Abstract
Sinorhizobium meliloti is a soil bacterium which can establish a nitrogen-fixing symbiosis with the legume Medicago sativa. Recent work has identified a pair of genes, sinR and sinI, which represent a potential quorum-sensing system and are responsible for the production of N-acyl homoserine lactones (AHLs) in two S. meliloti strains, Rm1021 and Rm41. In this work, we characterize the sinRI locus and show that these genes are responsible for the synthesis of several long-chain AHLs ranging from 12 to 18 carbons in length. Four of these, 3-oxotetradecanoyl HL, 3-oxohexadecenoyl HL, hexadecenoyl HL, and octadecanoyl HL, have novel structures. This is the first report of AHLs having acyl chains longer than 14 carbons. We show that a disruption in sinI eliminates these AHLs and that a sinR disruption results in only basal levels of the AHLs. Moreover, the same sinI and sinR mutations also lead to a decrease in the number of pink nodules during nodulation assays, as well as a slight delay in the appearance of pink nodules, indicating a role for quorum sensing in symbiosis. We also show that sinI and sinR mutants are still capable of producing several short-chain AHLs, one of which was identified as octanoyl HL. We believe that these short-chain AHLs are evidence of a second quorum-sensing system in Rm1021, which we refer to here as the mel system, for “S. meliloti.”
Quorum sensing is a widespread phenomenon among gram-negative bacteria (for recent reviews see references 9, 16, and 33). This form of cell density-dependent gene regulation is mediated by sensing the concentrations of low-molecular-weight compounds called autoinducers, which are produced by the bacteria. A few organisms such as Photobacterium fischeri and Pseudomonas aeruginosa serve as model systems for quorum sensing. P. fischeri, in which autoinduction was first identified, has a relatively simple quorum-sensing system. An autoinducer synthase, LuxI, produces the N-(3-oxohexanoyl)-l-homoserine lactone (oxo-C6-HL), which is recognized by the LuxR regulator (11, 22, 25). LuxR then induces expression of the lux operon, which causes bioluminescence (47, 48). This model has recently become slightly more complicated with the identification of a second autoinducer synthase, AinS, and the regulatory protein LuxO, both of which serve to modulate quorum-sensing-induced luminescence in P. fischeri (25, 26, 34). On the other hand, P. aeruginosa has two quorum-sensing systems (lasR/lasI and rhlR/rhlI) organized into a complex hierarchy, which together regulate numerous genes required for virulence (2, 36).
The most common and well-characterized gram-negative bacterial autoinducers are N-acyl homoserine lactones (AHLs) (see above reviews). AHLs consist of a variable acyl chain attached to a conserved homoserine lactone head group. The acyl chains can vary in length from 4 to 14 carbons. They can also vary in the nature of the substituent on the third carbon, from hydrogen to a hydroxyl or oxo group. One last characteristic that can confer variability is the presence of a double bond in the acyl chain. The largest AHLs identified so far carry 14 carbon acyl chains and are synthesized by the soil bacteria Rhizobium leguminosarum, Pseudomonas fluorescens, and Rhodobacter sphaeroides (27, 38, 43).
The production of AHLs by several rhizobial strains was identified several years ago in survey studies (6, 46). Further research has begun to characterize quorum sensing in Rhizobium etli and R. leguminosarum and the role that it plays in the symbiotic relationship with their legume hosts. Both R. etli and R. leguminosarum were shown elsewhere to produce several AHLs, including the growth-inhibiting AHL termed bacteriocin small (6, 41, 43, 46). The production of small in both species is controlled by the cinR and cinI genes; however, the structure of the AHL is slightly different between the species. R. leguminosarum produces N-3-hydroxy-7-cis-tetradecenoyl-l-homoserine lactone (OH-C14:1-HL), while R. etli produces a partially characterized saturated long-chain 3-hydroxy-acyl homoserine lactone (8, 29, 43). In R. leguminosarum, cinRI influences the synthesis of other AHLs as well as plasmid transfer (29). For R. etli, cinRI was shown elsewhere to be expressed during symbiosis and also to be involved in bacteroid development and nitrogen fixation (8).
Sinorhizobium meliloti is a gram-negative soil bacterium capable of establishing a symbiotic relationship with the alfalfa plant, Medicago sativa. A complex sequence of signals between the plant and the bacteria is required for the successful development of nodules and subsequent nitrogen fixation (for reviews see references 3 and 49). As quorum sensing has been shown to regulate aspects of symbiosis in R. leguminosarum and R. etli, it is possible that a similar scenario exists in S. meliloti. To address this possibility, our lab has begun characterization of quorum sensing in two commonly studied strains of S. meliloti, AK631 and Rm1021 (32). Thin-layer chromatography (TLC) analysis with the Chromobacterium and Agrobacterium indicator strains showed the presence of several AHLs in AK631 and Rm1021 culture extracts. We identified the presence of two quorum-sensing systems (the tra system and the sin system) in S. meliloti strain AK631 (32). The tra system, named for its homology to the tra systems in Agrobacterium tumefaciens and Rhizobium strain NGR234, resides on a large plasmid called pRme41a. This plasmid is unique to Rm41 (the AK631 parent strain) and therefore represents a quorum-sensing system that is present in AK631 but not Rm1021. The sin system, named for S. meliloti, is present in both AK631 and Rm1021 and is located on the chromosome. Since Rm1021 carries the sin system, but not the tra system, it produces only a subset of the AHLs made by AK631. In this study, we further characterize AHL production in Rm1021. We show that the sinR/sinI locus is responsible for the production of several novel AHLs, which have acyl side chains ranging from 12 to 18 carbons. These are the longest AHLs identified so far. Disruption of the sinI gene abolishes production of these long-chain AHLs, while synthesis of the other short-chain AHLs remains unaffected. We have identified one of these short-chain AHLs as octanoyl homoserine lactone (C8-HL) and propose that it and the other small AHLs are part of a second quorum-sensing system in Rm1021. Furthermore, we show that disruption of the sin system correlates with a delay in the appearance of nitrogen-fixing (pink) nodules, as well as an overall decrease in the number of pink nodules, indicating a role for quorum sensing in establishing a successful symbiosis with M. sativa.
MATERIALS AND METHODS
Bacterial strains and media.
