Abstract
1. Sodium taurocholate or cholate was administered systemically at a constant rate of about 2·9 μmole/min.kg body wt. to anaesthetized dogs in which the common bile duct had been cannulated. In steady-state conditions blood was sampled from systemic and hepatic veins and the fraction of bile salt removed in a single passage through the liver was determined. Total hepatic blood flow was estimated by application of the Fick principle.
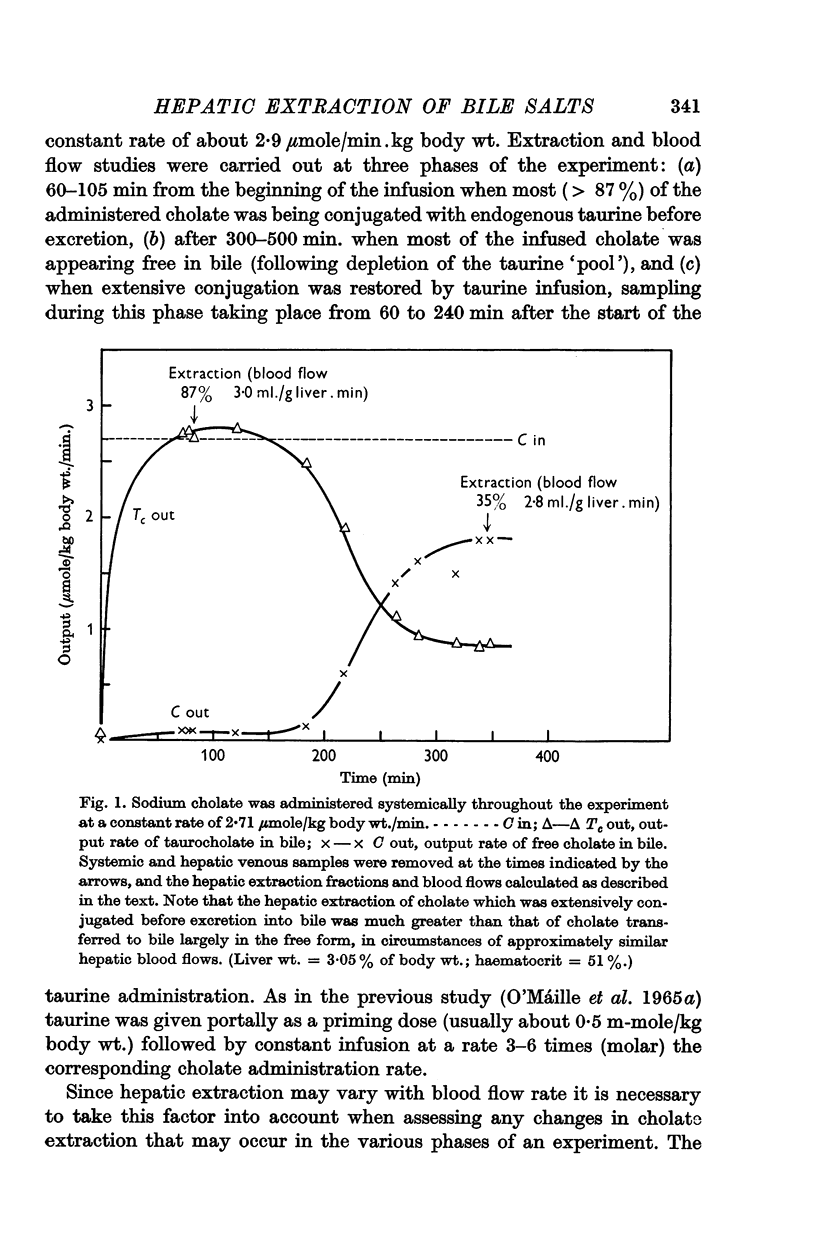
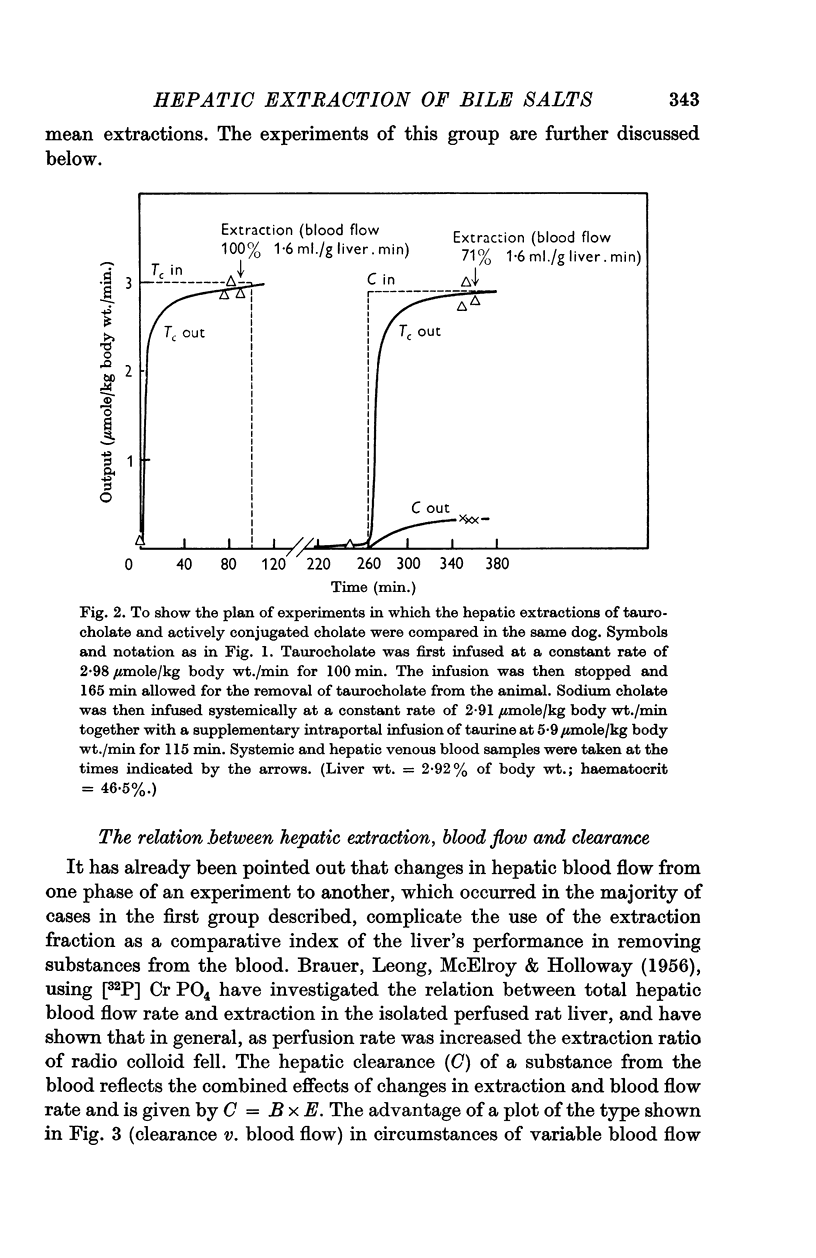
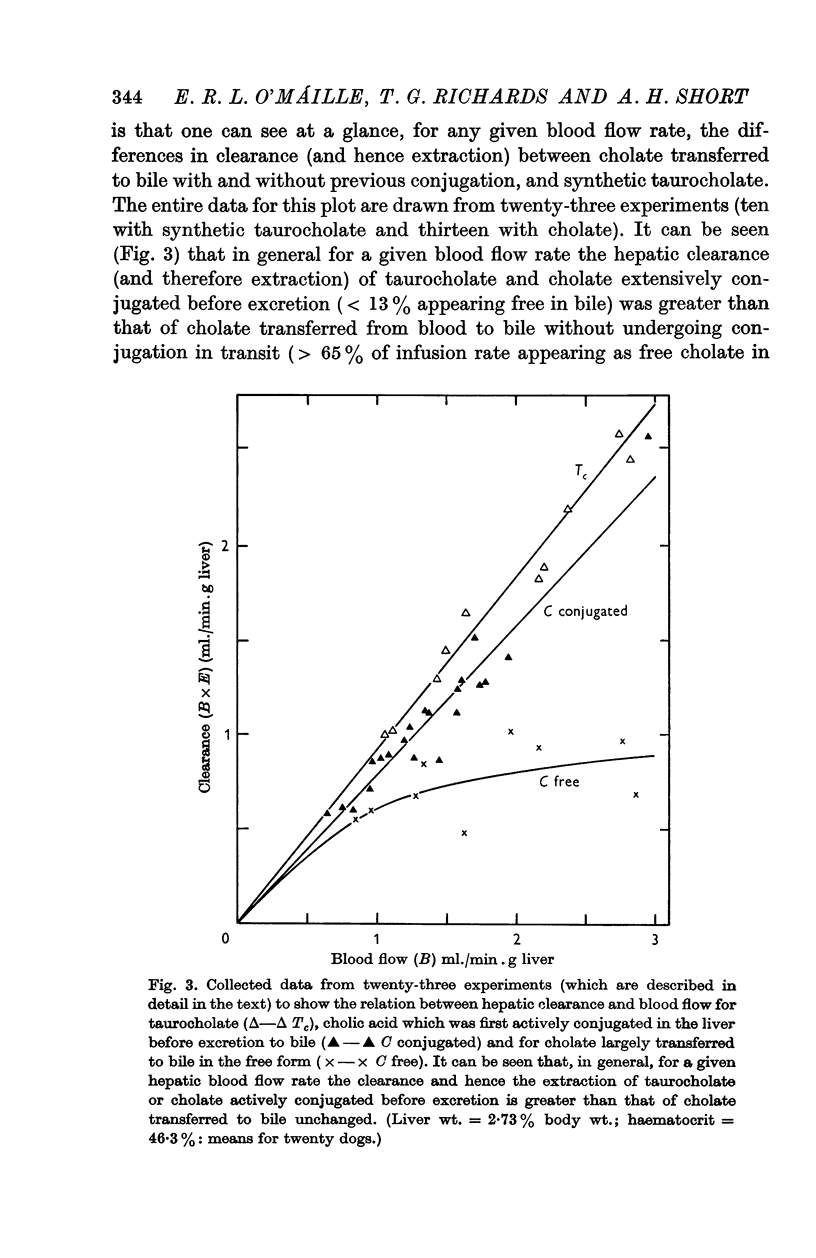
