Abstract
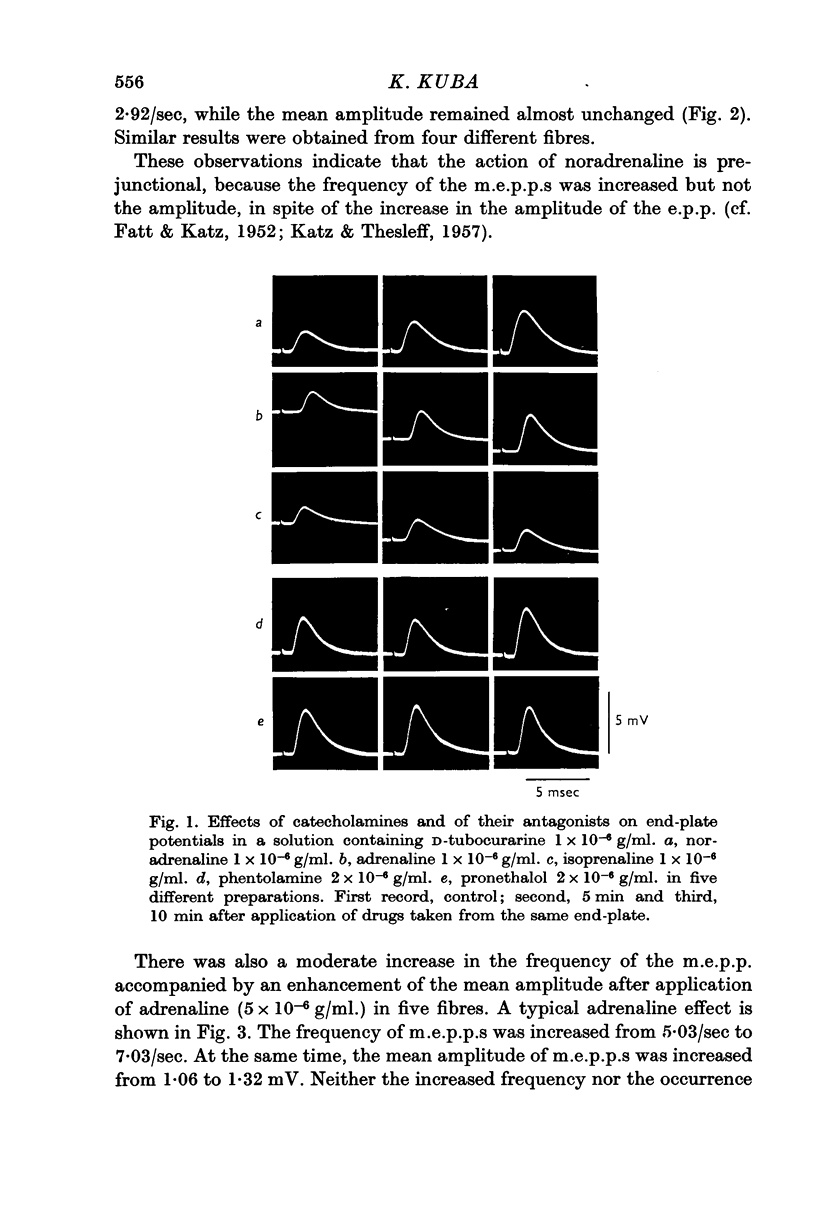
1. The effects of noradrenaline, adrenaline and isoprenaline on neuromuscular transmission in the rat diaphragm and the influence of adrenergic blocking agents on these actions were investigated.
2. The resting membrane potential of the muscle fibre was increased by adrenaline (5 × 10-6-10-5 g/ml.) and isoprenaline (5 × 10-6 g/ml.) up to 3-4 mV, but noradrenaline (5 × 10-6-10-5 g/ml.) had little effect.
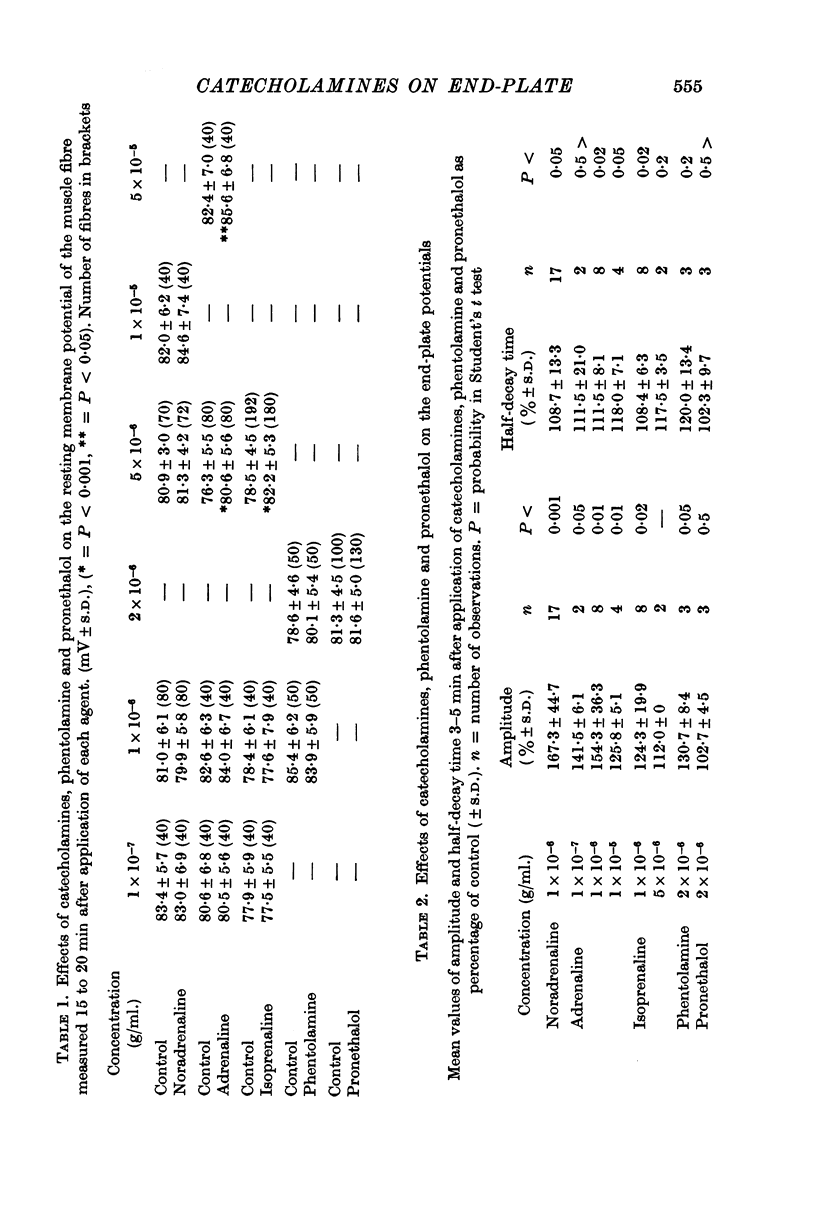
3. The amplitude and the half-decay time of the end-plate potential (e.p.p.) were increased by noradrenaline (1 × 10-6 g/ml.), adrenaline (1 × 10-7-10-5 g/ml.) and isoprenaline (1-5 × 10-6 g/ml.). The potentiation of the amplitude of the e.p.p. was greater with noradrenaline than with adrenaline and isoprenaline.