Table 1 lists the strains used in this work. All S. meliloti strains were cultured in Luria-Bertani broth supplemented with 2.5 mM CaCl2 and 2.5 mM MgSO4 (LB/MC). Antibiotics were added, as appropriate, to final concentrations of 500 μg of streptomycin/ml, 200 μg of neomycin/ml, 10 μg of tetracycline/ml, and 50 μg of gentamicin/ml. Concentrations were adjusted for the combination of tetracycline (5 μg/ml) and neomycin (100 μg/ml). A. tumefaciens NTL4(pZLR4) (46) was grown on LB containing 50 μg of gentamicin/ml. Escherichia coli strains were grown on LB containing 25 μg of kanamycin/ml, 5 μg of gentamicin/ml, 10 μg of tetracycline/ml, 100 μg of ampicillin/ml, 20 μg of chloramphenicol/ml, or 100 μg of spectinomycin/ml as needed. Concentrations were adjusted for the combination of tetracycline (5 μg/ml) and kanamycin (10 μg/ml). S. meliloti and A. tumefaciens strains were grown at 30°C, while E. coli was grown at 37°C.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Sinorhizobium meliloti | ||
| Rm1021 | SU47 str-21 | 28 |
| Rm11511 | Rm1021 sinI::KM | This work |
| Rm11512 | Rm1021 sinR::GM | This work |
| Agrobacterium tumefaciens | ||
| NTL4(pZLR4) | NT1 derivative carrying a traG::lacZ reporter fusion | 31 |
| Escherichia coli | ||
| MT616 | MT607 (pRK600) | 20 |
| DH5α | See source | Life Technologies |
| Plasmids | ||
| pSinR | pPCR-Script Amp SK (+) carrying the sinR ORF | This work |
| pSinR::GM | pSinR carrying sinR::GM | This work |
| pSinRlong | pPCR-Script Amp SK (+) carrying sinR and surrounding region | This work |
| pSinRlong::GM | pSinRlong carrying sinR::GM | This work |
| pSinIlong | pPCR-Script Amp SK (+) carrying sinI and surrounding region | This work |
| pSinIlong::KM | pSinIlong carrying sinI::KM | This work |
| pJQ200SmSp | Shuttle vector carrying sacB | 32 |
| pSinIlong::KM/sacB | pJQ200SmSp carrying sinI::KM | This work |
| pSinRlong::GM/sacB | pJQ200SmSp carrying sinR::GM | This work |
Construction of sinR and sinI mutants.
The sinR and sinI mutations were created by the modified PCR described in reference 19, whereby the disrupted open reading frame (ORF) is used as the primer and is incorporated into a larger template containing the wild-type copy. To generate the sinR disruption, the sinR ORF was first amplified from Rm1021 chromosomal DNA with the following primers: 5′-ATGGCTAATCAACAGGCTGTCC-3′ and 5′-GATGGTGGGGATCAGAGCATGTCG-3′. The PCR product was polished with Pfu DNA polymerase and then ligated into the EcoRV site of pPCR-Script Amp SK (+) (Stratagene), creating pSinR. The ORF was disrupted by ligating the HindIII fragment containing the gentamicin cassette from pUC-GM (44) into the HindIII site of pSinR, creating pSinR::GM. This disrupted ORF was then reamplified and used as the primer in a modified PCR described in reference 19. The template for the modified PCR was designed by amplifying a larger region of the chromosome containing the sinR ORF with the primers 5′-AAAGGAATTCCGGCCTCAGAATCCGCC-3′ and 5′-CCGAGCAGGATCGGGAA-3′ and cloning the PCR product into pPCR-Script Amp SK (+), as described above, creating pSinRlong. The PCR conditions for the modified PCR were as follows: 5 min at 95°C; followed by 18 cycles of 30 s at 95°C, 30 s at 55°C, and 6 min at 72°C; and then a final 10-min elongation step at 72°C. The PCR product was purified, and 5 μl was transformed into DH5α. This method results in the insertion of SinR::GM into pSinRlong, creating pSinRlong::GM. The sinI disruption was constructed by first amplifying a large region containing the sinI ORF with the primers 5′-CCTCGAAGAGGGGCTCG-3′ and 5′-GGCGGATCCCCTGCAGCGGCTGGTAACG-3′ and cloning the PCR product into pPCR-Script Amp SK (+), as described above, creating pSinIlong, which was used as the template for the modified PCR. Next, the kanamycin cassette from Tn5 was amplified, generating ends homologous to sinI, by using the following primers: 5′-GGAATCGAGCCGCTTCTGCATCCAAGCACTCAGGGCGCAAGGGCTG-3′ and 5′-ATGCGCGATCCTGGGAGATTTCGGGGGGTGGGCGAAGAACTCCAGCAT-3′. This PCR product was used as the primer along with the pSinIlong template in the modified PCR described above to generate pSinIlong::KM. Next, pJQ200SmSp was digested with BamHI and filled in with Klenow fragment. The SinIlong::KM and SinRlong::GM disruptions were then amplified with PfuTurbo; cloned into pJQ200SmSp, creating pSinIlong::KM/sacB and pSinRlong::GM/sacB; and transformed into DH5α. These constructs were used to transfer the mutations to Rm1021 via a triparental mating with MT616 as the helper strain (20). S. meliloti clones carrying the chromosomal disruption were selected by plating them on media containing the appropriate antibiotics and 5% sucrose (39). The resulting strains, Rm11511 and Rm11512, carry the sinI::KM and sinR::GM disruptions, respectively. An Rm1021 library was made by digesting Rm1021 chromosomal DNA with EcoRI and ligating it into the EcoRI site of pLAFR1. A complementing plasmid (p4B) carrying the sinRI locus was isolated by screening the library by PCR for the presence of sinI and sinR.
Preparation and TLC analysis of crude AHL preparations.
Five-milliliter S. meliloti cultures were grown to saturation (optical density at 600 nm of approximately 2.4). Whole cultures (cells and supernatant) were extracted with an equal volume of dichloromethane. The extracts were dried and resuspended in 500 μl of dichloromethane. The AHLs were analyzed by TLC and with the A. tumefaciens indicator strain NTL4(pZLR4) as described previously (32).
The following AHL standards were purchased from Sigma: N-(3-oxo-hexanoyl)-dl-homoserine lactone (oxo-C6-HL), N-hexanoyl-dl-homoserine lactone (C6-HL), and N-octanoyl-dl-homoserine lactone (C8-HL).
