Abstract
Adaptive mutation to Lac+ in Escherichia coli strain FC40 depends on recombination functions and is enhanced by the expression of conjugal functions. To test the hypothesis that the conjugal function that is important for adaptive mutation is the production of a single-strand nick at the conjugal origin, we supplied an exogenous nicking enzyme, the gene II protein (gIIp) of bacteriophage f1, and placed its target sequence near the lac allele. When both gIIp and its target site were present, adaptive mutation was stimulated three- to fourfold. Like normal adaptive mutations, gIIp-induced mutations were recA+ and ruvC+ dependent and were mainly single-base deletions in runs of iterated bases. In addition, gIIp with its target site could substitute for conjugal functions in adaptive mutation. These results support the hypothesis that nicking at the conjugal origin initiates the recombination that produces adaptive mutations in this strain of E. coli, and they suggest that nicking may be the only conjugal function required for adaptive mutation.
When populations of microorganisms are exposed to nonlethal selection, mutations that relieve the selective pressure can appear, a phenomenon called adaptive mutation (4, 5, 15). The mutational process is not directed to specific targets, because nonselected mutations also occur (14). But the process does require the presence of the selective agent; e.g., simply starving cells for a carbon source does not result in mutations (4, 5).
Adaptive mutation has been extensively studied in Escherichia coli strain FC40. This strain cannot utilize lactose (i.e., it is Lac−) because of a +1-bp frameshift mutation that affects the lacZ and lacY genes (6, 40). Although the Lac− allele, Φ(lacI33-lacZ), is slightly leaky, the amount of β-galactosidase produced is not sufficient to allow the cells to grow on medium containing lactose as the only carbon source (4, 13). For ease of genetic manipulation, the lac and proAB operons are carried on an episome, F′128, and the corresponding region is deleted from the chromosome.
During nonselective growth, Lac+ revertants of FC40 appear at a rate of about 10−9 per cell per generation; when incubated on lactose minimal medium, Lac+ revertants arise for about a week at a constant rate of about 10−7 per cell per day (4, 14). The Lac+ mutations arising during lactose selection are distinguished from the Lac+ mutations that arise during nonselected growth in several ways: (i) whereas mutations arising during nonselected growth consist of a variety of deletions and duplications that can revert the Φ(lacI33-lacZ) allele, adaptive Lac+ mutations are almost exclusively −1-bp frameshifts in runs of iterated bases (20, 50); (ii) unlike mutations occurring during nonselective growth, adaptive mutations require recombination functions, specifically E. coli's RecA-RecBCD pathway for double-strand break (DSB) repair (4, 32); (iii) to achieve the high level of recombination-dependent mutation in FC40, the Lac− allele must be on the episome and one or more conjugal functions must be expressed (21, 27, 47). Although we have argued that actual conjugation is not required (21, 22), others maintain that conjugal transfer of the DNA is essential to the mutagenic process (27, 30, 47).
During bacterial conjugation, transfer of the episomal DNA is initiated by a site-specific nick in the DNA at the conjugal origin, oriT. However, even in stationary-phase cells that are not conjugating, the nick persists (25). Several years ago it was proposed that this nicking was the initiating event for adaptive mutation in FC40 (21). This hypothesis was inspired by the fact that recombination between homologous alleles residing on the F′ and on the chromosome is 20- to 50-fold higher than recombination between the same alleles when both are on the chromosome (43). This enhancement requires the presence of oriT in cis to one of the alleles, the expression of the conjugal nicking functions, and the RecA-RecBCD pathway for recombination (7, 8). These requirements are the same as those for adaptive mutation in FC40.
To test whether a DNA nick stimulates adaptive mutation, we utilized the nicking function of the filamentous bacteriophage, f1. Rolling circle replication of filamentous coliphage genomes is initiated by the gene II protein (gIIp), which recognizes a 140-bp sequence at the phage origin of replication and nicks the plus-strand DNA at a specific site within this sequence. gIIp also unwinds and displaces the plus strand and then cleaves and religates this strand for packaging (58). A 37-bp sequence containing the nick site is sufficient to allow nicking but not to allow the other activities of gIIp (11, 53). When gIIp is expressed in yeast carrying this minimum target sequence, recombination and gene conversion are stimulated in both directions from the nick site (52). Recent evidence suggests that the nick must be converted into a DSB by replication in order to stimulate recombination (29).
We placed the minimum gIIp nick sequence close to the reversion site of the Φ(lacI33-lacZ) allele. We then supplied, from a plasmid, gene II under control of an exogenous promoter. With this system we were able to demonstrate that adaptive mutation is stimulated when both gIIp and its nick site are present in the cell and, further, that gIIp activity can substitute for conjugal functions in adaptive mutation. These results suggest that nicking may be the only conjugal function that is required for adaptive mutation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains and plasmids are listed in Table 1. All these strains were originally derived from P90C (CSH142 [41]; P90C and the two F′ strains used were obtained from J. H. Miller). FC40, the revertible strain, and FC29, the nonrevertible scavenger strain, have been described elsewhere (4). All genetic constructions used standard protocols (41). The traD411::Km allele was introduced onto episomes as described previously (21, 36) (the allele was obtained from K. Ippen-Ihler [deceased]). The recA938::Tn9-200 allele (56) (obtained from the Coli Genetic Stock Center) and the Δ(ruvC64)::Km allele (39) (obtained from R. Lloyd) were introduced by transduction with bacteriophage P1(vir); transductants were selected for drug resistance and then screened for sensitivity to UV light. In some cases the alleles were transduced into the F′ strain and in other cases they were transduced into its F− parent FC36 (4), and then the appropriate episome was introduced by conjugation. Even when the product strains were the same, these different constructions have different numbers as shown in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Name | Derivation | Relevant properties | Source or reference |
|---|---|---|---|
| Parental and control strains | |||
| P90C | F−ara Δ(gpt-lac)5 thiA | Parental strain | 41 |
| FC29 | P90C/ F′ Δ(lacI lacZ) LacY+ Pro+ | Nonrevertible Lac− scavenger | 4; J. H. Miller, personal communication |
| FC36 | P90C Rifr | Spontaneous Rifr mutant of P90C | 4 |
| FC40 | FC36/F′ Φ(lacI33-lacZ) LacY+ Pro+ | Revertible Lac− strain | 4, 6 |
| FCJ64 | FC29 traD411::Km | traD nonrevertible Lac− scavenger | P. L. Foster, unpublished |
| FCJ65 | FC40 traD411::Km | traD revertible Lac− strain | 21 |
| CRS183 | FC40 recA938::Cm | recA revertible Lac− strain | This study |
| Strains with the gIIp nick site next to Φ(lacI33-lacZ) | |||
| CRS18, CRS241 | FC40 with gIIp nick site | FC40 + nick site | This study |
| CRS186, CRS237 | CRS18 recA938::Cm | recA FC40 + nick site | This study |
| CRS174 | P90C/F′ Φ(lacI33-lacZ) with nick site, traD411::Km | traD revertible Lac− strain + nick site | This study |
| CRS189 | CRS174 recA938::Cm | recA traD revertible Lac− strain + nick site | This study |
| CRS242 | CRS241 traD411::Km | traD FC40 + nick site | This study |
| CRS238 | CRS18 ΔruvC::Km | ruvC FC40 + nick site | This study |
| Strains carrying the empty vector, pCRS3 | |||
| CRS232 | CRS18/pCRS3 | FC40 + nick site/pPBAD vector | This study |
| CRS234 | F29/pCRS3 | Scavenger/pPBAD vector | This study |
| CRS243 | CRS241/pCRS3 | FC40 + nick site/pPBAD vector | This study |
| CRS245 | CRS242/pCRS3 | traD FC40 + nick site/pPBAD vector | This study |
| CRS249 | FCJ64/pCRS3 | traD scavenger/pPBAD vector | This study |
| CRS247 | FC40/pCRS3 | FC40/pPBAD vector | This study |
| CRS251 | FCJ65/pCRS3 | traD FC40/pPBAD vector | This study |
| Strains carrying the gene II plasmid, pCRS4 | |||
| CRS10 | CRS18/pCRS4 | FC40 + nick site/pPBAD-gene II | This study |
| CRS177 | CRS174/pCRS4 | traD revertible Lac− strain + nick site/pPBAD-gene II | This study |
| CRS179, CRS248 | FC40/pCRS4 | FC40/pPBAD-gene II | This study |
| CRS184 | CRS183/pCRS4 | recA FC40/pPBAD-gene II | This study |
| CRS187 | CRS186/pCRS4 | recA FC40 + nick site/pPBAD-gene II | This study |
| CRS190 | CRS189/pCRS4 | traD recA revertible Lac− strain + nick site/pPBAD-gene II | This study |
| CRS235 | F29/pCRS4 | Scavenger/pPBAD-gene II | This study |
| CRS244 | CRS241/pCRS4 | FC40 + nick site/pPBAD-gene II | This study |
| CRS246 | CRS242/pCRS4 | traD FC40 + nick site/pPBAD-gene II | This study |
| CRS250 | FCJ64/pCRS4 | traD scavenger/pPBAD-gene II | This study |
| CRS252 | FCJ65/pCRS4 | traD FC40/pPBAD-gene II | This study |
| Plasmids | |||
| pCRS3 | pBR322 carrying araC-PBAD regulatory region; Cbr Tcr | Control vector | This study |
| pCRS4 | pCRS3 carrying gene II; Cbr Tcr | Gene II under control of PBAD | This study |
Strains were grown at 37°C in Luria-Bertani (LB) medium or M9 minimal medium (41). M9 medium contained 0.1% glycerol and 0.02% thiamine and, when necessary, it was supplemented with 0.2% fucose or arabinose. M9-lactose plates were described previously (4). LB and MacConkey-lactose media were as described elsewhere (41). Antibiotic concentrations were as follows: kanamycin, 20 μg/ml in minimal medium and 40 or 60 μg/ml in LB; chloramphenicol, 10 μg/ml; rifampin, 100 μg/ml; carbenicillin, 10 or 50 μg/ml in minimal medium and 100 μg/ml in LB. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was added at 40 μg/ml.