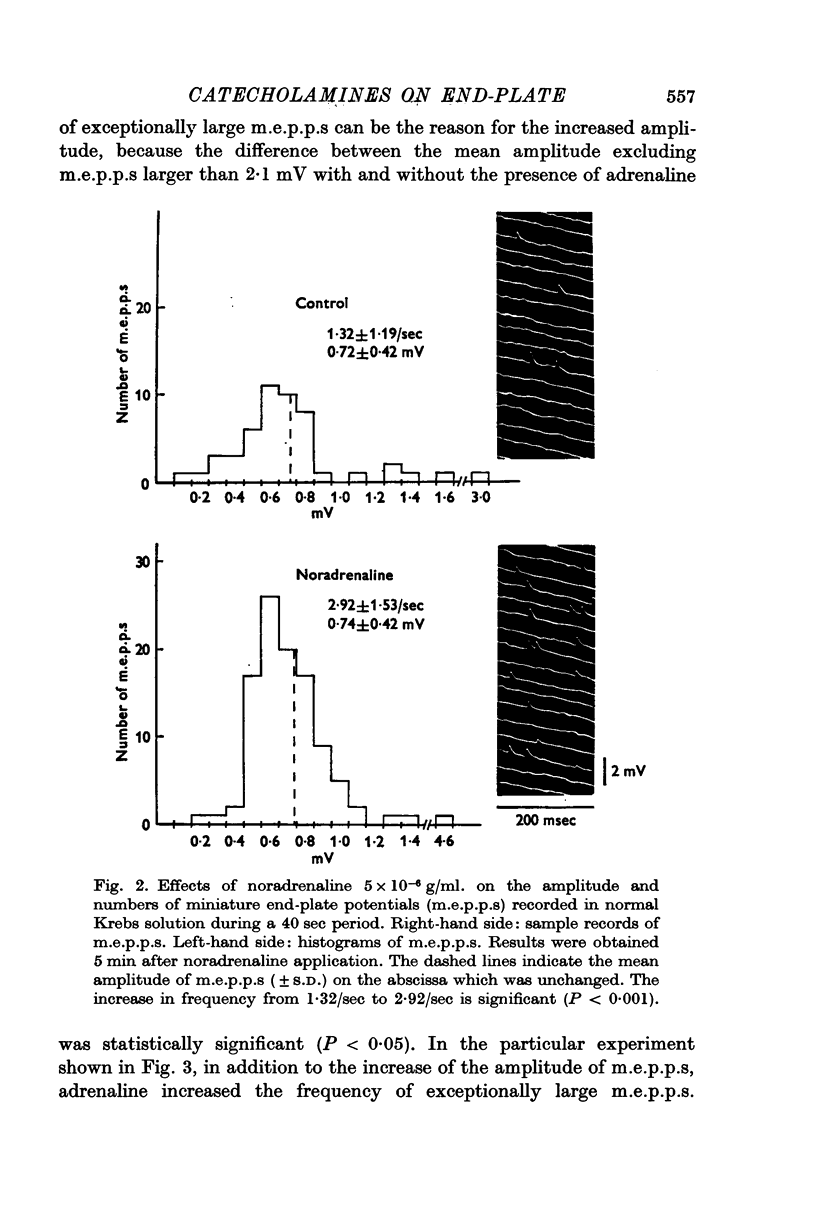
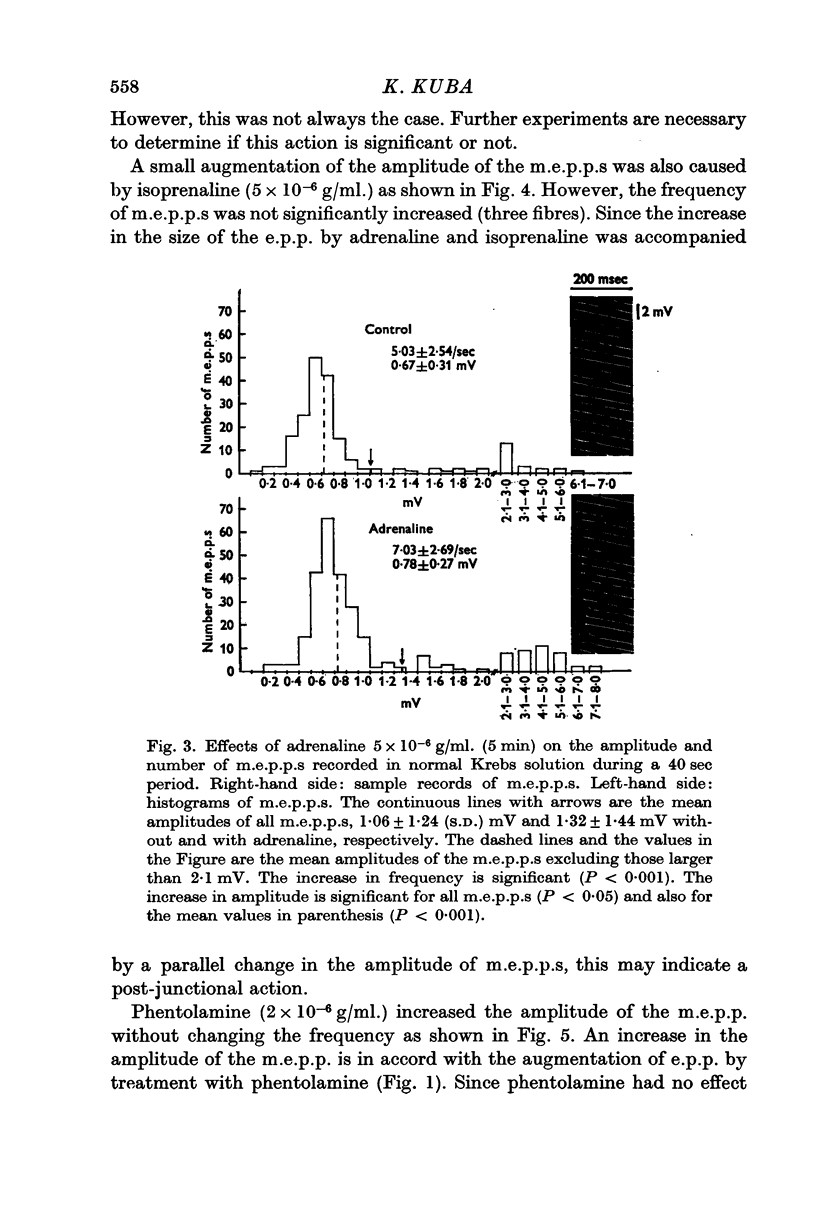
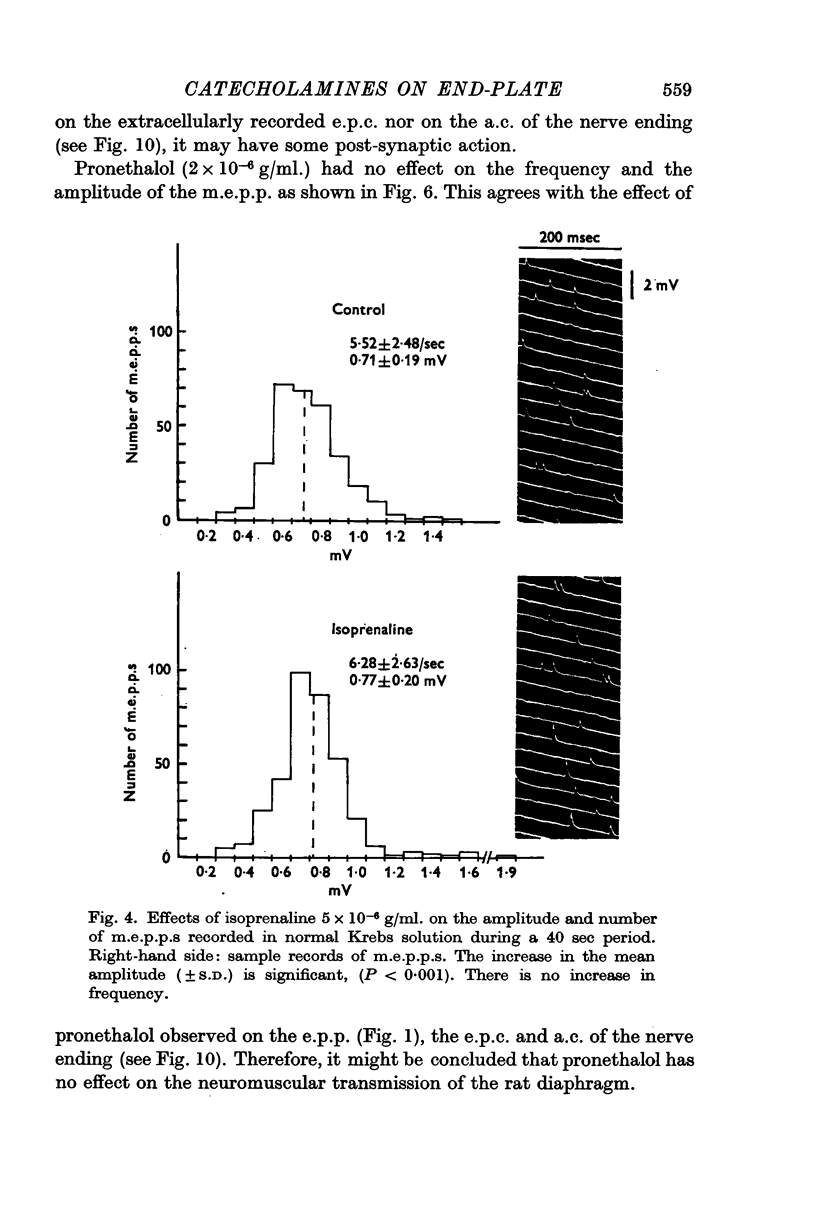
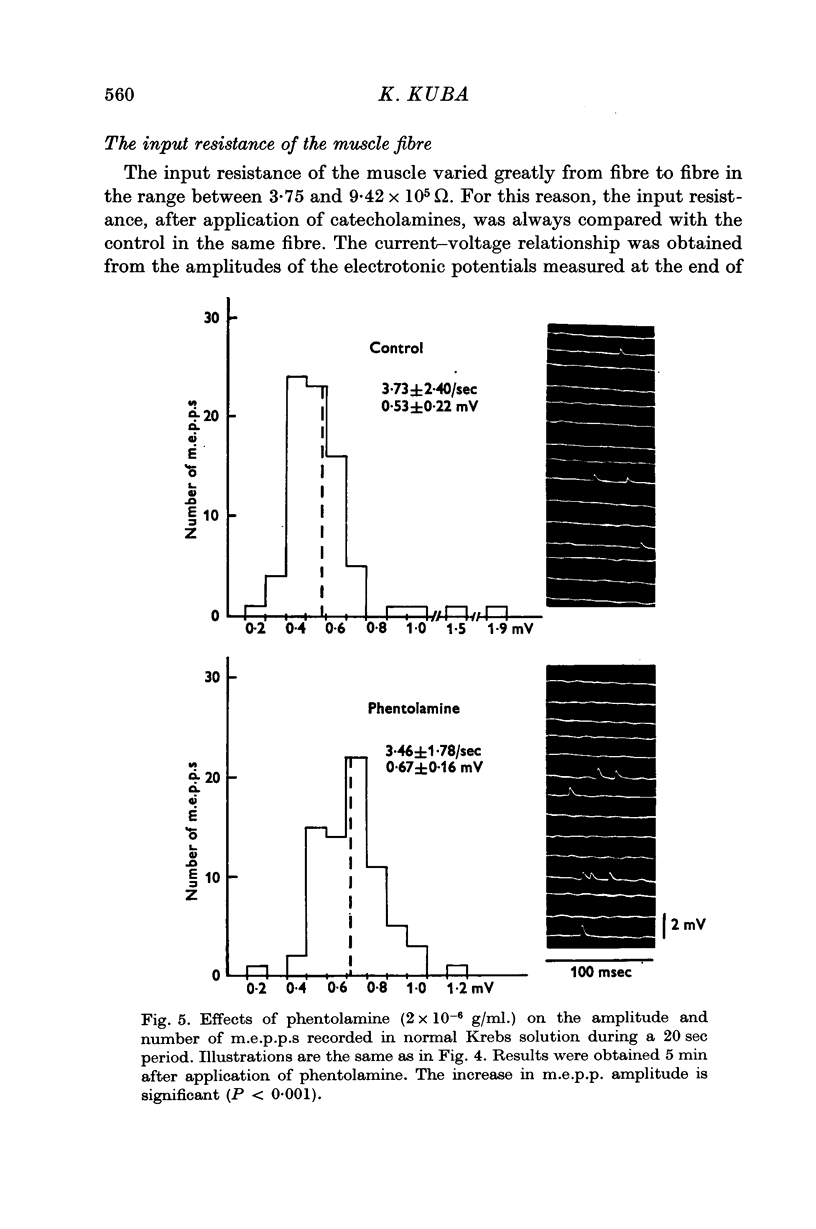
