAGAL AFFERENT FIBRES. Ergeb Physiol. 1963;52:74–156. [PubMed] [Google Scholar]
- Paintal A. S. Effects of temperature on conduction in single vagal and saphenous myelinated nerve fibres of the cat. J Physiol. 1965 Sep;180(1):20–49. [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S., Riley R. L. Responses of aortic chemoreceptors. J Appl Physiol. 1966 Mar;21(2):543–548. doi: 10.1152/jappl.1966.21.2.543. [DOI] [PubMed] [Google Scholar]
- ROSS L. L. Electron microscopic observations of the carotid body of the cat. J Biophys Biochem Cytol. 1959 Oct;6:253–262. doi: 10.1083/jcb.6.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]