Experimental protocols.
The protocol used for small-scale adaptive mutation experiments was as described previously (23). Cells of each strain were grown to saturation (24 h) in M9-glycerol medium plus antibiotics plus fucose or arabinose, as appropriate. The cultures were centrifuged and resuspended in saline, and the cell numbers were determined on LB plus antibiotics plates (the antibiotics were used to check that the cells retained their plasmids). Aliquots of 10 μl were spread on each quadrant of an M9-lactose plate. These were incubated at 37°C, and Lac+ colonies were counted daily starting 2 days after the bacteria were plated. For 36-h experiments, the original cultures were diluted 105-fold into fresh medium and allowed to again reach saturation. The cell numbers were determined, and 10-μl aliquots were plated on M9-lactose as before. There were no significant differences in cell numbers among strains and treatments.
The protocol for large-scale adaptive mutation experiments was as described elsewhere (4, 13). Cells were grown to saturation in M9-glycerol medium plus antibiotics, then diluted 105-fold into fresh medium, divided into five independent cultures, and allowed to again reach saturation. Scavenger strains were FC29 or FCJ64 carrying the same plasmids as the revertible strains; these cells were also grown in M9-glycerol medium plus antibiotics. At saturation, culture titers were determined on LB medium and on LB medium plus antibiotics; the experiment was continued only if the two titers were comparable, meaning that nearly all the cells maintained their plasmids. An appropriate number of cells from each of the independent cultures was mixed with 109 cells of the scavenger and spread onto three M9-lactose plates, which were incubated at 37°C. Lac+ colonies on one set of plates were counted daily starting 2 days after the bacteria were plated. The other sets were used to determine the number of Lac− cells, as previously described (19). Plugs were taken daily from the plates, and the cell numbers were determined on LB plus rifampin plates. These plates were then replicated onto MacConkey-lactose plates to determine if any of the colonies were Lac+; if Lac+ colonies were present, their numbers were subtracted from the total.
For β-galactosidase assays, cells were grown to saturation in M9-glycerol medium plus appropriate drugs. Assays were performed as described previously (19, 41).
DNA manipulations.
All DNA manipulations were carried out using standard protocols (2). Plasmid DNA was extracted with Wizard kits (Promega Corp.). Restriction enzymes, bacterial alkaline phosphatase, and T4 polynucleotide ligase were used according to the manufacturer's instructions (New England BioLabs).
Construction of a plasmid with a regulated gene II.
Plasmid pTII, carrying gene II from bacteriophage f1 (26), was digested with BamHI and EcoRI, yielding a fragment containing gene II from its ribosome binding site to 3 bp downstream from its terminator. To facilitate further subcloning, the polylinker-containing vector, pCR2.1 (Invitrogen, Co.), was digested with EcoRI, removing a 15-bp segment, and religated. This plasmid was named pCRS1. The BamHI/EcoRI fragment containing gene II was cloned in BamHI/EcoRI-cut pCRS1, and this plasmid was called pCRS2.
We wished to put gene II under the control of the araC-PBAD regulatory system, but the pBAD series of vectors designed for this purpose contains the M13 intergenic region that includes the gIIp target site (31). To eliminate this problem, the araC-PBAD control region was cut out of pBAD18-Cm (31) with ClaI and PvuI and ligated into the same restriction sites of plasmid pBR322 (3), generating plasmid pCRS3. pCRS2 was digested with KpnI and XbaI, obtaining gene II with its ribosomal binding site, and ligated in the same restriction sites of plasmid pCRS3, maintaining the proper orientation to the araC-PBAD regulatory system. This plasmid was called pCRS4. Plasmid pCRS4 and the empty vector, pCRS3, were transformed into the derivatives of E. coli FC40 generated for the purpose of this study (Table 1).
We confirmed gIIp activity by showing that pCRS4 increased the ability of f1 bacteriophage R12, which has a nonsense mutation in gene II, to form plaques on a nonsuppressing host, E. coli strain K38 (26) (bacteriophage and bacteria were obtained from P. Model). The number of plaques was additionally increased when arabinose was added, confirming that gene II was under control of PBAD (data not shown).
Construction of a plasmid with the gIIp target site in Φ(lacI33-lacZ).
The target for gIIp was a 45-bp sequence (Table 2, oligonucleotides 3 and 4) from the origin of bacteriophage f1 that contains the minimum recognition site necessary for nicking but not the sites required for plus-strand synthesis or ligation by gIIp (11, 52). These oligonucleotides also have Sau3AI sites on their ends. Oligonucleotides 3 and 4 were used for PCR, and the product was ligated into the TA cloning vector pCR2.1 (Invitrogen Co.). The clone was analyzed by PCR with oligonucleotide pair 1 and 4 and pair 3 and 2 (Table 2) and then sequenced using oligonucleotides 1 and 2 (Table 2) as primers. This plasmid was named pCRS5.
TABLE 2.
Oligonucleotides used in this studya
| Oligo-nucleotide no. | Sequence |
|---|---|
| 1 | 5′CAGGAAACAGCTATGAC |
| 2 | 5′GTTTTCCCAGTCACGAC |
| 3 | 5′GATCCTCTTT*AATAGTGGACTCTTGTTCCAAACTGGAACAACCCG |
| 4 | 3′GAGAAATTATCACCTGAGAACAAGGTTTGACCTTGTTGGGCCTAG |
| 5 | 5′ATCTCTCCAGGATCCTCGAATGGTGCAAAACC |
| 6 | 5′GCAGGAGCTCGTTATC |
Oligonucleotides 1 and 2 are, respectively, the universal M13 reverse primer (Invitrogen Corp.) and M13-40 forward primer (New England Biolabs). Oligonucleotides 3 and 4 contain the recognition site for nicking by the gIIp of bacteriophage f1; the site at which the DNA is nicked is indicated by an asterisk (11, 52). The terminal Sau3AI sites are underlined. Oligonucleotide 5 has a left tail of 8 bases, the restriction site for BamHI (underlined), and then 17 bases of the lacI gene (bases 8 to 24 of Ecolac [GenBank J01636.1]). Oligonucleotide 6 is 16 bases of lacZ (bases 3244 to 3228 of Ecolac).