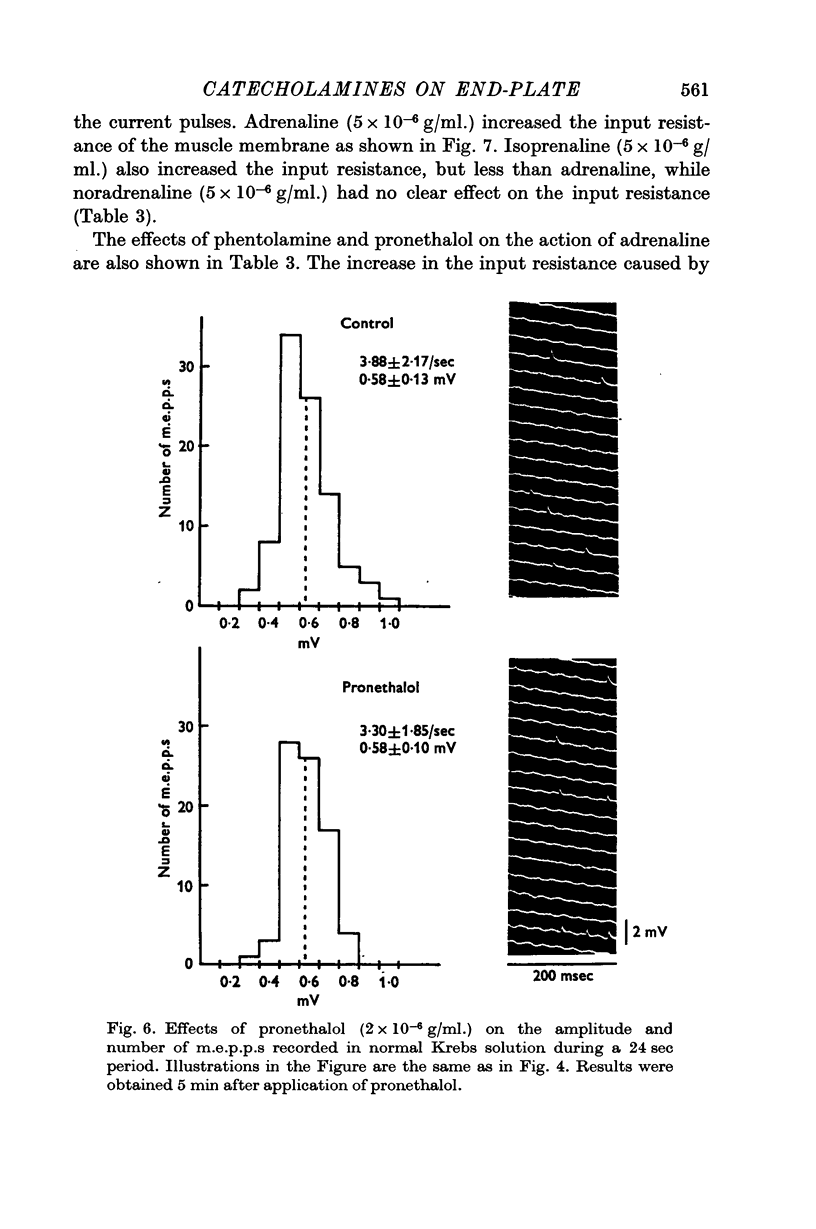
4. Noradrenaline (5 × 10-6 g/ml.) increased the frequency of miniature end-plate potentials (m.e.p.p.), but not their amplitude. However, isoprenaline (5 × 10-6 g/ml.) increased the amplitude of m.e.p.p.s without change in frequency. Adrenaline (5 × 10-6 g/ml.) increased both frequency and amplitude of m.e.p.p.s.
