Abstract
1. The effect of changes in the pH of the extracellular solution on the membrane conductance of frog sartorius and toe muscle fibres was measured with intracellular micro-electrodes.
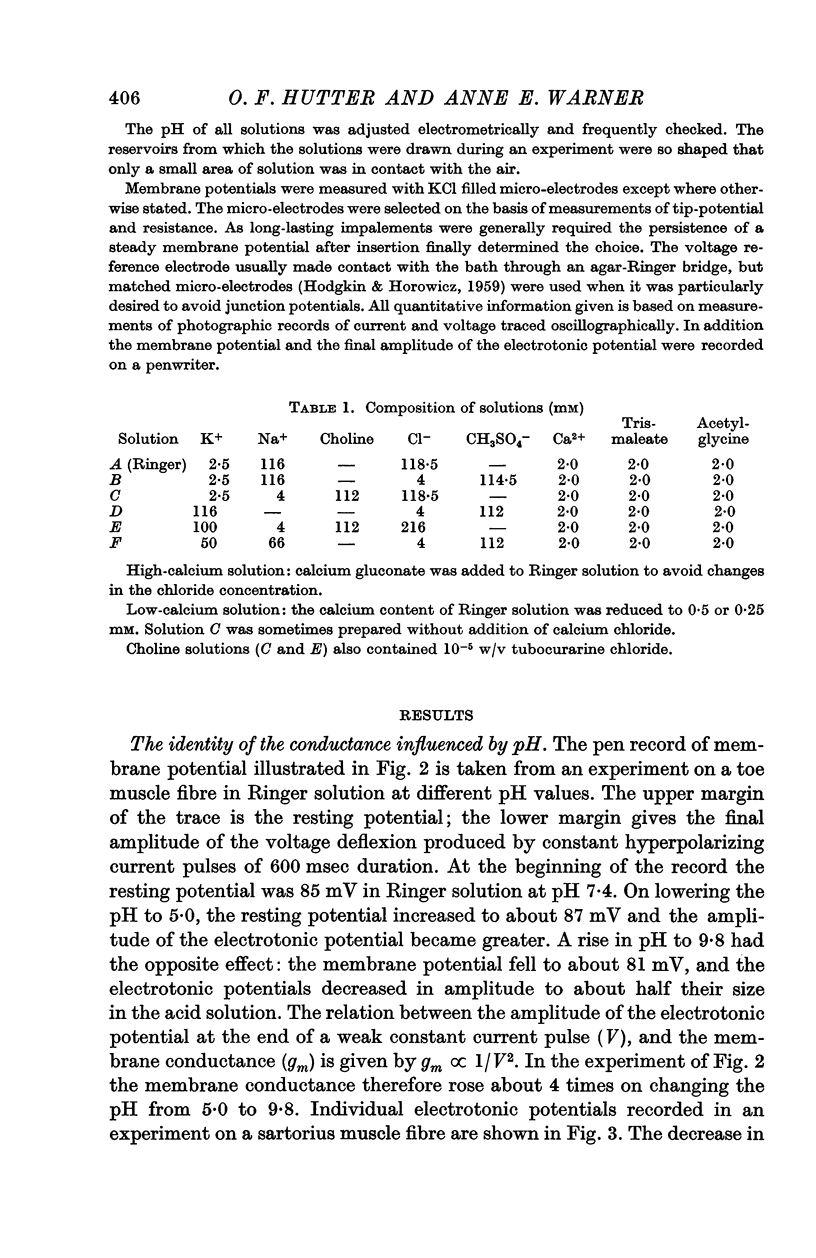
2. In Ringer solution the membrane conductance was found to be highly sensitive to changes in pH between 5·0 and 9·8. In alkaline solution the conductance rose; in acid solution it fell.
3. After replacement of chloride by the relatively impermeant methylsulphate ion the membrane conductance showed little change when pH was altered. It is concluded that chloride is the ion species principally concerned in the pH sensitivity of the resting membrane conductance.
4. The relation between pH and the chloride conductance was sigmoid, with the steepest part of the curve lying in the region of neutrality.
5. The membrane conductance of muscles equilibrated in a 100 mM-K 216 mM-Cl solution was also sensitive to changes of extracellular pH. As in Ringer solution, the membrane conductance rose in alkaline and fell in acid solutions in a sigmoid fashion.
6. Sartorius muscles in isotonic potassium methylsulphate solution showed no change in membrane conductance at different pH values.