Plasmid pFC530 carrying Φ(lacI33-lacZ) was created by replacing the BamHI-SacI fragment of plasmid pMC1403 (51) with a 3-kb BamHI-SacI fragment of Φ(lacI33-lacZ) generated by long, accurate PCR from strain FC40 (P.-E. Yeh and P. L. Foster, unpublished data). pFC530 was digested with BamHI and ClaI, yielding a 1,962-bp fragment carrying Φ(lacI33-lacZ) and the now-unique restriction site BclI (compatible with SauAI). This fragment was cloned into BamHI-ClaI-cut plasmid pBR322 (3), creating plasmid pCRS6. Because BclI is blocked by methylation, pCRS6 was transformed into the dam strain GM2163 (obtained from M. Marinus).
To generate a nonpolar insertion of the gIIp target site in Φ(lacI33-lacZ), the 45-bp Sau3AI fragment from plasmid pCRS5 was ligated into the BclI site of plasmid pCRS6. This insertion site corresponds to nucleotide 443 of the Ecolac sequence in GenBank (J01636.1), 593 bases to the 5′ side of the lacI33 mutation, a +1 frameshift at nucleotide 1036 (6, 20). To identify the correct orientation of the insert, clones were analyzed by PCR using the primer pairs 5 and 4 and 5 and 3 (Table 2). A plasmid with the correct orientation was named pCRS7. Finally, to generate a suicide plasmid with the construction, the BamHI-ClaI fragment of plasmid pCRS7 was cloned into the same restriction sites of plasmid pSG76-K, which requires the pir gene for replication and carries a recognition site for the endonuclease I-SceI (45) (pSG76-K and the host strain were obtained from G. Posfai). The new plasmid was called pCRS8.
Construction of E. coli strains carrying lacI33::lacZ with the gIIp nick site.
Φ(lacI33-lacZ) with the gIIp nick site was introduced into the episome of E. coli FC40 by in vivo gene replacement stimulated by a DNA DSB (44). First, the pir-dependent suicide plasmid, pCRS8, was transformed into FC40, and recombination between the plasmid and the episome was selected on LB-Km. Then, plasmid pST98-ASceP was introduced by transformation. pST98-ASceP is a temperature-sensitive suicide plasmid (it cannot replicate at 42°C) that constitutively expresses the I-SceI nuclease (plasmid obtained from G. Posfai). I-SceI will make a DNA DSB at its recognition site, which was recombined onto the episome in the previous step. The DSB stimulates intramolecular recombination between the introduced and the resident alleles. Recombinants that had eliminated the plasmid sequences were identified by loss of kanamycin resistance at the nonpermissive temperature. To verify that the desired gene replacement had taken place, clones were analyzed by PCR using oligonucleotides 5 and 4 (Table 2). This primer pair yields a PCR product only if the nick site is in the correct orientation. The strain carrying the gIIp nick site was called CRS18. The episome from CRS18 was mated into an F− Met− Nalr strain and then back into C36, to create strain CRS241.
PCR amplification and nucleotide sequencing.
PCR amplifications were carried out using the Platinum PCR Supermix (Gibco-BRL Corp.) for 30 cycles consisting of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min, except for amplification with primer pair 5 and 4, which was at 94°C for 1 min, 62°C for 1 min, and 72°C for 1 min. For sequencing reactions an ABI Prism Big Dye terminator (Applied Biosystems Inc.) was used for 25 cycles consisting of 96°C for 30 s, 50°C for 15 s, and 60°C for 4 min. Products were purified with DyeEx Spin columns (Qiagen, Inc.) and sequenced using ABI 377 or 3700 DNA sequencers.
Statistical analyses.
In most cases, standard statistical methods were used (57). Because the results presented below in Fig. 4 and 5 are ratios of numbers, both of which have associated errors, somewhat more complicated statistics were needed. The ratios and the standard errors for the ratios were calculated using the formulas described by Rice (49). The results were confirmed by simulating the experiments with the program Mathematica (Wolfram Research, Inc.). Simulations were run assuming that the data were normally distributed and using the observed means and variances of the data. In all but a few cases where the distributions included numbers very close to zero, a close fit to the calculated result was observed after 10,000 trials (A. Hanson, personal communication).
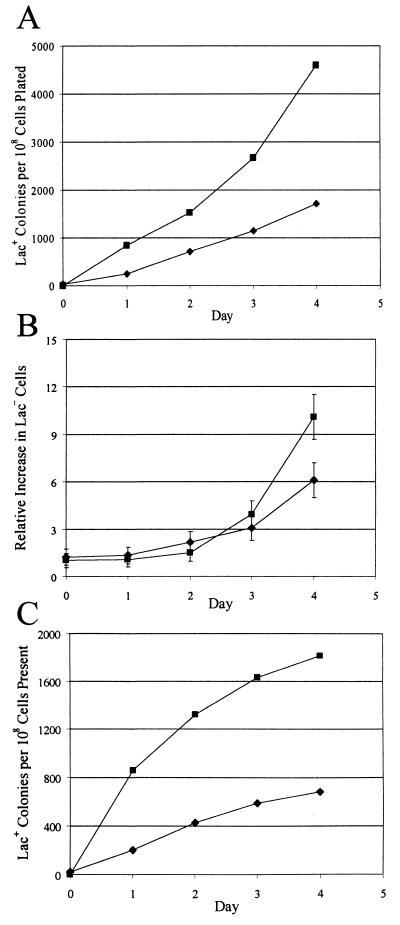
FIG. 4.
The presence of gene II and its nick site increases adaptive reversion to Lac+. The graphs show the results of a large-scale experiment (see Materials and Methods). Approximately 107 (strain CRS243) or 106 (strain CRS244) cells from each of five independent cultures, plus 109 scavenger cells, were spread onto M9-lactose plates. (A) The accumulation of Lac+ colonies divided by the number of cells originally plated. (B) The number of Lac− cells on the plates relative to the number originally plated. (C) The accumulation of Lac+ colonies divided by the number of Lac− cells on the plate 2 days earlier. Because it takes 2 days for a Lac+ colony to form, the curves in panels A and C have been shifted back 2 days. Diamonds, CRS243 (FC40 with the gIIp nick site carrying pCRS3, the empty vector); squares, CRS244 (FC40 with the gIIp nick site carrying pCRS4, the vector with gene II). The scavenger cells were CRS234 and CRS235, respectively. Data are the means and standard errors of the means. Some error bars are smaller than the symbols.
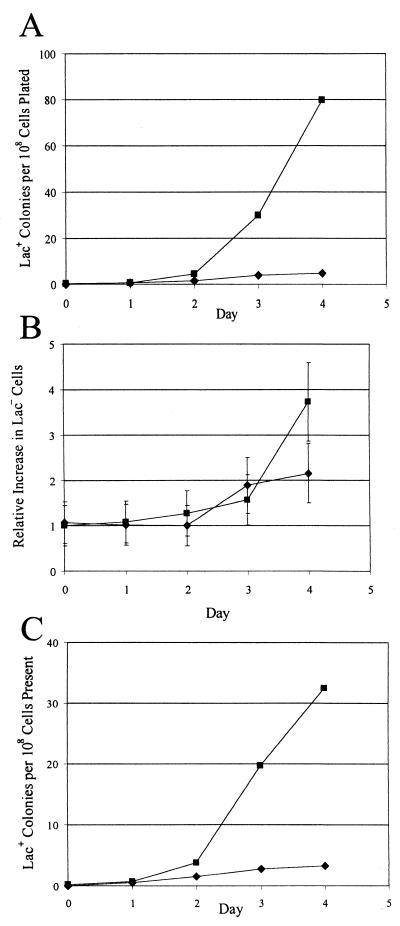
FIG. 5.