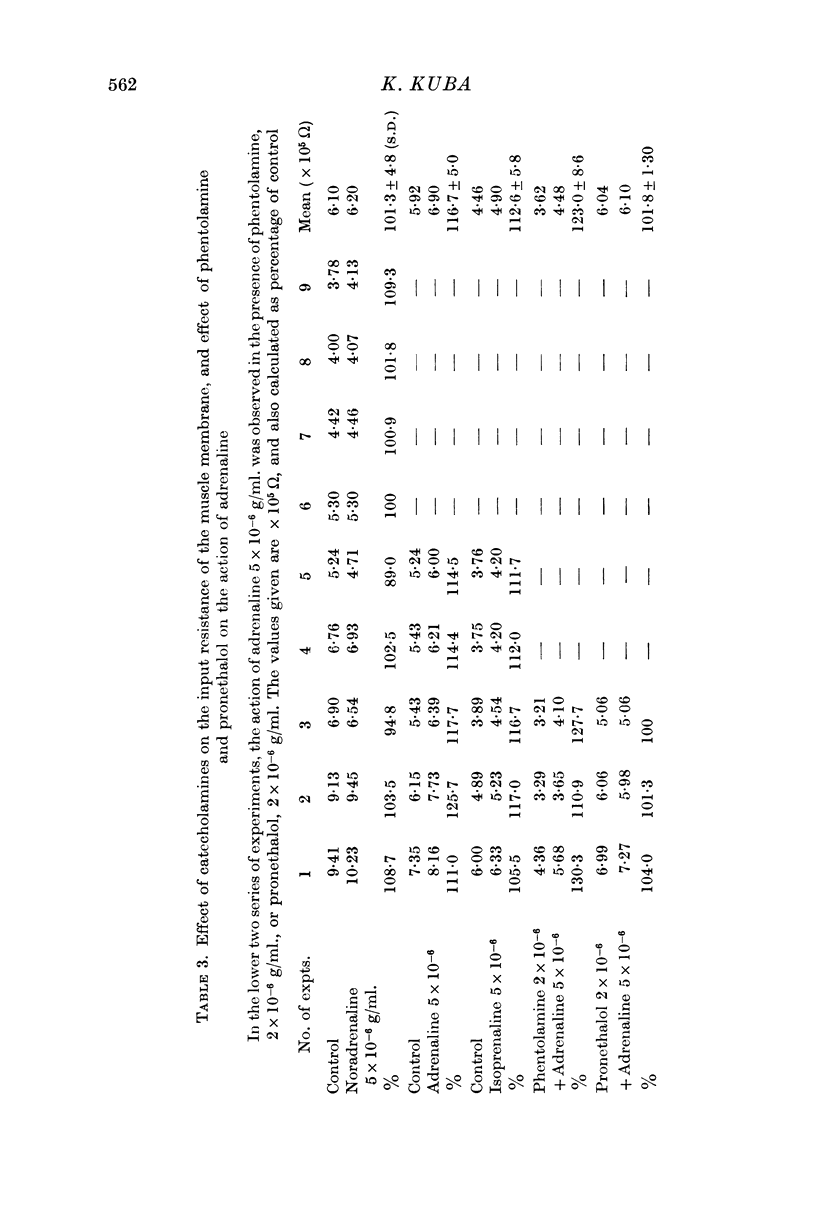
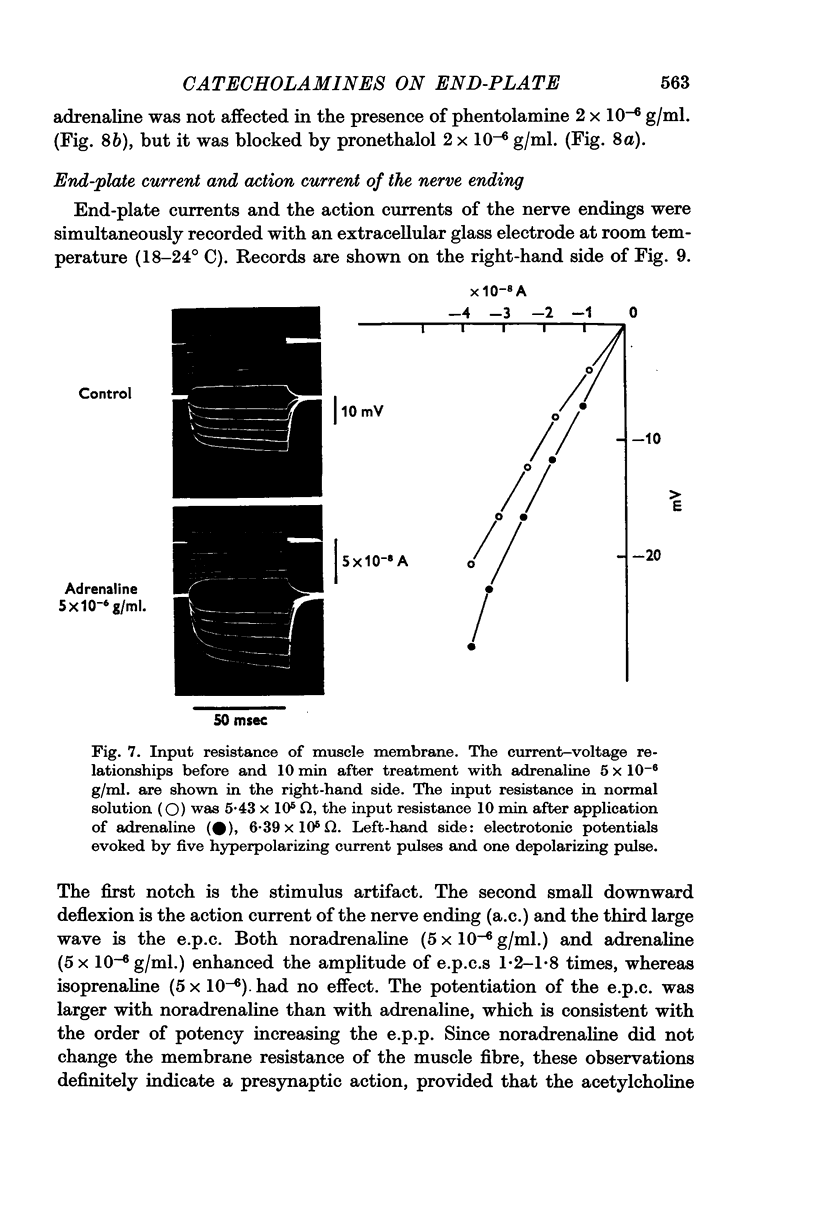
5. Adrenaline (5 × 10-6 g/ml.) and isoprenaline (5 × 10-6 g/ml.) increased the input resistance of the muscle membrane. The effect was blocked by the β-blocker, pronethalol (2 × 10-6 g/ml.), but not by the α-blocker, phentolamine (2 × 10-6 g/ml.). Noradrenaline did not change the input resistance of the muscle fibre.