2. The hepatic extraction fraction for synthetic taurocholate in ten experiments was 92%±5% (S.D.) over the blood flow range encountered (1·1-2·8 ml./min.g liver). The extraction of cholate extensively conjugated in the liver before excretion into bile was 79%±8% (S.D.) (twenty-one observations, thirteen experiments). In circumstances of similar hepatic blood flow the extraction of cholate transferred to bile in the free form (after acute taurine depletion) was significantly less than that of either synthetic taurocholate or cholate which could be actively conjugated before excretion. These results, which are discussed and criticized, support previous work on the advantage of conjugation in the transfer of cholic acid from blood to bile.
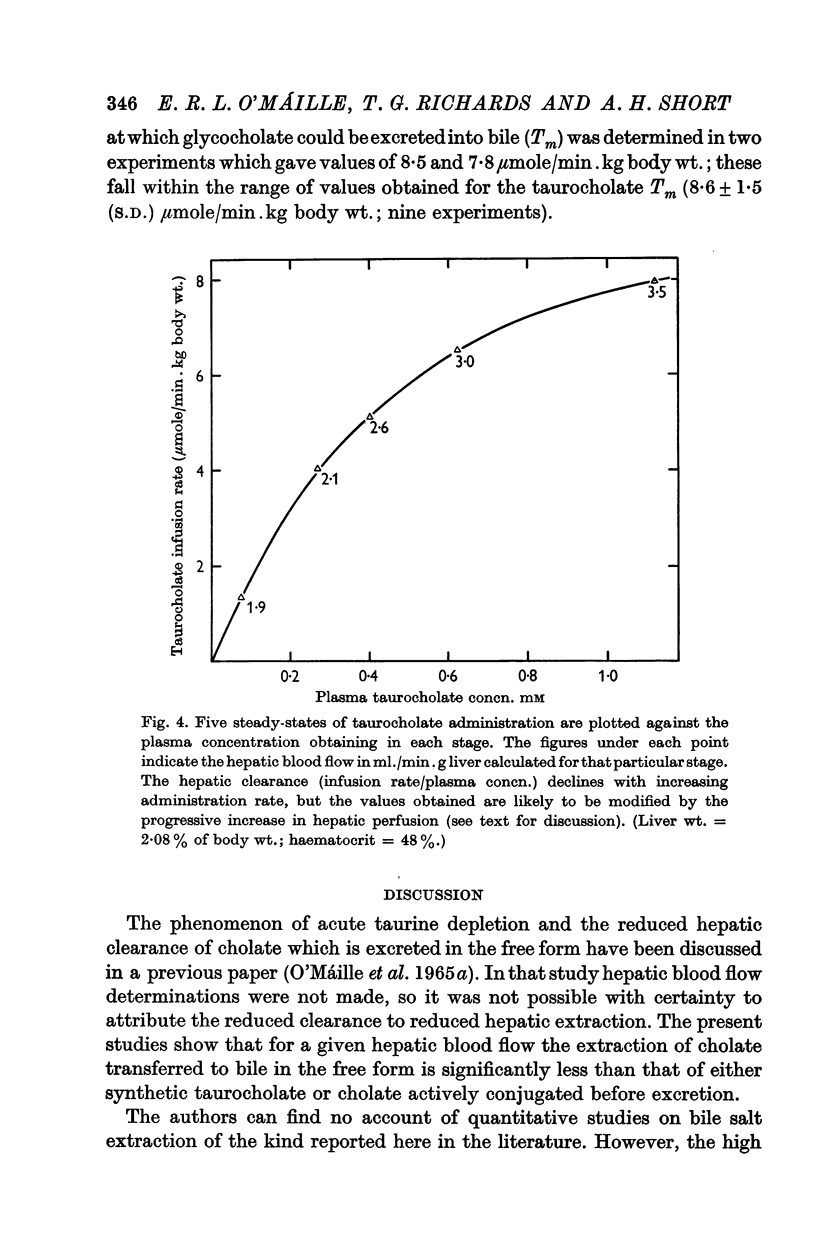
3. The hepatic clearance of bile salt decreases with increasing administration rate, but the values obtained may be influenced by changes in hepatic blood flow. With regard to taurocholate an increase in total hepatic flow was observed when its administration rate exceeded about 5 μmole/min.kg body wt.