The presence of gene II and its nick site compensates for loss of conjugal functions in adaptive reversion to Lac+. The figures show the results of a large-scale experiment (see Materials and Methods). Approximately 108 cells of each strain from each of five independent cultures, plus 109 scavenger cells, were spread on M9-lactose plates. (A) The accumulation of Lac+ colonies divided by the number of cells originally plated. (B) The number of Lac− cells on the plates relative to the number originally plated. (C) The accumulation of Lac+ colonies divided by the number of Lac− cells on the plate 2 days earlier. Because it takes 2 days for a Lac+ colony to form, the curves in panels A and C have been shifted back 2 days. Diamonds, CRS245 (FC40 traD411::Km with the gIIp nick site carrying pCRS3, the empty vector); squares, CRS246 (FC40 traD411::Km with the gIIp nick site carrying pCRS4, the vector with gene II). The scavenger cells were CRS249 and CRS250, respectively. Data are the means and standard errors of the means. Some error bars are smaller than the symbols.
RESULTS
Induction of the gIIp nickase in the presence of its target site increases the rate of adaptive mutation to Lac+.
Adaptive mutation in FC40 can be assayed in large-scale (4) or in small-scale (19) experiments. For small-scale assays, cultures are inoculated from single colonies and grown to saturation in M9-glycerol medium. Then, 10-μl aliquots (approximately 2 × 107 cells) are spread on each quadrant of a minimal lactose plate, which is incubated at 37°C. Most of the colonies that appear 2 days later are due to mutations that occurred during the prior growth of the culture; the colonies that appear on days 3 to 5 are due to adaptive mutations (4). The mean number of Lac+ colonies appearing on days 3, 4, and 5 gives a reasonable estimate of the rate of adaptive mutation (19).
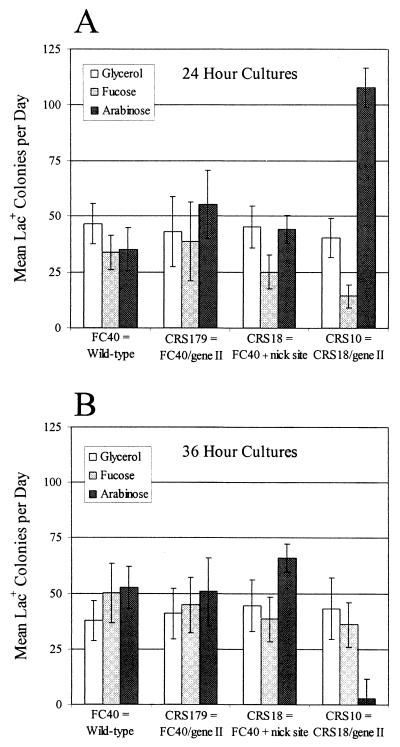
To determine if induction of a DNA nick increases adaptive mutation, we did small-scale assays with cells grown for 24 h in M9-glycerol medium supplemented with nothing, fucose (a nonmetabolizable repressor of the pBAD promoter [42]), or arabinose (an inducer of the pBAD promoter [31]). With strains FC40 and CRS179 (FC40/pCRS4; pCRS4 is the pBAD plasmid carrying gene II) grown under the different conditions, the rates at which Lac+ colonies appeared were similar, indicating that, in these assays, the presence and expression of gIIp from plasmid pCRS4 did not affect adaptive reversion to Lac+ (Fig. 1A, bars 1 to 6). When strain CRS18, which carries the gIIp nick site near the lacI33 mutation, was grown in the different media, again a similar rate of mutation to Lac+ was obtained, indicating that the nick site alone did not affect reversion to Lac+ (Fig. 1A, bars 7 to 9). But when strain CRS10 (CRS18/pCRS4), which carries both the nick site and gene II, was fully induced for gIIp expression, the rate at which Lac+ colonies appeared increased threefold relative to FC40 (Fig. 1A, bars 10 to 12). These results indicate that induction of a DNA nick increases adaptive mutation to Lac+.
FIG. 1.
Induction of a DNA nickase increases adaptive reversion to Lac+. The bars show the daily accumulation of Lac+ colonies during incubation on lactose plates from days 3 to 5 during a small-scale experiment (see Materials and Methods). The cultures were grown to saturation in M9-glycerol medium (plus appropriate antibiotics) supplemented with nothing (white), fucose (light grey), or arabinose (dark grey). FC40 is wild type; CRS179 is FC40/pCRS4; CRS18 is FC40 with the gIIp nick site; CRS10 is CRS18/pCRS4. pCRS4 is the plasmid carrying gene II. (A) Cultures grown to saturation for 24 h before plating; (B) the same cultures diluted 105-fold and grown again to saturation for 36 h before plating. Data are the means and standard errors of the means of one to three cultures of each strain and each treatment counted daily for 3 days.
For large-scale experiments, cells are grown from very small inocula and take up to 48 h to reach saturation (4, 13). We found that such long periods of continuous induction resulted in a decrease in adaptive Lac+ mutation in strain CRS10, which has the nick site and gene II. Figure 1B shows a small-scale assay done with the cultures described above but diluted 105-fold into fresh media and grown for an additional 36 h. While the number of Lac+ revertants was similar among strains FC40, CRS179, and CRS18 (Fig. 1B, bars 1 to 9), the reversion rate was significantly reduced in strain CRS10 grown with arabinose (Fig. 1B, bars 10 to 12). Large-scale experiments gave similar results (data not shown). One explanation is that prolonged continuous nicking of the episomal DNA resulted in loss of all or part of the episome, including the reversion site. Microscopic examination of CRS10 cells grown in the presence of arabinose revealed many filamentous cells, which is symptomatic of induction of the SOS response (24).
Induction of the gIIp nickase in the presence of its target site compensates for the loss of conjugal functions in adaptive mutation.
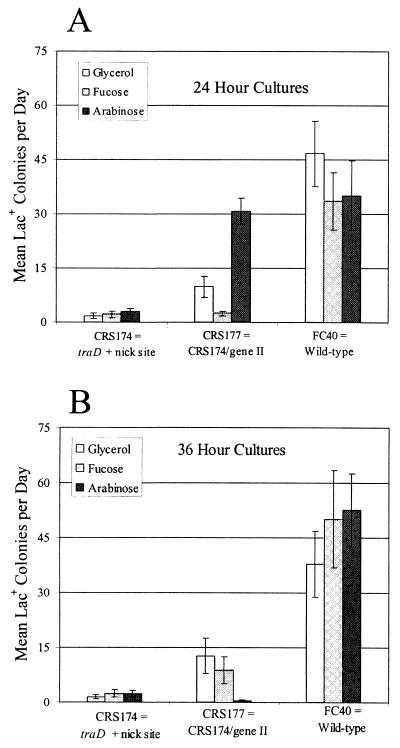
If conjugal functions are not expressed in strain FC40, the rate of adaptive mutation to Lac+ falls about 10-fold (21). Using the small-scale assay described above, we tested whether gIIp plus its target site could restore adaptive mutation to a traD mutant, which is defective for conjugal functions (see Discussion). As shown in Fig. 2A, CRS174, the traD strain with just the gIIp target site, had the expected low rate of adaptive mutation (Fig. 2A, bars 1 to 3). But strain CRS177, which has both gIIp and its target site, had an increased rate of adaptive mutation (Fig. 2A, bars 4 to 6). Indeed, when gIIp was fully induced, the reversion rate of CRS177 was 10-fold higher than that of strain CRS174 and nearly 70% that of the wild-type FC40 strain. These results indicate that induction of a DNA nick compensates for loss of conjugal functions.