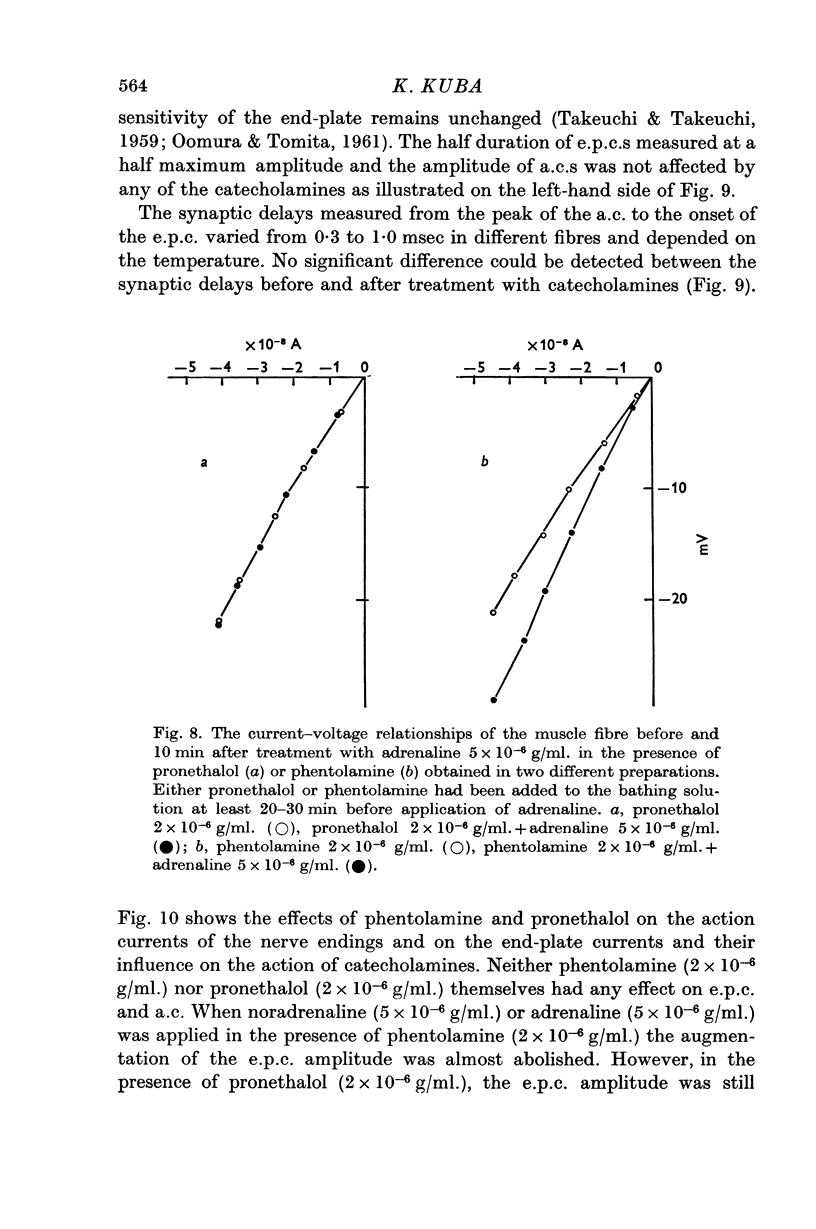
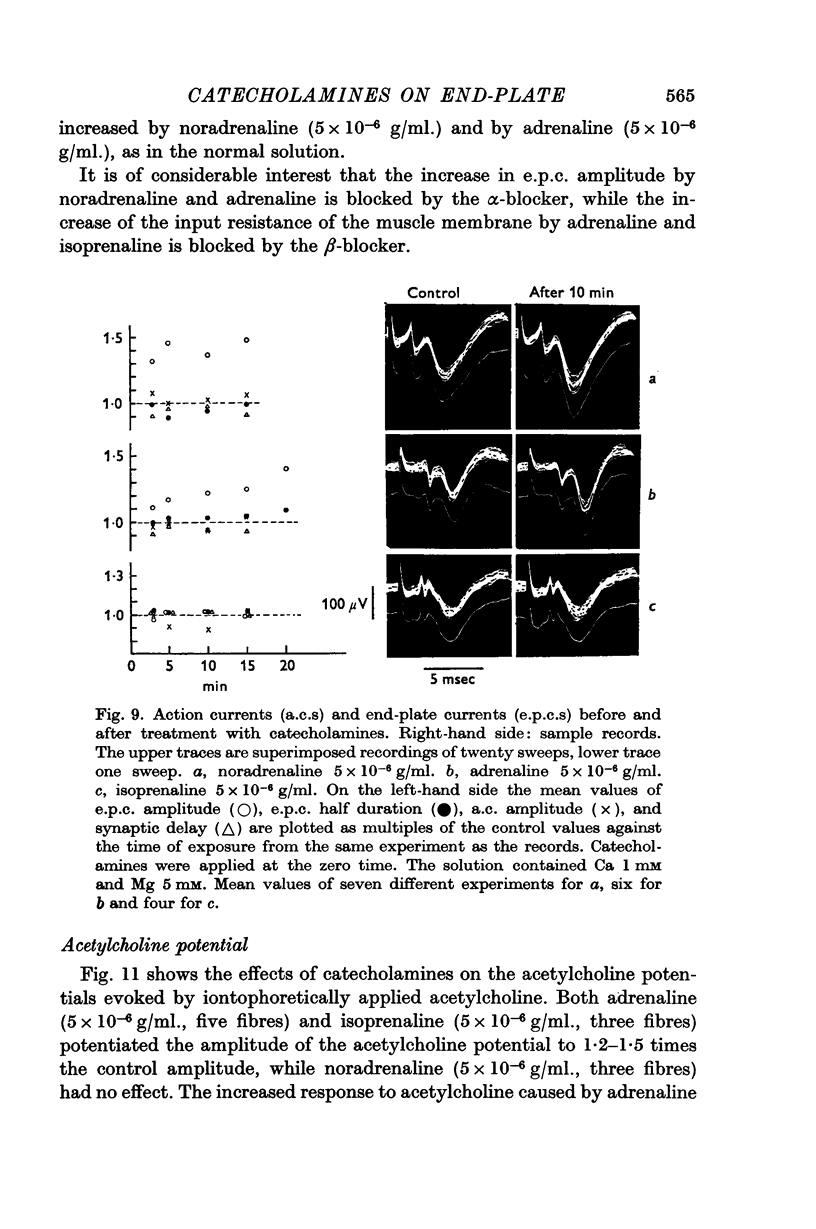
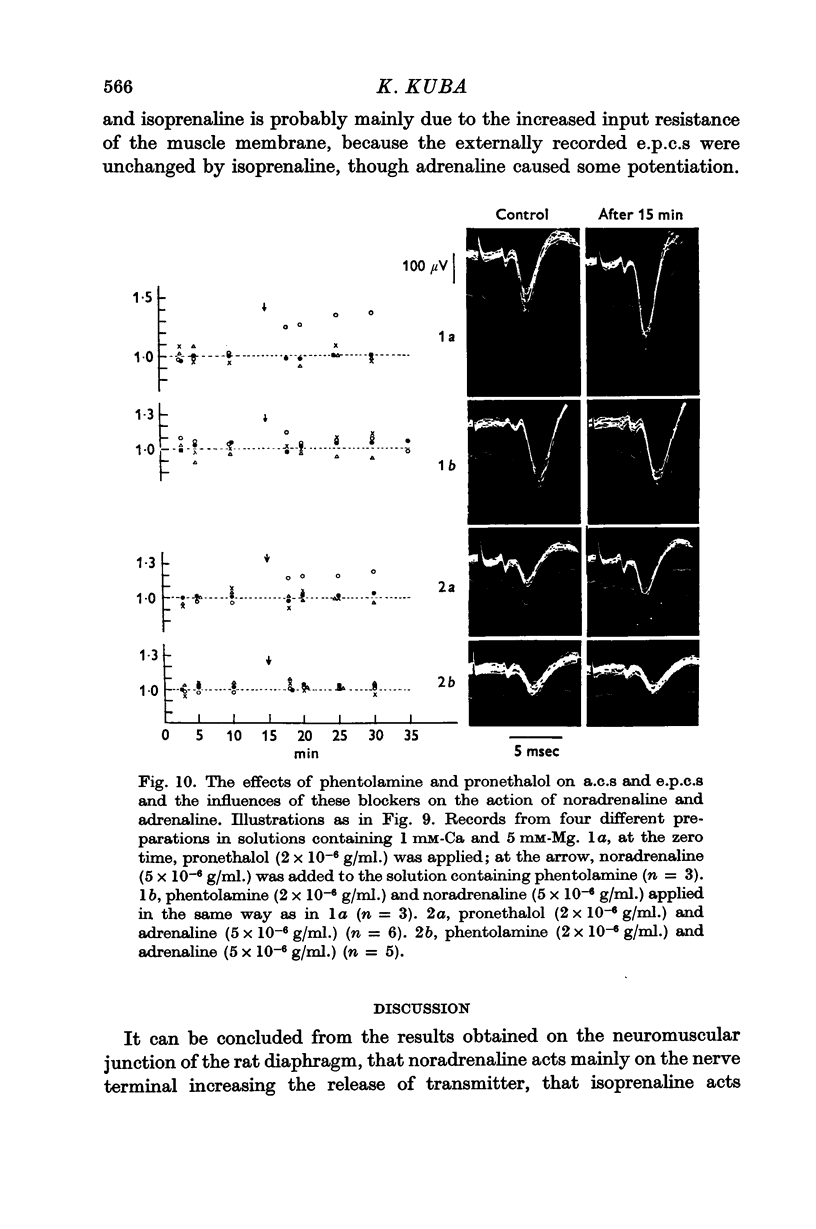
6. Noradrenaline (5 × 10-6 g/ml.) and adrenaline (5 × 10-6 g/ml.) augmented the extracellularly recorded end-plate current (e.p.c.), but they had no effect on the half duration, nor on the action current (a.c.) of the nerve terminal, nor on the synaptic delay. Isoprenaline (5 × 10-6 g/ml.) had no effect on any of these parameters. The actions of noradrenaline and adrenaline on e.p.c. were abolished by phentolamine (2 × 10-6 g/ml.), but not by pronethalol (2 × 10-6 g/ml.).