4. The secretory maximum for glycocholate, a bile salt not normally found in dog bile, was of the same order as that for taurocholate.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUER R. W., LEONG G. F., MCELROY R. F., HOLLOWAY R. J. Circulatory pathways in the rat liver as revealed by P32 chromic phosphate colloid uptake in the isolated perfused liver preparation. Am J Physiol. 1956 Mar;184(3):593–598. doi: 10.1152/ajplegacy.1956.184.3.593. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. Failure of conduction and of synaptic transmission in degenerating mammalian C fibres. J Physiol. 1965 Jul;179(1):95–112. doi: 10.1113/jphysiol.1965.sp007650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT W. H. The breakdown of adenosine triphosphate accompanying cholic acid activation by guinea-pig liver microsomes. Biochem J. 1957 Feb;65(2):315–321. doi: 10.1042/bj0650315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT W. H. The enzymic activation of cholic acid by guinea-pig-liver microsomes. Biochem J. 1956 Mar;62(3):427–433. doi: 10.1042/bj0620427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNDY S. M., SJOVALL J. Studies on bile acids in rat systemic blood. Bile acids and steroids. 107. Proc Soc Exp Biol Med. 1961 Jun;107:306–309. doi: 10.3181/00379727-107-26610. [DOI] [PubMed] [Google Scholar]
- Josephson B., Rydin A. The resorption of the bile acids from the intestines. Biochem J. 1936 Dec;30(12):2224–2228. doi: 10.1042/bj0302224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Máille E. R., Richards T. G., Short A. H. Acute taurine depletion and maximal rates of hepatic conjugation and secretion of cholic acid in the dog. J Physiol. 1965 Sep;180(1):67–79. [PMC free article] [PubMed] [Google Scholar]
- O'Máille E. R., Richards T. G., Short A. H. Factors determining the maximal rate of organic anion secretion by the liver and further evidence on the hepatic site of action of the hormone secretin. J Physiol. 1966 Oct;186(2):424–438. doi: 10.1113/jphysiol.1966.sp008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVECRONA T., SJOVALL J. Bile acids in rat portal blood: bile acids and steroids 77. Acta Physiol Scand. 1959 Jun 24;46:284–290. doi: 10.1111/j.1748-1716.1959.tb01758.x. [DOI] [PubMed] [Google Scholar]
- PORTMAN O. W., SHAH S. The determination of concentrations of bile acids in peripheral, portal and hepatic blood of Cebus monkeys. Arch Biochem Biophys. 1962 Mar;96:516–523. doi: 10.1016/0003-9861(62)90329-6. [DOI] [PubMed] [Google Scholar]
- SHOEMAKER W. C. Measurement of hepatic blood flow in the unanesthetized dog by a modified bromsulphalein method. J Appl Physiol. 1960 May;15:473–478. doi: 10.1152/jappl.1960.15.3.473. [DOI] [PubMed] [Google Scholar]
- SHOEMAKER W. C., WALKER W. F., VAN ITALLIE T. B., MOORE F. D. A method for simultaneous catheterization of major hepatic vessels in a chronic canine preparation. Am J Physiol. 1959 Feb;196(2):311–314. doi: 10.1152/ajplegacy.1959.196.2.311. [DOI] [PubMed] [Google Scholar]
- SIPERSTEIN M. D., MURRAY A. W. Enzymatic synthesis of cholyl coA and taurocholic acid. Science. 1956 Mar 2;123(3192):377–378. doi: 10.1126/science.123.3192.377. [DOI] [PubMed] [Google Scholar]