FIG. 2.
Induction of a DNA nickase increases adaptive reversion to Lac+ in a strain that is defective in conjugal functions. The bars show the daily accumulation of Lac+ colonies during incubation on lactose plates from days 3 to 5 during a small-scale experiment (see Materials and Methods). The cultures were grown to saturation in M9-glycerol medium (plus appropriate antibiotics) supplemented with nothing (white), fucose (light grey), or arabinose (dark grey). CRS174 is P90C carrying F′Φ(lacI33-lacZ) traD411::Km and the gIIp nick site; CRS177 is CRS174/pCRS4. pCRS4 is the plasmid carrying gene II. The data for FC40 from Fig. 1 are repeated for comparison. (A) Cultures grown to saturation for 24 h before plating; (B) the same cultures diluted 105-fold and grown again to saturation for 36 h before plating. Data are the means and standard errors of the means of two cultures of each strain and each treatment counted daily for 3 days
As observed for the wild-type strain, when the traD mutant strain CRS177, which has both the nick site and gene II, was grown under inducing conditions for about 60 h, the adaptive reversion rate to Lac+ was decreased (Fig. 2B, bars 4 to 6). That is, the continuous production of DNA nicks was detrimental to traD mutant cells, as it was to wild-type cells.
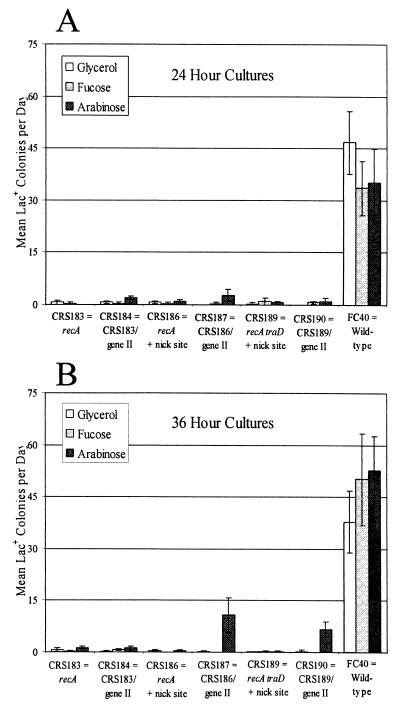
Most nickase-induced Lac+ adaptive mutations are recA+ dependent.
Adaptive mutations in E. coli FC40 are reduced 100-fold when RecA is defective (4, 32). In small-scale assays of saturated cultures grown for only 24 h, adaptive mutation was likewise severely reduced in recA derivatives of strains carrying gIIp and its target site (Fig. 3A.) Thus, nickase-induced adaptive mutations are recA+ dependent. In contrast to recA+ strains, when gIIp was fully induced by growing the recA mutant strains with the nick site and the gene II plasmid, CRS187 (tra+) and CRS190 (traD), for 36 to 60 h in the presence of arabinose, adaptive mutation to Lac+ was increased (Fig. 3B). However, these mutation rates were still less than those of rec+ strains (see Discussion).
FIG. 3.
Nickase-induced Lac+ mutations are recA+ dependent. The bars show the daily accumulation of Lac+ colonies during incubation on lactose plates from days 3 to 5 during a small-scale experiment (see Materials and Methods). The cultures were grown to saturation in M9-glycerol medium (plus appropriate antibiotics) supplemented with nothing (white), fucose (light grey), or arabinose (dark grey). CRS183 is FC40 recA938::Cm; CRS184 is CRS183/pCRS4; CRS186 is FC40 recA938::Cm with the gIIp nick site; CRS187 is CRS186/pCRS4; CRS189 is P90C recA938::Cm carrying F′Φ(lacI33-lacZ) traD411::Km and the gIIp nick site; CRS190 is CRS189/pCRS4. pCRS4 is the plasmid carrying gene II. The data for FC40 from Fig. 1 are repeated for comparison. (A) Cultures grown to saturation for 24 h before plating; (B) the same cultures diluted 105-fold and grown again to saturation for 36 h before plating. Data are the means and standard errors of the means of one to two cultures of each strain and each treatment counted daily for 3 days. Some error bars are too small to be seen.
The pBAD plasmid increases the apparent “leakiness” of the lac allele.
The lac allele used in these experiments, Φ(lacI33-lacZ), is slightly leaky, producing about 2 Miller units of β-galactosidase, an amount insufficient to allow these cells to grow on lactose (4, 13). However, in certain mutant backgrounds (e.g., recD mutants) and in Salmonella enterica serovar Typhimurium, this allele is actually or effectively leakier, allowing those cells to grow on lactose plates (19, 27). Roth and colleagues have argued that the process of adaptive mutation occurs even when cells are growing slowly under selective conditions (27, 35). In the small-scale experiments described above, the Lac− cells of wild-type strains carrying pCRS4 made visible lawns on lactose plates. Although such growth cannot account for large differences in mutation rates, it can obscure small differences.
As shown in Table 3, cells carrying either pCRS4 or pCRS3 produced more β-galactosidase than cells without a plasmid. This increase required neither gene II nor its target but was a property of the pBAD plasmid. Whether the plasmid caused the lac allele to be expressed at a higher level or caused an increase in the number of episomes or of lac alleles was not investigated. Because of this increased leakiness, we confirmed the key results of the experiments presented above with experiments during which we monitored the growth of Lac− cells on lactose medium (see below).
TABLE 3.
β-galactosidase activities produced by various Lac− strains
| Strain | Properties | Plasmid | β-galactosi- dase activity (Miller units) | Fold increase due to plasmid |
|---|---|---|---|---|
| FC40 | Wild type | None | 2.3 | |
| CRS247 | Wild type | pCRS3 (vector) | 3.7 | 1.6 |
| CRS248 | Wild type | pCRS4 (gene II) | 3.0 | 1.3 |
| CRS241 | Wild type + nick site | None | 2.0 | |
| CRS243 | Wild type + nick site | pCRS3 (vector) | 2.7 | 1.4 |
| CRS244 | Wild type + nick site | pCRS4 (gene II) | 2.4 | 1.2 |
| J65 | traD | None | 1.4 | |
| CRS251 | traD | pCRS3 (vector) | 2.9 | 2.1 |
| CRS252 | traD | pCRS4 (gene II) | 2.1 | 1.6 |
| CRS242 | traD + nick site | None | 1.5 | |
| CRS245 | traD + nick site | pCRS3 (vector) | 2.8 | 1.9 |
| CRS246 | traD + nick site | pCRS4 (gene II) | 2.3 | 1.5 |
The increase in adaptive mutation does not require full induction of the gIIp nickase.
The previous experiments indicated that gIIp is induced to some extent even when the cells are grown in glycerol and not exposed to arabinose (e.g., compare CRS174 and CRS177 in Fig. 2A). To minimize the detrimental effects of full induction of gIIp, we grew cells in M9-glycerol medium and determined the rate of adaptive mutation to Lac+ of strains carrying the gIIp nick site and either pCRS3, the empty vector, or pCRS4, the vector with gene II. As mentioned above, these plasmids allow cells to grow slowly on lactose plates. Therefore, we also determined the number of Lac− cells on the lactose plates and divided the number of new Lac+ colonies appearing each day by the number of Lac− cells present 2 days earlier (it takes 2 days for a Lac+ revertant to grow into a visible colony [4, 13]). As shown in Fig. 4C, the mutation rate of cells carrying pCRS3 (the empty vector) was about fivefold higher than that previously observed with FC40 (13). We attribute this increase to the extra β-galactosidase being produced (see above), which is roughly correlated with mutation rates (28). However, even when corrected for cell growth, the presence of gIIp expressed from plasmid pCRS4 increased the rate of adaptive mutation by three- to fourfold (Fig. 4C).