In vivo labeling of AHLs.
AHLs produced by Rm1021 and its derivatives were labeled by a previously described method (12, 42). Five milliliters of supplemented minimal medium (22 g of Na2HPO4, 6 g of KH2PO4, 1 g of NaCl, 27.8 mg of CaCl2, 246 mg of MgSO4, 2 g of NH4Cl, 4 g of glucose, and 1 mg of biotin per liter) was inoculated with 50 μl of an overnight culture and grown at 30°C. Growth was monitored by measuring the optical density at 600 nm. At approximately late log phase, 5 μCi of [α-14C]methionine was added, and the cells were incubated with the label at 30°C for 4 h. The labeled AHLs were extracted from whole cultures with ethyl acetate. The entire extract was separated on a reverse-phase C18 high-pressure liquid chromatography (HPLC) column (Varian) with a linear gradient from 0 to 95% methanol at a flow rate of 1 ml/min. One-milliliter fractions were collected, scintillation fluid (ScintiSafe Plus) was added, and each fraction was analyzed in a scintillation counter (Beckman LS 6500).
Purification of AHLs.
A 3-liter culture of Rm1021 was grown in LB/MC to an optical density at 600 nm of 2.4 and extracted with an equal volume of dichloromethane. The extract was dried by rotary evaporation, resuspended in approximately 10 ml of dichloromethane, and dried again in a Speed Vac (Labconco). The residue was finally resuspended in 1 ml of dichloromethane. Half of this concentrated extract was separated on a reverse-phase C18 column as described above, and the fractions were dried in a Speed Vac. The fractions were tested for activity with the Agrobacterium indicator, and the active fractions were analyzed by mass spectrometry.
Identification of AHLs.
Electrospray ionization mass spectra (ESI MS/MS) were recorded with a Micromass (Beverly, Mass.) Quattro I triple quadrupole mass spectrometer. The source temperature was 80°C. Argon was used as the collision gas, and the collision energy was kept at 21 V for all experiments. The cone was set at 18 V, and the capillary was set at 3.88 kV. Samples were infused into the electrospray source as 75% (vol/vol) acetonitrile-25% (vol/vol) 0.22 M formic acid solutions at 4 μl/min. For each spectrum, 20 scans at 3 s each were acquired and coadded by multiple channel analysis, and the resulting spectrum was smoothed. For all samples, the precursor ions of the mass 102 ion (P) as well as the precursor ion of the neutral loss of mass 101 (NL) were identified. The fragmentation spectra of all those precursor ions whose mass corresponded to those of acyl homoserine lactones with acyl groups that could be expected to be derived from fatty acid biosynthesis (35) were recorded as well and compared to the fragmentation spectra of the corresponding authentic compounds when these were available.
Plant assays.
S. meliloti strains were incubated with M. sativa on standard Jensen's medium as previously described (28). Plants were checked on a weekly basis, and after 30 days plant height was measured and pink (nitrogen-fixing) and white nodules were counted. Growth conditions were 25°C, 60% relative humidity, and a 16-h light cycle.
Growth curve analysis.
A 2-ml culture of each S. meliloti strain was inoculated with a single colony and grown in LB/MC in the presence of the appropriate antibiotics at 30°C until the culture was saturated. The strains were then subcultured (1:1,000) into LB/MC, and cell density was measured by monitoring the optical density at 600 nm. All growth curves were repeated at least twice.
RESULTS
Disruption of sinI abolishes production of long-chain AHLs.
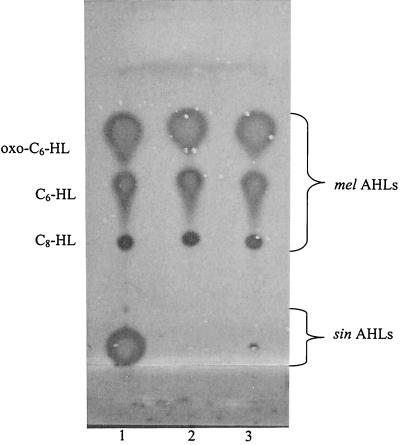
Our lab has previously identified the sinRI locus as an AHL-producing system in Rm1021 (32). This locus corresponds to the SMc00170 and SMc00168 annotations in the S. meliloti Rm1021 genome database (5, 18). We showed that the sinRI genes, when cloned into E. coli, were sufficient for the production of several AHLs (32). Although not identical, the pattern of AHLs produced by E. coli carrying the sinRI genes was very similar to the pattern of AHLs made by Rm1021. Therefore, it was suggested that the sinRI locus was responsible for all of the AHLs made by Rm1021. In this study we wanted to further characterize the sinRI locus, in order to absolutely determine which AHLs were synthesized or regulated by the sinRI gene products. To address this issue we introduced a disruption into the SinI autoinducer synthase. Figure 1, lane 1, shows that wild-type Rm1021 produced several AHLs detected by the Agrobacterium indicator. A sinI disruption (lane 2) resulted in the dramatic loss of the AHLs which migrate close to the origin of the TLC plate. Interestingly, the pattern of the short-chain AHLs did not change. A broth-only control did not show any spots (data not shown). These results indicate that SinI is responsible only for the production of the long-chain AHLs, which remain close to the TLC origin. We will refer to these as the sin AHLs for clarity. In E. coli carrying the sinRI locus, we previously observed these long-chain AHLs along with several shorter-chain AHLs (32). The observation that the pattern of the shorter AHLs did not exactly match the pattern made by Rm1021, along with the result shown here that disruption of sinI in Rm1021 did not affect the short-chain AHLs, suggests that an additional synthase is responsible for the remaining short-chain AHLs that were detected in Rm1021 sinI. The additional short-chain AHLs detected in E. coli may be an artifact resulting from expressing sinRI in a nonnative background.
FIG. 1.
Analysis of Rm1021 sinI and sinR mutations. The TLC plate was overlaid with the A. tumefaciens indicator. Lane 1, 500 μl of Rm1021 extract; lane 2, 500 μl of Rm1021 sinI extract; lane 3, 500 μl of Rm1021 sinR extract. The mobilities of AHL standards (not shown) are indicated on the left.
SinR regulates production of the sin AHLs.