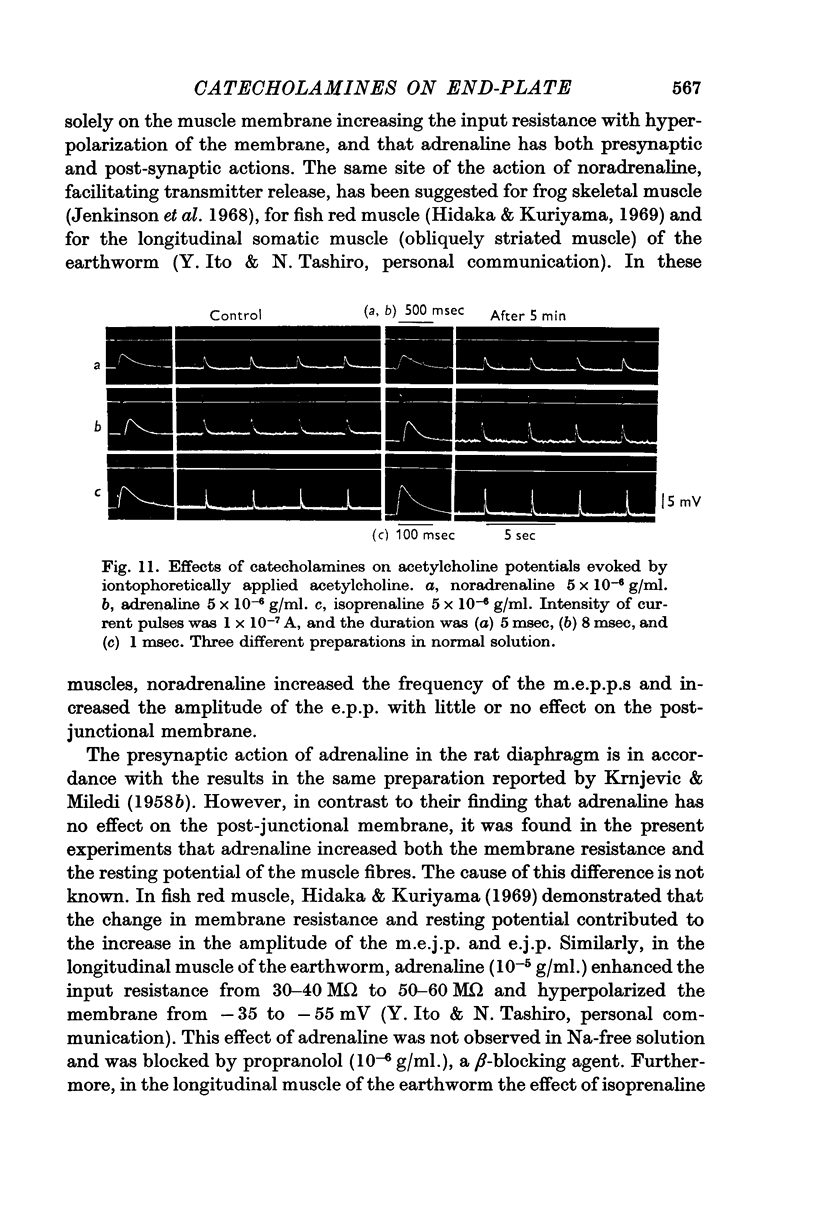
7. Adrenaline (5 × 10-6 g/ml.) and isoprenaline (5 × 10-6 g/ml.) enhanced the amplitude of the acetylcholine potential elicited by iontophoretic application of acetylcholine. No such effect was produced by noradrenaline (5 × 10-6 g/ml.).
8. It was concluded that noradrenaline acts on the nerve ending increasing the release of transmitter, and that isoprenaline acts on the post-synaptic membrane enhancing the input resistance, while adrenaline has both presynaptic and post-synaptic actions. The effect on the nerve ending is concerned with the α-action, whereas that on post-synaptic membrane with β-action of the catecholamines.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman W. C., Raper C. Adrenotropic receptors in skeletal muscle. Ann N Y Acad Sci. 1967 Feb 10;139(3):741–753. doi: 10.1111/j.1749-6632.1967.tb41241.x. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., LOEWENSTEIN W. R. Nature of neuromuscular facilitation by sympathetic stimulation in the frog. J Physiol. 1955 Dec 29;130(3):559–571. doi: 10.1113/jphysiol.1955.sp005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T., Kuriyama H. Effects of catecholamines on the cholinergic neuromuscular transmission in fish red muscle. J Physiol. 1969 Mar;201(1):61–71. doi: 10.1113/jphysiol.1969.sp008742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H., Stamenović B. A., Whitaker B. D. The effect of noradrenaline on the end-plate potential in twitch fibres of the frog. J Physiol. 1968 Apr;195(3):743–754. doi: 10.1113/jphysiol.1968.sp008486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Acetylcholine in mammalian neuromuscular transmission. Nature. 1958 Sep 20;182(4638):805–806. doi: 10.1038/182805b0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Some effects produced by adrenaline upon neuromuscular propagation in rats. J Physiol. 1958 Apr 30;141(2):291–304. doi: 10.1113/jphysiol.1958.sp005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K. The action of phenol on neuromuscular transmission in the red muscle of fish. Jpn J Physiol. 1969 Dec;19(6):762–774. doi: 10.2170/jjphysiol.19.762. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Pharmacology of excitation-contraction coupling in vascular smooth muscle and in avian slow muscle. Fed Proc. 1969 Sep-Oct;28(5):1634–1642. [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]