The same experiment with traD mutant strains is shown in Fig. 5. The traD mutant cells grew much less well on the lactose plates than did traD+ cells (Fig. 5B), and the mutation rate of the traD mutant with the nick site carrying the empty vector (strain CRS245) (Fig. 4C) was comparable to that which we previously observed with traD mutants (21). As observed in small-scale experiments, the presence of gIIp (strain CRS246) increased the adaptive mutation rate 10-fold (Fig. 5C).
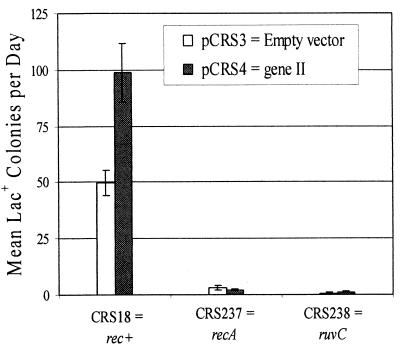
Nickase-induced adaptive Lac+ mutations are ruvC dependent.
Many of the genes encoding proteins that are involved in recombination are repressed by LexA, the repressor of the genes that constitute E. coli's SOS response to DNA damage (24). Because RecA is required to induce these genes (via its coprotease activity against LexA), loss of adaptive mutations in a recA mutant does not show definitively that the mutations are dependent on recombination. ruvC, which encodes an enzyme that resolves recombination intermediates (Holliday junctions), is not under LexA control (24). In otherwise wild-type cells, adaptive mutation to Lac+ is ruvC as well as recA dependent (23, 33). Therefore, we used a ruvC mutant strain to determine whether nickase-induced Lac+ mutations are truly recombination dependent or are simply a consequence of induction of the SOS response. As shown in Fig. 6, all nickase-induced Lac+ mutations were dependent on ruvC+ as well as on recA+ and thus require the resolution of a Holliday junction to be produced or retained.
FIG. 6.
Nickase-induced Lac+ mutations are both recA+ and ruvC+ dependent. The bars show the daily accumulation of Lac+ colonies during incubation on lactose plates from days 3 to 5 during a small-scale experiment. The cultures were grown to saturation in M9-glycerol medium plus antibiotics. White, cells carrying pCRS3, the empty vector; dark grey, cells carrying pCRS4, the plasmid with gene II. Parental strains were CRS18 (FC40 with the gIIp nick site), CRS237 (FC40 recA938::Cm with the gIIp nick site), and CRS238 (FC40 ruvC::Km with the gIIp nick site). Data are the means and standard errors of the means of three or four cultures of each strain counted daily for 3 days. Some error bars are too small to be seen.
Nickase-induced Lac+ mutations are stable.
Most of the Lac+ colonies that appear on lactose plates during an adaptive mutation experiment are composed of cells that have reverted the Lac− allele. However, a few Lac+ colonies appear that are composed of cells that have amplified the unreverted Lac− allele. These make up only a small percentage of the Lac+ colonies during a normal 5-day experiment (13, 18, 19) but can represent nearly 50% of the Lac+ colonies if plates are incubated for 7 to 10 days (34, 46). The hallmark of amplification is that the Lac+ phenotype is unstable (54). Instability can be detected by allowing individual Lac+ cells to grow on medium containing X-Gal and seeing if white (Lac−) sectors form within blue (Lac+) colonies.
We tested approximately 32 Lac+ colonies each from strains CRS243 (carrying the empty vector) and CRS244 (carrying the plasmid with gene II) appearing on days 4 to 6 during the experiment shown in Fig. 4, and an additional 30 colonies of each strain from a repeat of this experiment. One out of 62 colonies of CRS243 and 4 out of 62 colonies of CRS244 were unstable, a difference that is not significant (χ2 = 0.83; P = 0.36). Similar results were obtained with the traD strains: none of 21 Lac+ colonies of CRS245 (carrying the empty vector) and 2 of 32 Lac+ colonies of CRS246 (carrying the plasmid with gene II) were unstable, which was again a nonsignificant difference (χ2 = 0.19; P = 0.67). We conclude that induction of a DNA nick does not increase the number of cells that amply the Lac− allele, at least not to the extent that the cells become phenotypically Lac+.
The spectrum of nickase-induced Lac+ mutations is the same as that of adaptive mutations in wild-type cells.
Table 4 gives the sequence of 35 Lac+ mutations of CRS244 from the experiment shown in Fig. 4. As found in the wild-type strain (20, 50), the spectrum of mutations in CRS244 was dominated by −1-bp deletions in runs of iterated bases. In particular, −1-bp frameshifts at the hotspot at bp 1036, which normally account for about 50% of the adaptive mutations (18), were 53% of the nickase-induced mutations.
TABLE 4.
The spectrum of nickase-induced Lac+ mutations
| Mutationa | Site | No. of Lac+ revertants
|
||||
|---|---|---|---|---|---|---|
| Day 3 | Day 4 | Day 5 | Day 6 | Total | ||
| −G at 1020 | GGG | 2 | 1 | 3 | ||
| −C at 1036 | CCCC | 6 | 6 | 5 | 5 | 22 (53%) |
| −C at 1064 | CCC | 1 | 1 | |||
| −A at 1056 | AAAAA | 3 | 1 | 4 | ||
| −T at 1077 | T | 1 | 1 | |||
| −C at 1085 | CC | 1 | 1 | |||
| Size change | Deletionb | 1 | 1 | 2 | ||
| Other | Not in targetc | 1 | 1 | |||
| Total | 9 | 9 | 8 | 9 | 35 | |
The numbers refer to the bases in Ecolac (GenBank J01636.1), except that the extra C at 1036 to 1038, which constitutes the lacI33 mutation, is not numbered. Only the base in the coding DNA strand is given.
Detectable by a mobility shift of the PCR product.
DISCUSSION
Expressing the bacteriophage f1 gIIp in a strain carrying the gIIp nick site near the revertible lac allele stimulated Lac+ adaptive mutation. In addition, induction of the nickase was able to restore mutation to a conjugal-defective traD mutant. These results support the hypothesis that the recombination events required for adaptive mutation are normally initiated by a DNA single-strand nick produced by conjugal proteins at oriT, the conjugal origin of the F′ episome (23, 35). Further, nicking appears to be the most important, and perhaps the only, conjugal function required for adaptive mutation in strain FC40.
oriT is about 80 kb away from the lacI33 site (P. L. Foster, unpublished data), whereas we placed the gIIp nick site about 600 bp away from lacI33. Although the oriT and gIIp nicks are on the same DNA strand, they are on opposite sides of the lacI33 site (dividing the episome at its vegetative origins). It will be interesting to see if the adaptive mutation rate varies as a function of distance and/or orientation relative to the site of mutation.
It may appear surprising that the nicking function of gIIp can, at least partially, substitute for the loss of TraD in adaptive mutation. The traD allele that we used is not polar (K. Ippen-Ihler, personal communication), and TraD is not required for nicking by TraI, the enzyme that nicks at oriT (12). However, TraI is stimulated by TraM (37), and TraM interacts with and may be activated by TraD (10). Thus, our finding that the traD defect can be complemented for adaptive mutation by an exogenous nicking system supports the idea that TraD is required for the maximal level of nicking at oriT in vivo.