To establish the role of the SinR regulator in AHL production, we introduced a disruption into sinR and analyzed the AHL pattern (Fig. 1). As with Rm1021 sinI, an Rm1021 sinR mutant (lane 3) also had a dramatic decrease in the sin AHLs; however, a faint spot could be detected near the origin. This suggests that there is some residual SinI synthase activity in the sinR mutant. This is not surprising, since the typical quorum-sensing model begins with a basal level of expression of the AHL synthase gene, which results in the eventual accumulation of AHLs and activation of the quorum-sensing system (13, 15, 17, 45). As with Rm1021 sinI, the sinR mutant also did not affect the synthesis of the short-chain AHLs. This implies that a second quorum-sensing system exists and is not under the control of the sin system. For simplicity, we will refer to this new system as the mel system (for “S. meliloti”). Further evidence for the presence of the mel system is the fact that the sin mutants were still able to activate the Agrobacterium indicator in cross streaks on petri plates (data not shown).
Labeling of the sin AHLs.
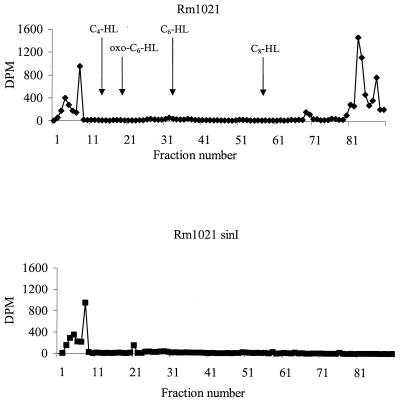
To confirm the AHL phenotypes of Rm1021 and the sinI mutant, we took advantage of a previously described in vivo labeling method (12, 42). This results in each newly synthesized AHL being labeled in the homoserine lactone ring. Figure 2 shows the labeled AHL profile for Rm1021. There were at least two prominent peaks at the end of the gradient (fractions 83 and 88) and an additional smaller peak at fraction 69. There were two peaks eluting at fractions 4 and 7, which correspond to unincorporated label. Interestingly, none of the short-chain AHLs seen on the TLC plate were labeled. Since the extracts analyzed by TLC were obtained from cultures growing in full media, while the labeling experiments were done with minimal media, it was possible that growth conditions may have altered the AHL pattern. To address this possibility, we compared the AHL pattern of Rm1021 grown in full media to the pattern when it was grown in minimal medium. In minimal medium conditions, the mel AHLs were not detected by TLC analysis (data not shown), which may explain our inability to detect them in the labeling experiment.
FIG. 2.
Profile of labeled AHLs in Rm1021 and Rm1021 sinI. Five-milliliter cultures were incubated with 5 μCi of [14C]methionine. The labeled AHLs were extracted, separated by HPLC, and then quantitated by scintillation counting. Elution times of AHL standards are indicated in parentheses: C4-HL (13 min), oxo-C6-HL (18 min), C6-HL (34 min), and C8-HL (55 min).
To verify that SinI is responsible for the production of the long-chain AHLs, we added [α-14C]methionine to Rm1021 sinI and analyzed it as described above. Figure 2 shows that the peaks at 69, 83, and 88 are missing in a sinI mutant. This profile is in agreement with the TLC pattern seen in Fig. 1. It is important to note that, while the sin AHLs correspond to a prominent spot near the origin of the TLC plate, they are resolved into at least three prominent peaks during HPLC. This suggests that sinI is capable of synthesizing more than one AHL.
Identification of AHLs produced by Rm1021.
In order to determine the identity of the sin AHLs, we grew a large volume of an Rm1021 culture and extracted it with dichloromethane. This extract was separated as described for the labeling experiments and then tested for activity with the Agrobacterium indicator. Fractions having activity were analyzed by mass spectrometry to identify their AHL structures.
The ESI MS/MS spectra of authentic AHLs showed certain consistent trends in peak height and fragmentation patterns, which aided in the identification of the AHLs produced by Rm1021. The ratio of the peak height observed for the precursor ion in the neutral loss scan (neutral loss of mass 101) to that of the precursor ion observed in the parent scan (parents of 102) was characteristic of the functionality present in the fatty acid moiety. For 2,3-unsaturated AHLs the ratio was consistently greater than 10. For unsubstituted AHLs, the ratio ranged from about 4 for short-chain acyl groups to about 0.3 for an 18-carbon chain. For 3-oxo-substituted acyl groups, the ratio was nearly 1, and for 3-hydroxy-substituted acyl groups, the ratio was considerably below 0.1. A double bond that was not conjugated to the carbonyl group seemed to have little effect on these ratios.
The fragmentation patterns of the various classes of compounds showed consistent trends as well. The 2,3-unsaturated AHLs gave a prominent peak at mass MH-101 but none at mass 102, presumably reflecting the preference towards the formation of a stable conjugated acylium ion. The unsubstituted AHLs had a small peak at mass 102 and a considerably larger one at mass MH-101. The 3-oxo-substituted AHLs showed small peaks at both mass 102 and mass MH-101. The 3-hydroxy-substituted AHLs had a peak at mass 102 and a smaller peak at mass MH-101. All compounds showed a small peak at mass MH-18, presumably representing loss of water, and one at MH-28, suggesting loss of carbon monoxide. Though none of these criteria in themselves allow the unambiguous identification of a substance giving rise to a certain peak in the ESI MS/MS, these criteria, along with the finding that the mass of the ion under question could reasonably be supposed to come from fatty acid synthesis, can allow a tentative identification, providing the means to screen crude samples quickly and with a high degree of selectivity and sensitivity. Comparison with an authentic compound then gives a definite identification.
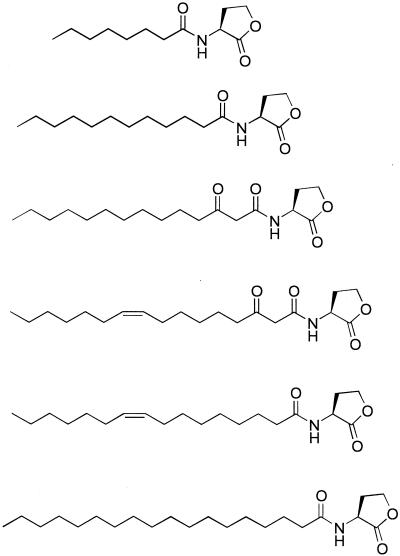
Various novel AHLs were identified in the Rm1021 HPLC fractions (Table 2). Among them is oxo-C14-HL, C16:1-HL (Fig. 3) (also reported previously by M. P. Johnston, A. Eberhard, S. Gallacher, and L. A. Glover [submitted for publication]), oxo-C16:1-HL (Fig. 4), and C18-HL. Comparison of the natural-sample spectra with those of authentic samples supports our assignments in all cases. In the cases of oxo-C14-HL and oxo-C16:1-HL, an authentic standard was not available, so the spectra were compared to those of synthetic oxo-C12-HL and oxo-C18:1-HL, respectively. The smallest AHL that was identified was octanoyl-HL (C8-HL). Accordingly, we detected a spot on the TLC plate with mobility similar to that of the C8-HL standard (Fig. 1).
