Abstract
1. A method for measuring the time course of ionic fluxes during a non-propagated membrane action potential is described; the technique combines intracellular perfusion of the squid giant axon with radioactive tracer methods and with a method for controlling the membrane potential during small time intervals.
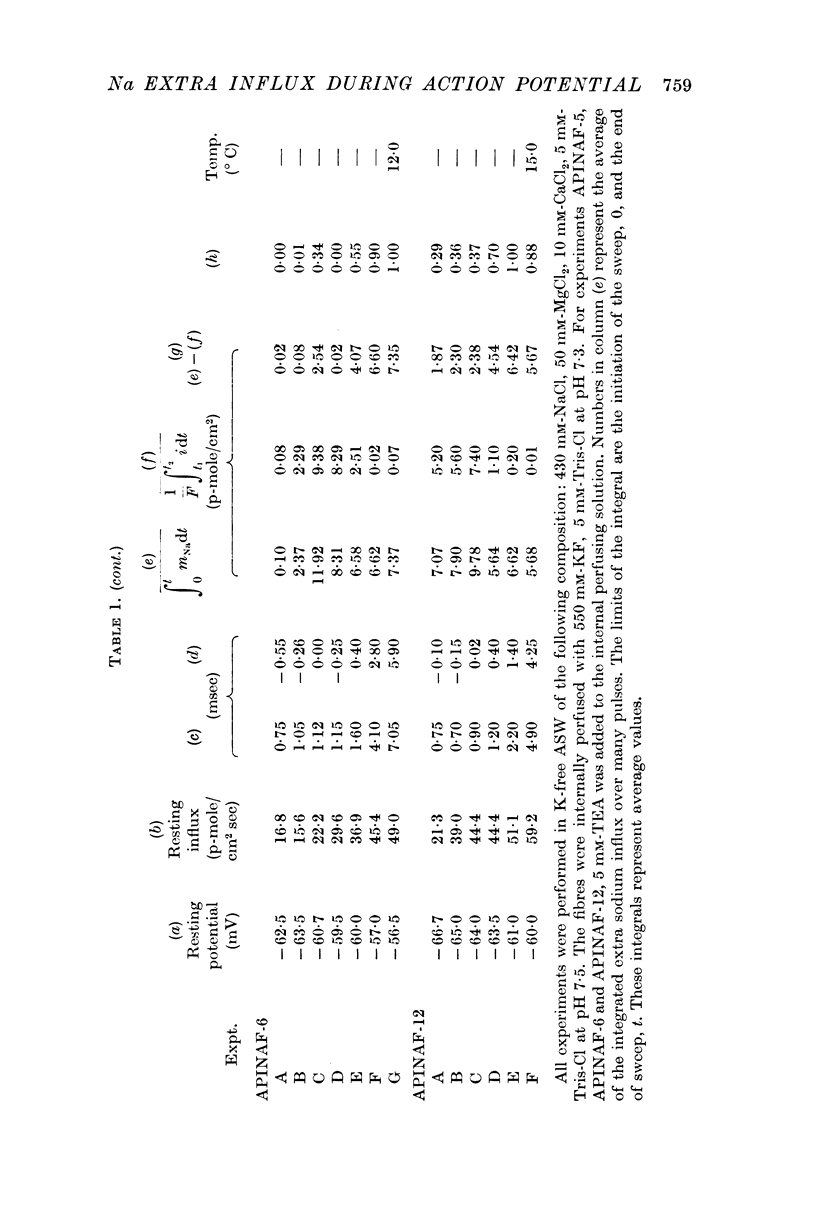
2. The method is used to determine the temporal course of the sodium extra influx during an action potential.
3. The results agree with the time course of the permeability change to sodium ions calculated with the Hodgkin & Huxley equations.
4. An average extra sodium influx of 7·13 p-mole/cm2 per action potential was determined at 12° C and of 5·23 p-mole/cm2 per action potential at 15° C.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. doi: 10.1085/jgp.50.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Bezanilla F., Rojas E. Sodium influxes in internally perfused squid giant axon during voltage clamp. J Physiol. 1969 May;201(3):657–664. doi: 10.1113/jphysiol.1969.sp008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Sodium and potassium conductance changes during a membrane action potential. J Physiol. 1970 Dec;211(3):729–751. doi: 10.1113/jphysiol.1970.sp009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Time course of the sodium influx in squid giant axon during a single voltage clamp pulse. J Physiol. 1970 Mar;207(1):151–164. doi: 10.1113/jphysiol.1970.sp009054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Canessa-Fischer M. Sodium movements in perfused squid giant axons. Passive fluxes. J Gen Physiol. 1968 Aug;52(2):240–257. doi: 10.1085/jgp.52.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E., Atwater I., Bezanilla F. Analysis of the effects of calcium or magnesium on voltage-clamp currents in perfused squid axons bathed in solutions of high potassium. J Gen Physiol. 1969 Oct;54(4):532–552. doi: 10.1085/jgp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw T. I. Cation movements in perfused giant axons. J Physiol. 1966 Jan;182(1):209–216. doi: 10.1113/jphysiol.1966.sp007819. [DOI] [PMC free article] [PubMed] [Google Scholar]