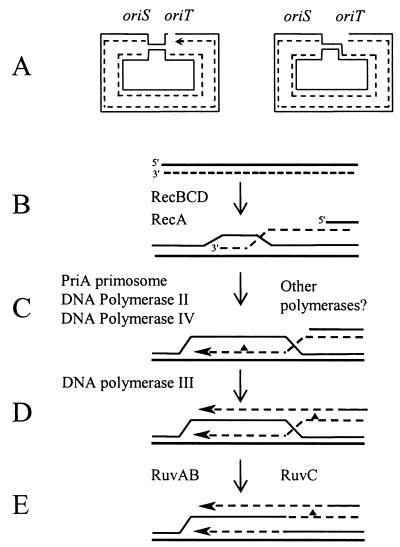
Adaptive mutations in FC40 are dependent on the recombination pathway that repairs DNA DSBs (19, 32). This requirement, plus the requirement for conjugal functions but not actual conjugation (21), were reconciled by Kuzminov (38), who proposed that when a replication fork encountered the persistent nick at oriT, the fork collapses, producing a DSB (Fig. 7A). Repair of this DSB by recombination with the sister chromosome would reestablish the replication fork (Fig. 7B to D). We have further postulated (16) that the reestablished replisome initially contains not the normal replicative DNA polymerase, DNA Pol III, but either DNA Pol II or DNA Pol IV. If accurate Pol II gains access, the new DNA synthesis is error free; if error prone Pol IV gains access, a track of inaccurate DNA synthesis is produced, accounting for some (but not all) of the adaptive mutations. Eventually DNA Pol III continues DNA synthesis, and the errors that it makes account for the rest of the mutations (Fig. 7D). The results presented here support the hypothesis that the initiating event is a DNA nick.
FIG. 7.
A model for adaptive mutation in E. coli strain FC40. (A) When incubated in lactose, replication is occasionally initiated at the unidirectional vegetative origin, oriS, or at the bidirectional vegetative origin, oriV (not shown). The persistent nick at the conjugal origin, oriT, causes the replication fork to collapse, creating a double-strand DNA end. New DNA is indicated as a dashed line; the arrow marks the 3′ end. (B) The double-strand DNA end is a substrate for recombinational repair. RecA and RecBCD catalyze invasion of the 3′ end into the homologous duplex of the sister chromosome (or another episome.) (C) A “restart” replication complex is assembled, consisting of the primosome proteins, including PriA (9) and either DNA polymerase II (48) or IV (16). If Pol IV gains access to the DNA, it frequently makes an error, symbolized by a triangle. Other polymerases may also be involved. (D) The Pol III replication fork is reestablished. (Note: Pol III also can make replication errors.) (E) The Holliday junction is resolved and the error is preserved.
Our results also support a different mechanism for adaptive reversion that involves amplification. First proposed in 1992 (17), amplification of the nonreverted Lac− allele has been shown to account for some, but not all, Lac+ colonies that appear during lactose selection (13, 34, 46). But Roth and colleagues (1, 35) have proposed that amplification is a necessary precondition for all Lac+ revertants. According to their model, nicking at oriT leads to amplification of the lac allele and cells with amplifications produce slowly growing clones on lactose medium. True Lac+ mutations then appear among the amplified arrays, and the arrays then disappear (35). Although the data presented here do not distinguish between the Roth model and the model presented in Fig. 7, the fact that exogenous nicking increased adaptive mutation without significantly increasing the number of unstable Lac+ colonies (see above) argues against the importance of amplification.
When the Φ(lacI33-lacZ) allele is transferred to its normal location on the chromosome, the rate of adaptive mutation drops 100-fold and the mutations are no longer recA dependent (21, 47). We attempted to stimulate adaptive mutation on the chromosome by transferring the DNA region carrying Φ(lacI33-lacZ) and the gIIp target site to the chromosome, and then supplying gIIp from plasmid pCRS4. These experiments were unsuccessful—no additional mutations were induced in the presence of gene II with or without arabinose. Two possible reasons that this experiment failed are (i) nicking does not stimulate mutation on the chromosome to an extent that can be detected (at least at the site monitored), and (ii) gIIp cannot act at the site on the chromosome where its recognition sequence was placed. We eliminated a third possibility, that other conjugal functions are required for adaptive mutation, by supplying all the conjugal functions from an additional plasmid and showing that adaptive mutation on the chromosome did not increase (data not shown). It has been previously suggested that the E. coli chromosome has both hot and cold spots for recombination-dependent mutation (55)
Finally, we found that prolonged induction of gene II was highly detrimental, resulting in filamentous cells and few Lac+ mutations. However, in recA cells, prolonged induction resulted in a small increase (about threefold) in lac reversion (Fig. 3). This was true whether the recA cells were traD+ or traD deficient. We interpret this result to mean two things. First, the detrimental effects of continuous induction of gIIp depend on RecA function. It is likely that continuous DNA nicking induces the SOS response and consequent lethal filamentation. As mentioned above, RecA is required to induce the SOS response and may also be required to process the nicked DNA into the SOS-inducing signal, single-stranded DNA. Second, when the DNA is nicked there is at least one pathway for adaptive mutation that is independent of RecA. However, this pathway is normally a minor contributor to adaptive mutation.
Acknowledgments
We thank L.-M. Guzman, K. A. Ippen-Ihler (deceased), R. G. Lloyd, M. G. Marinus, J. H. Miller, P. Model, G. Posfai, and J. N. Strathern for bacterial and phage strains and plasmids. A. J. Hanson, E. A. Housworth, and F. W. Stahl kindly helped us with the statistical analysis. We thank J. Cairns for his continuing interest and collaboration.
This work was supported by NSF grant MCB-9996308 to P.L.F. C.R. received a scholarship from the Direccion General de Asuntos del Personal Academico of the Universidad Nacional Autonoma de Mexico.
REFERENCES
- 1.Andersson, D. I., E. S. Slechta, and J. R. Roth. 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282:1133-1135. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1988. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heynecker, H. W. Boyer, J. H. Crosa, and S. Falkow. 1977. Construction and characterization of new cloning vehicles II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 4.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns, J., J. Overbaugh, and S. Miller. 1988. The origin of mutants. Nature 335:142-145. [DOI] [PubMed] [Google Scholar]
- 6.Calos, M. P., and J. H. Miller. 1981. Genetic and sequence analysis of frameshift mutations induced by ICR-191. J. Mol. Biol. 153:39-66. [DOI] [PubMed] [Google Scholar]
- 7.Carter, J. R., D. R. Patel, and R. D. Porter. 1992. The role of oriT in tra-dependent enhanced recombination between mini-F-lac-oriT and lambda plac5. Genet. Res. 59:157-165. [DOI] [PubMed] [Google Scholar]
- 8.Carter, J. R., and R. D. Porter. 1991. traY and traI are required for oriT-dependent enhanced recombination between lac-containing plasmids and lambda plac5. J. Bacteriol. 173:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, M. M. 2001. Recombinational DNA repair of damaged replication forks in Escherichia coli: questions. Annu. Rev. Genet. 35:53-82. [DOI] [PubMed] [Google Scholar]
- 10.Disque-Kochem, C., and B. Dreiseikelmann. 1997. The cytoplasmic DNA-binding protein TraM binds to the inner membrane protein TraD in vitro. J. Bacteriol. 179:6133-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dotto, G. P., K. Horiuchi, K. S. Jakes, and N. D. Zinder. 1982. Replication origin of bacteriophage f1, two signals required for its function. J. Mol. Biol. 162:335-343. [DOI] [PubMed] [Google Scholar]
- 12.Everett, R., and N. Willetts. 1980. Characterization of an in vivo system for nicking at the origin of conjugal DNA transfer of the sex factor F. J. Mol. Biol. 136:129-150. [DOI] [PubMed] [Google Scholar]
- 13.Foster, P. L. 1994. Population dynamics of a Lac− strain of Escherichia coli during selection for lactose utilization. Genetics 138:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster, P. L. 1997. Nonadaptive mutations occur on the F′ episome during adaptive mutation conditions in Escherichia coli. J. Bacteriol. 179:1550-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster, P. L. 1999. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu. Rev. Genet. 33:57-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster, P. L. 2000. Adaptive mutation in Escherichia coli. Cold Spring Harbor Symp. Quant. Biol. 65:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster, P. L., and J. Cairns. 1992. Mechanisms of directed mutation. Genetics 131:783-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, P. L., G. Gudmundsson, J. M. Trimarchi, H. Cai, and M. F. Goodman. 1995. Proofreading-defective DNA polymerase II increases adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:7951-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster, P. L., and W. A. Rosche. 1999. Increased episomal replication accounts for the high rate of adaptive mutation in recD mutants of Escherichia coli. Genetics 152:15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster, P. L., and J. M. Trimarchi. 1994. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science 265:407-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster, P. L., and J. M. Trimarchi. 1995. Adaptive reversion of an episomal frameshift mutation in Escherichia coli requires conjugal functions but not actual conjugation. Proc. Natl. Acad. Sci. USA 92:5487-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster, P. L., and J. M. Trimarchi. 1995. Conjugation is not required for adaptive reversion of an episomal frameshift mutation in Escherichia coli. J. Bacteriol. 177:6670-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster, P. L., J. M. Trimarchi, and R. A. Maurer. 1996. Two enzymes, both of which process recombination intermediates, have opposite effects on adaptive mutation in Escherichia coli. Genetics 142:25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. American Society for Microbiology, Washington, D.C.