TABLE 2.
Identification of AHL structures
See Results.
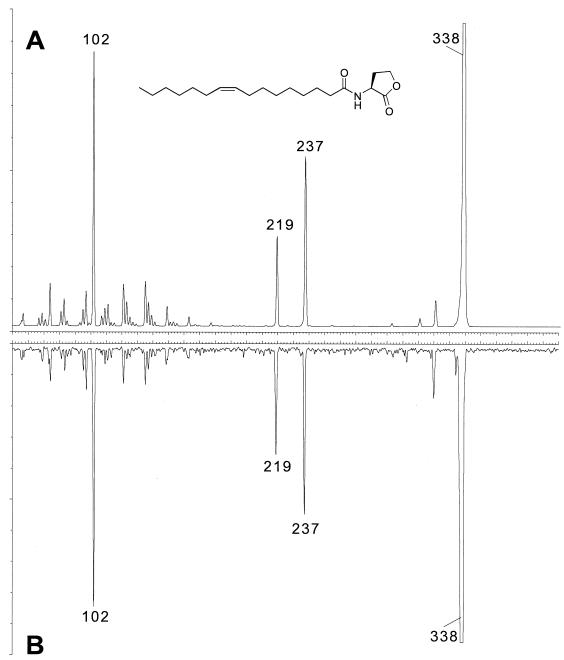
FIG. 3.
Comparison of the collisionally induced dissociation spectrum for the precursor ion observed at m/z = 338 in the natural sample to that of an authentic sample. (A) Synthetic N-(tetrahydro-2-oxo-3-furanyl)-9-cis-hexadecenamide recorded for precursor ion m/z = 338. (B) Same scan carried out on the semipurified natural material.
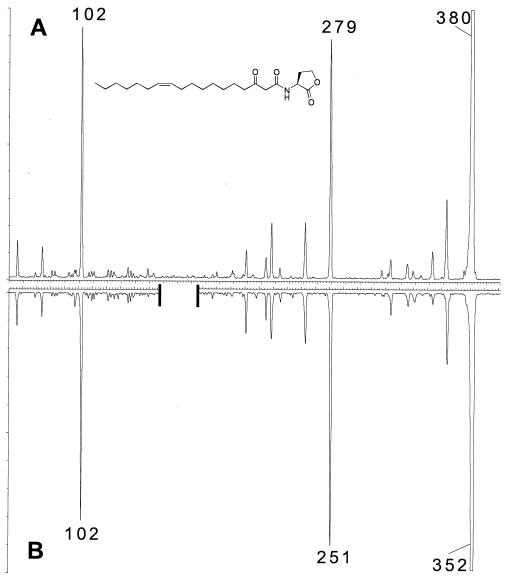
FIG. 4.
Comparison of the collisionally induced dissociation spectrum for the precursor ion observed at m/z = 352 in the natural sample to that of an analogous authentic sample (3-oxo-C18:1-HL). (A) Synthetic 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)-9-cis-octadecenamide for precursor ion m/z = 380. (B) Semipurified natural material. To allow ready visualization of the correspondence, the figure was broken by 28 mass units to account for the two extra carbon atoms that the 3-oxo-C18:1-HL authentic sample has in the region of the spectrum that involved fragmentation of the acyl chain.
Analysis of sin mutants in free-living conditions.
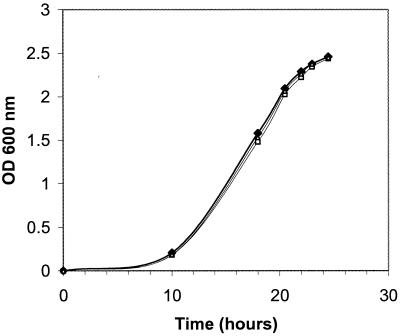
Previous research has shown that the production of OH-C14:1-HL by R. leguminosarum, as well as production of the uncharacterized saturated long-chain AHL by R. etli, plays a role in regulating growth (8, 43). These particular AHLs were shown to induce stationary phase and to contribute to survival in stationary phase. Since the sin locus makes several long-chain AHLs, including one with a 14-carbon acyl chain, we were interested to see if disrupting sinI would have an effect on the growth rate. We measured the growth of Rm1021, Rm1021 sinI, and Rm1021 sinR in LB/MC (Fig. 5). It is clear that neither of the mutations had an effect on growth in these conditions, since the growth curves were identical to that of wild-type Rm1021. Similar results were obtained with mannitol-glutamate minimal media (data not shown). This suggests that under these conditions the sin AHLs do not seem to play a role in regulating growth phase in Rm1021.
FIG. 5.
Effect of sinI and sinR disruptions on growth. All strains were grown in LB/MC, and growth was monitored by measuring the optical density at 600 nm. Symbols: solid diamonds, Rm1021; open squares, Rm1021 sinI; open triangles, Rm1021 sinR.
Effect of sinI and sinR disruptions on symbiosis.
