Abstract
1. Axons perfused with a K-free solution containing 300 mM-NaF + sucrose to maintain isotonicity (referred to as 300 mM-NaF) and placed in K-free artificial sea-water usually depolarized spontaneously to around 0 mV. The membrane could be hyperpolarized to -70 to -100 mV with a small inwardly directed current; in one experiment the holding current was measured and was found to be less than 20 μA/cm2.
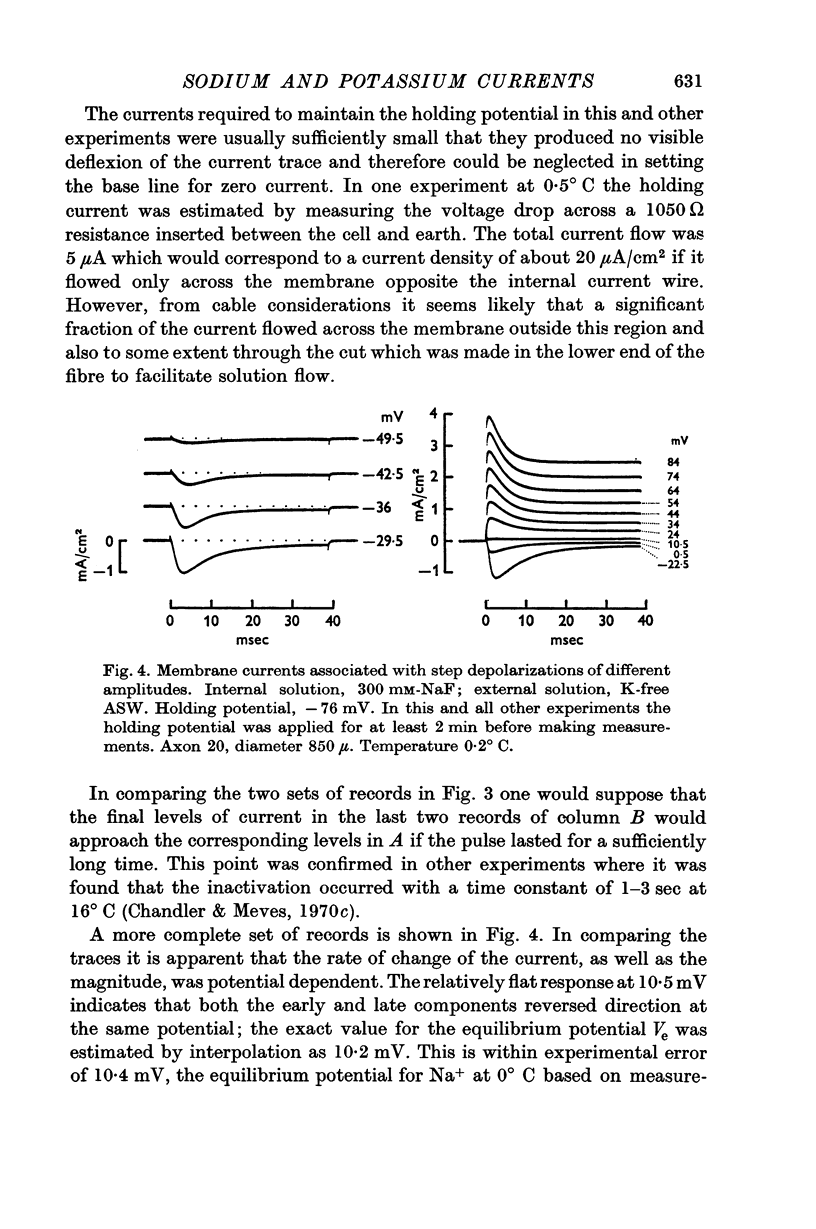
2. Membrane currents associated with a step depolarization from a potential which varied from -70 to -100 mV showed three phases: (a) an initial capacitative transient, (b) an early current which was inward for small depolarizations and outward for large ones, (c) a smaller maintained current. The currents in (b) and (c) are considered to be carried by Na ions since they both reversed direction at the same potential which was on the average within 0·3 mV of the equilibrium potential for Na ions, 10·4 mV at 0° C and 11 mV at 16·5° C, as estimated from measurements made with a cation-sensitive glass electrode.
3. The instantaneous current—voltage relation was determined at the time of peak current and at the end of a long prepulse when the current had reached a steady level. In both cases the curve was approximately linear with a slight deviation at negative potentials.
4. Prepulses, lasting 11-48 msec, to a potential of 33-64 mV (0-3·5° C) produced a shift in the equilibrium potential of 0·6-3·3 mV. This small change can be accounted for by assuming that Na ions accumulate in the Frankenhaeuser—Hodgkin space.
5. Both peak and steady-state components of Na current were blocked by tetrodotoxin (10-7 g/ml.) in the external solution.
6. The values of peak and steady-state Na conductance were strongly voltage-dependent for V less than -20 mV; for V more negative than -40 mV the peak and steady-state values increased e-fold for a change in potential of 4 and 6-8 mV respectively. At positive potentials the peak conductance was relatively independent of potential, whereas the steady-state curve showed an increase; at 50 mV the steady-state conductance was on the average 0·44 times the peak value for temperatures -0·3 to 4° C and 0·24 times the peak value for a temperature of 16·5° C.