7. In chloride-free solution a fall in pH tended to cause depolarization; a rise in pH had the opposite effect.
8. In Ringer solution the initial effect of a rise in pH was usually a transient depolarization. The indication is that the intracellular concentration of chloride ions may be slightly in excess of that which corresponds to the resting potential. The long-term effects of changes in pH on the membrane potential in Ringer solution were in the same direction as in the absence of chloride.
9. The transient potential changes produced on addition and withdrawal of chloride ions were found to be larger in alkaline solutions than in acid solutions. This is further evidence for a higher chloride permeability in alkaline solutions.
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. Potassium chloride movement and the membrane potential of frog muscle. J Physiol. 1960 Apr;151:154–185. [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINLEY F. J., Jr, MULLINS L. J. ION FLUXES AND TRANSFERENCE NUMBER IN SQUID AXONS. J Neurophysiol. 1965 May;28:526–544. doi: 10.1152/jn.1965.28.3.526. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARR C. W. Studies on the binding of small ions in protein solutions with the use of membrane electrodes. III. The binding of chloride ions in solutions of various proteins. Arch Biochem Biophys. 1953 Oct;46(2):417–423. doi: 10.1016/0003-9861(53)90212-4. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. E., Janse M. Intracellular chloride concentration in cat papillary muscles. Influence of external K concentration. Arch Int Physiol Biochim. 1965 Jan;73(1):174–175. [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., NELSON T. E., Jr, SANCHEZ V. Mechanism of the increased acetylcholine sensitivity of skeletal muscle in low pH solutions. J Cell Comp Physiol. 1962 Feb;59:35–44. doi: 10.1002/jcp.1030590105. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Sodium permeability in toad nerve and in squid nerve. J Physiol. 1960 Jun;152:159–166. doi: 10.1113/jphysiol.1960.sp006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J. THE CHLORIDE PERMEABILITY OF FROG SARTORIUS. J Physiol. 1965 Jan;176:123–135. doi: 10.1113/jphysiol.1965.sp007539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. The influence of the external medium on the internal pH of muscle. Proc R Soc Lond B Biol Sci. 1955 Aug 16;144(914):1–22. doi: 10.1098/rspb.1955.0030. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. Ionic movements and electrical activity in giant nerve fibres. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):1–37. doi: 10.1098/rspb.1958.0001. [DOI] [PubMed] [Google Scholar]
- HUBBARD S. J. The electrical constants and the component conductances of frog skeletal muscle after denervation. J Physiol. 1963 Mar;165:443–456. doi: 10.1113/jphysiol.1963.sp007069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. Anion conductance of cardiac muscle. J Physiol. 1961 Jul;157:335–350. doi: 10.1113/jphysiol.1961.sp006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., PADSHA S. M. Effect of nitrate and other anions on the membrane resistance of frog skeletal muscle. J Physiol. 1959 Apr 23;146(1):117–132. doi: 10.1113/jphysiol.1959.sp006182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. Action of some foreign cations and anions on the chloride permeability of frog muscle. J Physiol. 1967 Apr;189(3):445–460. doi: 10.1113/jphysiol.1967.sp008178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The effect of pH on the 36-Cl efflux from frog skeletal muscle. J Physiol. 1967 Apr;189(3):427–443. doi: 10.1113/jphysiol.1967.sp008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. CHLORIDE IN THE SQUID GIANT AXON. J Physiol. 1963 Dec;169:690–705. doi: 10.1113/jphysiol.1963.sp007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMB J. F. The chloride content of rat auricle. J Physiol. 1961 Aug;157:415–425. doi: 10.1113/jphysiol.1961.sp006733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LING G., GERARD R. W. The normal membrane potential of frog sartorius fibers. J Cell Physiol. 1949 Dec;34(3):383–396. doi: 10.1002/jcp.1030340304. [DOI] [PubMed] [Google Scholar]
- MEVES H., VOLKNER K. G. Die Wirkung von CO2 auf das Ruhemembranpotential und die elektrischen Konstanten der quergestreiften Muskelfaser. Pflugers Arch. 1958;265(5):457–476. doi: 10.1007/BF00369773. [DOI] [PubMed] [Google Scholar]
- PAGE E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol. 1962 Nov;46:201–213. doi: 10.1085/jgp.46.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZACHAR J., ZACHAROVA D., HENCEK M. THE RELATIVE POTASSIUM AND CHLORIDE CONDUCTANCES IN THE MUSCLE MEMBRANE OF THE CRAYFISH (ASTACUS FLUVIATILIS). Physiol Bohemoslov. 1964;13:129–136. [PubMed] [Google Scholar]