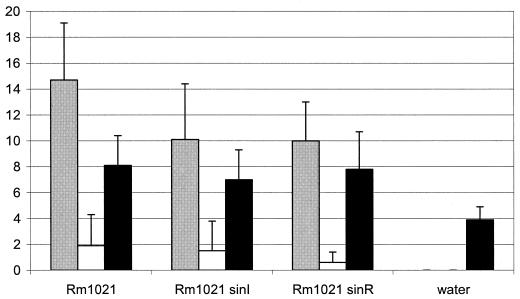
Studies of R. leguminosarum indicate that quorum sensing plays a role in symbiosis, such as controlling nodulation efficiency (7). To determine whether the sin system also has a role in symbiosis, we inoculated the sinI and sinR mutants onto M. sativa (alfalfa). Figure 6 shows the final results after incubating the plants for 1 month. While neither of the mutants had a Fix− phenotype, we did observe a slight, but consistent, decrease in the average number of pink nodules for both Rm1021 sinI and Rm1021 sinR. In addition, we monitored the progress of nodulation by checking the plants on a weekly basis. Interestingly, we found that the sinI and sinR mutants had a slight delay in the appearance of nodules (data not shown). After 1 week, plants inoculated with Rm1021 had developed numerous white nodules and a few pink nodules. Plants inoculated with the sinI and sinR mutants had few, if any, white nodules, and there were no pink nodules present. By the second week all plants had developed pink nodules; however, there were fewer pink nodules on plants inoculated with the sin mutants than on those inoculated with Rm1021. This suggests that the sinRI locus may regulate one or more genes involved in establishing a successful symbiotic relationship. Clearly, the role of the sin system, and the possible target genes of SinR, needs to be investigated to further determine the nature of quorum-sensing regulation in symbiosis.
FIG. 6.
Effect of sinI and sinR disruptions on alfalfa nodulation. Alfalfa plants were inoculated with Rm1021, Rm1021 sinI, and Rm1021 sinR. Gray bars, number of pink nodules; white bars, number of white nodules; black bars, plant height (centimeters). Data represent scoring of 20 plants per S. meliloti strain after 30 days of incubation.
DISCUSSION
Recent work in our lab identified the sinRI locus as an AHL-producing system common to S. meliloti strains AK631 and Rm1021 (32). We show here that the sinI and sinR genes are responsible for the production of several long-chain AHLs. When the autoinducer synthase sinI was disrupted, the long-chain AHLs were no longer present (Fig. 1 and 2). Likewise, disrupting the regulator, sinR, resulted in a dramatic decrease in the long-chain AHLs (Fig. 1). This is consistent with the role of SinR as a positive regulator for the sinI gene. The fact that there is some residual AHL synthesis suggests that there is a basal level of expression of the sinI gene even in the absence of SinR. This phenomenon has been observed before and is the basis for the quorum-sensing mechanism (13, 15, 17, 45). These results strongly suggest that the sinRI locus is responsible, either directly or indirectly, for the production of the long-chain AHLs. It remains a possibility that the phenotypes observed in the sin mutants may be an indirect effect.
To further characterize the AHLs made by the sinRI locus, we conducted an in vivo labeling experiment, which showed that multiple long-chain AHLs could be detected from Rm1021 cultures (Fig. 2). Subsequent mass spectrometry analysis determined the structures of these AHLs to be N-(tetrahydro-2-oxo-3-furanyl)-dodecanamide (C12-HL), 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)-tetradecanamide (oxo-C14-HL), 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)-9-cis-hexadecenamide (oxo-C16:1-HL), N-(tetrahydro-2-oxo-3-furanyl)-9-cis-hexadecenamide (C16:1-HL), and N-(tetrahydro-2-oxo-3-furanyl)-octadecanamide (C18-HL) (Table 2). To the best of our knowledge, this is the first report of naturally occurring oxo-C14-HL, oxo-C16:1-HL, and C18-HL. The C16:1-HL has also been observed in an uncharacterized marine bacterium designated 667-2 (Johnston et al., submitted). These novel AHLs are the longest ones identified to date. There may still be additional sin AHLs, which we have not been able to identify, but these will likely be minor species. Other organisms, such as R. leguminosarum, R. sphaeroides, and P. fluorescens, were shown elsewhere to produce AHLs with acyl chains that were 14 carbons in length (27, 38, 43). However, in those studies, only the culture supernatants were analyzed. Since the long-chain AHLs are highly hydrophobic, it is likely that they will partition to the cell membrane. Therefore, we reasoned that by extracting whole cultures (cells and supernatant) we would increase the recovery of long-chain AHLs. It is possible that AHLs with acyl chains longer than 14 carbons may be present in other organisms as well, and extracting whole cultures may make their recovery and detection more efficient.
In addition to the long-chain AHLs produced by the sinRI locus, Rm1021 makes several short-chain AHLs (Fig. 1). One of these AHLs was identified as N-(tetrahydro-2-oxo-3-furanyl)-octanamide (C8-HL). These AHLs seem to represent a quorum-sensing system separate from the sinRI system, since mutations in sinI and sinR had no effect on the levels of the remaining short-chain AHLs (Fig. 1). Although we have not identified the genes responsible for the synthesis of these AHLs, we have tentatively named this additional system the mel system (S. meliloti) for convenience. A search of the Rm1021 genome database did not reveal any other LuxI-type or LuxM-type AHL synthases. However, the genome does contain an ORF designated as a putative 1-acyl-sn-glycerol-3-phosphate acyltransferase (SMc00714) (5, 18). Recent work by Laue et al. showed that a homologous gene, termed hdtS, was responsible for the production of multiple AHLs in P. fluorescens (27). Since hdtS could direct AHL synthesis but was not homologous to either luxI or luxM, it was proposed that it represented a new class of AHL synthase. It is possible that the S. meliloti homolog (SMc00714) is a member of the HdtS family and is responsible for the synthesis of C8-HL and the other short-chain AHLs (25).
It is interesting that the length and variety of the acyl chains in the AHLs produced as a result of the SinI synthase activity. Clearly SinI is quite promiscuous in its ability to interact with the proper acyl carrier protein (ACP). ACPs are involved not only in the synthesis of fatty acyl chains but also in their transfer during phospholipid, lipid A, α-hemolysin (23, 24), and nodulation (Nod) factor biosynthesis (10, 30). In E. coli, all of these functions seem to be realized by a single and absolutely essential ACP, which is produced constitutively from the acpP gene (24, 40). In addition to AcpP, S. meliloti potentially encodes four additional ACPs. One of them is the gene product of nodF, which is involved, together with NodE, in the synthesis of polyunsaturated fatty acids that are host-specific substituents of Nod factors (50). The rkpF gene product in S. meliloti 41 is a novel ACP, which presumably functions, in combination with other gene products, in the synthesis of an unusual fatty acid or β-ketide (14, 37). An additional novel ACP, named AcpXL, seems to be involved in the transfer of the unusually long 27-hydroxyoctacosanoic acid to a sugar backbone during lipid A biosynthesis in R. leguminosarum (4). Finally, a fifth putative ACP is encoded by the SMc01553 locus in the S. meliloti genome (5, 18). From our work it seems that SinI produces mostly long-chain AHLs, while the putative MelI synthase is involved in the synthesis of mostly short-chain AHLs. It is possible that the SinI and MelI synthases could interact specifically with one or more of the specialized ACPs in S. meliloti. It would be interesting to determine if mutations in any of the putative ACP genes in S. meliloti (nodF, SMc01553, acpXL, or rkpF in Rm41) affect the biosynthesis of AHLs. Alternatively, the sinRI locus might regulate another synthase gene, which would direct the synthesis of one or more of the long-chain AHLs.