7. Following an 18-164 min perfusion period with 300 mM-NaF, the delayed K currents with 300 mM-KF were reduced in amplitude to less than one-tenth the initial level. This apparent removal of the delayed rectifier was not accompanied by any significant change in either the relation between peak early current and voltage or the associated equilibrium potential.
8. In an experiment in which tetrodotoxin was used to block the early channel, K currents were determined before and after NaF perfusion. In both cases the kinetics on depolarization followed the Hodgkin—Huxley n4 relationship and the rate constants were similar, although after NaF perfusion the amplitude was reduced to 0·07 times the control level.
9. In axons perfused with 300 mM-KF, following removal of the delayed rectifier by 300 mM-NaF, the ratio of steady-state Na current: peak Na current was estimated to be about half the value obtained with NaF. A similar decrease was obtained in an axon which was perfused with 300 mM-CsF; on subsequent perfusion with 300 mM-KF, following 35 min with CsF, about half the original delayed current was present.
10. The general conclusion is that in axons perfused with 300 mM-NaF the Na conductance is not fully inactivated by depolarizations which last for tens of milliseconds. The maintained component may underlie the plateau phase of long lasting action potentials which have been recorded under similar conditions.
Full text
PDF




























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Dyron F. M., Senft J. P. Internally perfused axons: effects of two different anions on ionic conductance. Science. 1966 Mar 18;151(3716):1392–1394. doi: 10.1126/science.151.3716.1392. [DOI] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Senft J. P. Effects of internal sodium on ionic conductance of internally perfused axons. Nature. 1966 Nov 5;212(5062):614–616. doi: 10.1038/212614a0. [DOI] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Senft J. P. Voltage clamp studies on the effect of internal cesium ion on sodium and potassium currents in the squid giant axon. J Gen Physiol. 1966 Nov;50(2):279–293. doi: 10.1085/jgp.50.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Bezanilla F., Rojas E. Sodium influxes in internally perfused squid giant axon during voltage clamp. J Physiol. 1969 May;201(3):657–664. doi: 10.1113/jphysiol.1969.sp008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., MEVES H. THE EFFECT OF DILUTING THE INTERNAL SOLUTION ON THE ELECTRICAL PROPERTIES OF A PERFUSED GIANT AXON. J Physiol. 1964 Apr;170:541–560. doi: 10.1113/jphysiol.1964.sp007348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN M., SHAHN E., WEISS M. F. The routine fitting of kinetic data to models: a mathematical formalism for digital computers. Biophys J. 1962 May;2:275–287. doi: 10.1016/s0006-3495(62)86855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L. The effect of internal sodium on the action potential in the presence of different internal anions. J Physiol. 1965 Dec;181(3):594–611. doi: 10.1113/jphysiol.1965.sp007785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Slow changes in membrane permeability and long-lasting action potentials in axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):707–728. doi: 10.1113/jphysiol.1970.sp009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Blaustein M. P., Anderson N. C., Narahashi T. Basis of tetrodotoxin's selectivity in blockage of squid axons. J Gen Physiol. 1967 May;50(5):1401–1411. doi: 10.1085/jgp.50.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T. Tetrodotoxin's highly selective blockage of an ionic channel. Fed Proc. 1967 Nov-Dec;26(6):1655–1663. [PubMed] [Google Scholar]
- NARAHASHI T. DEPENDENCE OF RESTING AND ACTION POTENTIALS ON INTERNAL POTASSIUM IN PERFUSED SQUID GIANT AXONS. J Physiol. 1963 Nov;169:91–115. doi: 10.1113/jphysiol.1963.sp007243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakajima S., Grundfest H. The action of tetrodotoxin on electrogenic components of squid giant axons. J Gen Physiol. 1965 Jul;48(6):975–996. [PubMed] [Google Scholar]
- Narahashi T., Anderson N. C., Moore J. W. Comparison of tetrodotoxin and procaine in internally perfused squid giant axons. J Gen Physiol. 1967 May;50(5):1413–1428. doi: 10.1085/jgp.50.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Atwater I. Effect of tetrodotoxin on the early outward currents in perfused giant axons. Proc Natl Acad Sci U S A. 1967 May;57(5):1350–1355. doi: 10.1073/pnas.57.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKI I., TAKENAKA T. EFFECTS OF VARIOUS POTASSIUM SALTS AND PROTEASES UPON EXCITABILITY OF INTRACELLULARLY PERFUSED SQUID GIANT AXONS. Proc Natl Acad Sci U S A. 1964 Sep;52:804–810. doi: 10.1073/pnas.52.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Luxoro M., Ruarte A. Electrophysiological studies of chilean squid axons under internal perfusion with sodium-rich media. Science. 1965 Nov 12;150(3698):899–901. doi: 10.1126/science.150.3698.899. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Singer I., Takenaka T. Effects of internal and external ionic environment on excitability of squid giant axon. A macromolecular approach. J Gen Physiol. 1965 Jul;48(6):1095–1123. doi: 10.1085/jgp.48.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]