- 25.Frost, L. S., and J. Manchak. 1998. F− phenocopies: characterization of expression of the F transfer region in stationary phase. Microbiology 144:2579-2587. [DOI] [PubMed] [Google Scholar]
- 26.Fulford, W., and P. Model. 1988. Regulation of bacteriophage f1 DNA replication I. New functions for genes II and X. J. Mol. Biol. 203:49-62. [DOI] [PubMed] [Google Scholar]
- 27.Galitski, T., and J. R. Roth. 1995. Evidence that F plasmid transfer replication underlies apparent adaptive mutation. Science 268:421-423. [DOI] [PubMed] [Google Scholar]
- 28.Galitski, T., and J. R. Roth. 1996. A search for a general phenomenon of adaptive mutability. Genetics 143:645-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galli, A., and R. H. Schiestl. 1998. Effects of DNA double-strand and single-strand breaks on intrachromosomal recombination events in cell-cycle-arrested yeast cells. Genetics 149:1235-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godoy, V. G., and M. S. Fox. 2000. Transposon stability and a role for conjugational transfer in adaptive mutability. Proc. Natl. Acad. Sci. USA 97:7393-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzman, L.-M., D. Belin, M. J. Carson, and J. R. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris, R. S., S. Longerich, and S. M. Rosenberg. 1994. Recombination in adaptive mutation. Science 264:258-260. [DOI] [PubMed] [Google Scholar]
- 33.Harris, R. S., K. J. Ross, and S. M. Rosenberg. 1996. Opposing roles of the Holliday junction processing systems of Escherichia coli in recombination-dependent adaptive mutation. Genetics 142:681-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hastings, P. J., H. J. Bull, and S. M. Rosenberg. 2000. Adaptive amplification: an inducible chromosomal instability mechanism. Cell 103:723-731. [DOI] [PubMed] [Google Scholar]
- 35.Hendrickson, H., E. S. Slechta, U. Bergthorsson, D. I. Andersson, and J. R. Roth. 2002. Amplification-mutagenesis: evidence that “directed” adaptive mutation and general hypermutability result from growth with a selected gene amplification. Proc. Natl. Acad. Sci. USA 99:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kathir, P., and K. Ippen-Ihler. 1991. Construction and characterization of derivatives carrying insertion mutations in F plasmid transfer region gene trbA, artA, traQ, and trbB. Plasmid 26:40-54. [DOI] [PubMed] [Google Scholar]
- 37.Kupelwieser, G., M. Schwab, G. Hogenaue, G. Koraimann, and E. L. Zechner. 1998. Transfer protein TraM stimulates TraI-catalyzed cleavage of the transfer origin of plasmid R1 in vivo. J. Mol. Biol. 275:82-94. [DOI] [PubMed] [Google Scholar]
- 38.Kuzminov, A. 1995. Collapse and repair of replication forks in Escherichia coli. Mol. Microbiol. 16:373-384. [DOI] [PubMed] [Google Scholar]
- 39.Mandal, T. N., A. A. Mahdi, G. J. Sharples, and R. G. Lloyd. 1993. Resolution of Holliday intermediates in recombination and DNA repair: indirect suppression of ruvA, ruvB, and ruvC mutations. J. Bacteriol. 175:4325-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, J. H. 1985. Mutagenic specificity of ultraviolet light. J. Mol. Biol. 182:45-65. [DOI] [PubMed] [Google Scholar]
- 41.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Newman, J. R., and C. Fuqua. 2002. Broad-host-range expression vectors that carry the L-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene 227:197-203. [DOI] [PubMed] [Google Scholar]
- 43.Porter, R. D., and K. B. Low. 1981. Specialized transduction with λplac5: dependence on recA and on configuration of lac and attλ. J. Virol. 38:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posfai, G., V. Kolisnychenko, Z. Bereczki, and F. R. Blattner. 1999. Markerless gene replacement in Escherichia coli stimulated by a double-strand break in the chromosome. Nucleic Acids Res. 27:4409-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Posfai, G., M. D. Koob, H. A. Kirkpatrick, and F. R. Blattner. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J. Bacteriol. 179:4426-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell, S. C., and R. M. Wartell. 2001. Different characteristics distinguish early versus late arising adaptive mutations in Escherichia coli FC40. Mutat. Res. 473:219-228. [DOI] [PubMed] [Google Scholar]
- 47.Radicella, J. P., P. U. Park, and M. S. Fox. 1995. Adaptive mutation in Escherichia coli: a role for conjugation. Science 268:418-420. [DOI] [PubMed] [Google Scholar]
- 48.Rangarajan, S., R. Woodgate, and M. F. Goodman. 2000. A phenotype for enigmatic DNA polymerase II: a pivotal role for Pol II in replication restart in UV-irradiated Escherichia coli. Proc. Natl. Acad. Sci. USA 96:9224-9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice, J. A. 1995. Mathematical statistics and data analysis. Wadsworth Publishing Company, Belmont, Calif.
- 50.Rosenberg, S. M., S. Longerich, P. Gee, and R. S. Harris. 1994. Adaptive mutation by deletions in small mononucleotide repeats. Science 265:405-407. [DOI] [PubMed] [Google Scholar]
- 51.Shapira, S. K., J. Chou, F. V. Richaud, and M. J. Casadaban. 1983. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of β-galactosidase. Gene 25:71-82. [DOI] [PubMed] [Google Scholar]
- 52.Strathern, J. N., K. G. Weinstock, D. R. Higgins, and C. B. McGill. 1991. A novel recombinator in yeast based on gene II protein from bacteriophage f1. Genetics 127:61-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taddei, F., I. Matic, and M. Radman. 1995. cAMP-dependent SOS induction and mutagenesis in resting bacterial populations. Proc. Natl. Acad. Sci. USA 92:11736-11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tlsty, D. T., A. M. Albertini, and J. H. Miller. 1984. Gene amplification in the lac region of E. coli. Cell 37:217-224. [DOI] [PubMed] [Google Scholar]
- 55.Torkelson, J., R. S. Harris, M.-J. Lombardo, J. Nagendran, C. Thulin, and S. M. Rosenberg. 1997. Genome-wide hypermutation in a subpopulation of stationary-phase cells underlies recombination-dependent adaptive mutation. EMBO J. 16:3303-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wertman, K. F., A. R. Wyman, and D. Botstein. 1986. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene 49:253-262. [DOI] [PubMed] [Google Scholar]
- 57.Zar, J. H. 1984. Biostatistical analysis. Prentice Hall, Englewood Cliffs, N.J.
- 58.Zinder, N. D., and K. Horiuchi. 1985. Multiregulatory element of filamentous bacteriophages. Microbiol. Rev. 49:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]