In addition to characterizing the sinRI locus, we are also interested in identifying potential downstream target genes which are regulated by quorum sensing. In R. leguminosarum, the long-chain AHL, OH-C14:1-HL, not only induces early onset of stationary phase but also influences nodulation efficiency (7, 21). Among the several long-chain AHLs made by S. meliloti is an oxo-C14-HL, which is similar to the OH-C14:1-HL in R. leguminosarum. However, disruption of sinI, which abolishes production of oxo-C14-HL, did not alter the dynamics of the growth curve (Fig. 5). Therefore, the long-chain AHLs do not seem to regulate growth phase in Rm1021. It should be noted, however, that the presence of the symbiotic plasmid pRL1JI is required for growth sensitivity to OH-C14:1-HL (1); therefore, we cannot rule out the possibility that the sinRI locus or the sin AHLs may regulate growth phase in other genetic backgrounds, such as Rm41. Since quorum sensing has also been shown to control aspects of symbiosis in other organisms, we decided to study the effects of the sinI and sinR disruptions on nodulation of the alfalfa plant, M. sativa. Interestingly, the sin mutants showed a slight decrease in the number of nitrogen-fixing (pink) nodules per plant, as well as a delay in the appearance of the pink nodules. These results indicate that, although nodulation is not abolished in the mutants, the process is definitely impaired. A similar phenomenon was observed for R. leguminosarum, where it was shown that the rhiABC operon is controlled by quorum sensing and, in turn, regulates nodulation efficiency (7, 21). Similarly, in R. etli the rai quorum-sensing system regulates the number of nodules, while the cin system controls symbiosome development and nitrogen fixation (8, 41). While we do not know which genes are specifically controlled by the S. meliloti sin system, our work suggests that one or more target genes are involved in establishing a successful symbiotic relationship. Further work will need to be done to identify these target genes. With the power of proteomics, it should be possible to identify genes that are expressed differently in the sin mutants compared to those in the wild type. Characterization of those genes should provide valuable insights into the role of quorum sensing in symbiosis.
Acknowledgments
We thank the members of this laboratory for their helpful discussions and insights on this project. A.E. thanks Ithaca College for a sabbatical leave for 2001-2002 and Athula Attygalle for help with ESI MS/MS. Access to the mass spectrometer was graciously provided by Jerrold Meinwald, Cornell University.
This work was supported by the National Science Foundation grant MCB-9733532 to J.E.G. This material was also based in part on work supported by the Texas Advanced Research Program under grant no. 009741-0022-2001.
REFERENCES
- 1.Brewin, N. J., J. E. Beringer, A. V. Buchanan-Wollaston, A. W. B. Johnston, and P. R. Hirsch. 1980. Transfer of symbiotic genes with bacteriocinogenic plasmids in Rhizobium leguminosarum. J. Gen. Microbiol. 116:261-270. [Google Scholar]
- 2.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broughton, W. J., S. Jabbouri, and X. Perret. 2000. Keys to symbiotic harmony. J. Bacteriol. 182:5641-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brozek, K. A., R. W. Carlson, and C. R. Raetz. 1996. A special acyl carrier protein for transferring long hydroxylated fatty acids to lipid A in Rhizobium. J. Biol. Chem. 271:32126-32136. [DOI] [PubMed] [Google Scholar]
- 5.Capela, D., F. Barloy-Hubler, J. Gouzy, G. Bothe, F. Ampe, J. Batut, P. Boistard, A. Becker, M. Boutry, E. Cadieu, S. Dreano, S. Gloux, T. Godrie, A. Goffeau, D. Kahn, E. Kiss, V. Lelaure, D. Masuy, T. Pohl, D. Portetelle, A. Pühler, B. Purnelle, U. Ramsperger, C. Renard, P. Thebault, M. Vandenbol, S. Weidner, and F. Galibert. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. USA 98:9877-9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 7.Cubo, M. T., A. Economou, G. Murphy, A. W. Johnston, and J. A. Downie. 1992. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J. Bacteriol. 174:4026-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels, R., D. E. De Vos, J. Desair, G. Raedschelders, E. Luyten, V. Rosemeyer, C. Verreth, E. Schoeters, J. Vanderleyden, and J. Michiels. 2002. The cin quorum sensing locus of Rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J. Biol. Chem. 277:462-468. [DOI] [PubMed] [Google Scholar]
- 9.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denarie, J., F. Debelle, and J. C. Prome. 1996. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65:503-535. [DOI] [PubMed] [Google Scholar]
- 11.Eberhard, A., A. L. Burlingame, C. Eberhard, G. L. Kenyon, K. H. Nealson, and N. J. Oppenheimer. 1981. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20:2444-2449. [DOI] [PubMed] [Google Scholar]
- 12.Eberhard, A., T. Longin, C. A. Widrig, and S. J. Stranick. 1991. Synthesis of the lux gene autoinducer in Vibrio fischeri is positively autoregulated. Arch. Microbiol. 155:294-297. [Google Scholar]
- 13.Engebrecht, J., K. Nealson, and M. Silverman. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773-781. [DOI] [PubMed] [Google Scholar]
- 14.Epple, G., K. M. van der Drift, J. E. Thomas-Oates, and O. Geiger. 1998. Characterization of a novel acyl carrier protein, RkpF, encoded by an operon involved in capsular polysaccharide biosynthesis in Sinorhizobium meliloti. J. Bacteriol. 180:4950-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuqua, C., and E. P. Greenberg. 1998. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1:183-189. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 17.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galibert, F., T. M. Finan, S. R. Long, A. Pühler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 19.Geiser, M., R. Cebe, D. Drewello, and R. Schmitz. 2001. Integration of PCR fragments at any specific site within cloning vectors without the use of restriction enzymes and DNA ligase. BioTechniques 31:88-92. [DOI] [PubMed] [Google Scholar]
- 20.Glazebrook, J., and G. C. Walker. 1991. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 204:398-418. [DOI] [PubMed] [Google Scholar]
- 21.Gray, K. M., J. P. Pearson, J. A. Downie, B. E. Boboye, and E. P. Greenberg. 1996. Cell-to-cell signaling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of a stationary phase and rhizosphere-expressed genes. J. Bacteriol. 178:372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanzelka, B. L., and E. P. Greenberg. 1995. Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J. Bacteriol. 177:815-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Issartel, J. P., V. Koronakis, and C. Hughes. 1991. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature 351:759-761. [DOI] [PubMed] [Google Scholar]
- 24.Jackowski, S., J. E. J. Cronan, and C. O. Rock. 1991. Lipid metabolism in procaryotes, p. 43-85. In J. E. Vance and D. E. Vance (ed.), Biochemistry of lipids, lipoproteins and membranes. Elsevier, Amsterdam, The Netherlands.
- 25.Kuo, A., N. V. Blough, and P. V. Dunlap. 1994. Multiple N-acyl-l-homoserine lactone autoinducers of luminescence in the marine symbiotic bacterium Vibrio fischeri. J. Bacteriol. 176:7558-7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo, A., S. M. Callahan, and P. V. Dunlap. 1996. Modulation of luminescence operon expression by N-octanoyl-l-homoserine lactone in ainS mutants of Vibrio fischeri. J. Bacteriol. 178:971-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laue, B. E., Y. Jiang, S. R. Chhabra, S. Jacob, G. S. Stewart, A. Hardman, J. A. Downie, F. O'Gara, and P. Williams. 2000. The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N-(3-hydroxy-7-cis-tetradecenoyl)homoserine lactone, via HdtS, a putative novel N-acylhomoserine lactone synthase. Microbiology 146:2469-2480. [DOI] [PubMed] [Google Scholar]
- 28.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 82:6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lithgow, J. K., A. Wilkinson, A. Hardman, B. Rodelas, F. Wisniewski-Dye, P. Williams, and J. A. Downie. 2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 37:81-97. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Lara, I. M., and O. Geiger. 2000. Expression and purification of four different rhizobial acyl carrier proteins. Microbiology 146:839-849. [DOI] [PubMed] [Google Scholar]
- 31.Luo, Z. Q., Y. Qin, and S. K. Farrand. 2000. The antiactivator TraM interferes with the autoinducer-dependent binding of TraR to DNA by interacting with the C-terminal region of the quorum-sensing activator. J. Biol. Chem. 275:7713-7722. [DOI] [PubMed] [Google Scholar]
- 32.Marketon, M. M., and J. E. González. 2002. Identification of two quorum-sensing systems in Sinorhizobium meliloti. J. Bacteriol. 184:3466-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto, C. M., Y. H. Lin, and E. A. Meighen. 2000. Control of bioluminescence in Vibrio fischeri by the LuxO signal response regulator. Mol. Microbiol. 36:594-607. [DOI] [PubMed] [Google Scholar]
- 35.More, M. I., L. D. Finger, J. L. Stryker, C. Fuqua, A. Eberhard, and S. C. Winans. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272:1655-1658. [DOI] [PubMed] [Google Scholar]
- 36.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 37.Petrovics, G., P. Putnoky, B. Reuhs, J. Kim, T. A. Thorp, K. D. Noel, R. W. Carlson, and A. Kondorosi. 1993. The presence of a novel type of surface polysaccharide in Rhizobium meliloti requires a new fatty acid synthase-like gene cluster involved in symbiotic nodule development. Mol. Microbiol. 8:1083-1094. [DOI] [PubMed] [Google Scholar]
- 38.Puskas, A., E. P. Greenberg, S. Kaplan, and A. L. Schaefer. 1997. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 179:7530-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 40.Rawlings, M., and J. E. Cronan, Jr. 1992. The gene encoding Escherichia coli acyl carrier protein lies within a cluster of fatty acid biosynthetic genes. J. Biol. Chem. 267:5751-5754. [PubMed] [Google Scholar]
- 41.Rosemeyer, V., J. Michiels, C. Verreth, and J. Vanderleyden. 1998. luxI- and luxR-homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J. Bacteriol. 180:815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaefer, A. L., B. L. Hanzelka, M. R. Parsek, and E. P. Greenberg. 2000. Detection, purification, and structural elucidation of the acylhomoserine lactone inducer of Vibrio fischeri luminescence and other related molecules. Methods Enzymol. 305:288-301. [DOI] [PubMed] [Google Scholar]
- 43.Schripsema, J., K. E. de Rudder, T. B. van Vliet, P. P. Lankhorst, E. de Vroom, J. W. Kijne, and A. A. van Brussel. 1996. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules, known as autoinducers and as quorum sensing co-transcription factors. J. Bacteriol. 178:366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweizer, H. D. 1993. Small broad-host-range gentamycin resistance gene cassettes for site-specific insertion and deletion mutagenesis. BioTechniques 15:831-834. [PubMed] [Google Scholar]
- 45.Seed, P. C., L. Passador, and B. H. Iglewski. 1995. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens, A. M., K. M. Dolan, and E. P. Greenberg. 1994. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc. Natl. Acad. Sci. USA 91:12619-12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevens, A. M., and E. P. Greenberg. 1997. Quorum sensing in Vibrio fischeri: essential elements for activation of the luminescence genes. J. Bacteriol. 179:557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Rhijn, P., and J. Vanderleyden. 1995. The Rhizobium-plant symbiosis. Microbiol. Rev. 59:124-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, G. P., F. Debelle, A. Savagnac, M. Ferro, O. Schiltz, F. Maillet, D. Prome, M. Treilhou, C. Vialas, K. Lindstrom, J. Denarie, and J. C. Prome. 1999. Structure of the Mesorhizobium huakuii and Rhizobium galegae Nod factors: a cluster of phylogenetically related legumes are nodulated by rhizobia producing Nod factors with α,β-unsaturated N-acyl substitutions. Mol. Microbiol. 34:227-237. [DOI] [PubMed] [Google Scholar]