Abstract
The Cpx (conjugative plasmid expression) stress response of Escherichia coli is induced in response to extracytoplasmic signals generated in the cell envelope, such as misfolded proteins in the periplasm. Detection of stress is mediated by the membrane-bound histidine kinase, CpxA. Signaling of the response regulator CpxR by activated CpxA results in the expression of several factors required for responding to cell envelope stress. CpxA was originally thought to be required for the expression of the positive regulator of the F plasmid transfer (tra) operon, TraJ. It was later determined that constitutive gain-of-function mutations in cpxA led to activation of the Cpx envelope stress response and decreased TraJ expression. In order to determine the nature of the downregulation of TraJ, the level of expression of TraJ, TraM, and TraY, the F-encoded regulatory proteins of the F tra region, was determined both in a cpxA* background and in a wild-type background in which the Cpx stress response was induced by overexpression of the outer membrane lipoprotein, NlpE. Our results suggest that TraJ downregulation is controlled by a posttranscriptional mechanism that operates in the cytoplasm in response to upregulation of the Cpx stress response by both the cpxA* gain-of-function mutation and the overexpression of NlpE.
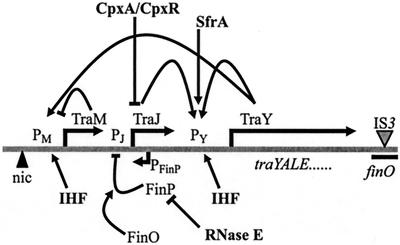
The cpx regulon was first identified by its effects on conjugative plasmid expression. Gain-of-function mutations in cpxA caused a decrease in F plasmid transfer due to a lack of pili on the cell surface (30). Conjugative pilus expression and transfer of the F-plasmid from donor to recipient Escherichia coli cells requires the proteins encoded by the 33.3-kb tra operon of the F plasmid (Fig. 1) (reviewed in reference 14). The major promoter of the tra operon, PY, is located at the start of the operon, and it is directly upregulated by the F regulatory protein, TraJ (13). TraJ indirectly upregulates expression of F TraM via TraY, which also increases expression of the tra genes from PY (37). The antisense RNA FinP, which is complementary to the 5′ untranslated region of the traJ mRNA, controls expression of TraJ by forming an RNA-RNA duplex with traJ mRNA, occluding the ribosome-binding site of the transcript and thus preventing TraJ translation and tra operon expression (Fig. 1) (35). In F-like plasmids, the negative regulatory effect mediated by the FinP/traJ mRNA interaction requires the FinO protein, but in F, an IS3 insertion in FinO renders the protein nonfunctional, leading to derepression of F transfer (Fig. 1) (60). Several host-encoded proteins also influence expression from PY. The transcriptional activator SfrA (47, 53) and integration host factor (49) both influence tra operon expression and F plasmid transfer. Silverman et al. (48) observed that cpxA deletion mutants exhibited quasi-wild-type levels of Flac transfer. However, Flac transfer was decreased in some cpxA gain-of-function mutants, and the levels of TraJ protein and expression from a PtraY-lacZ fusion construct were also reduced, suggesting that activation of the Cpx system led to a lowered level of TraJ. Decreased levels of TraJ, therefore, most likely caused the impaired transfer ability of F in cpxA gain-of-function mutants.
FIG. 1.
Schematic representation of the F tra region, including relevant promoters and genes as indicated. Arrows indicate positive regulatory effects, whereas black bars indicate negative regulatory effects. Plasmid-encoded gene products are shown in regular type, and host-encoded factors are shown in boldface. The drawing is not to scale. Further details are provided in the text.
Most cpx phenotypes have been associated with the cell envelope (29, 32), and subsequent work showed that the cpx regulon is controlled by a two-component signal transduction system, which senses and responds to cell envelope stress in E. coli (reviewed in reference 44). This system consists of the membrane-bound sensor kinase, CpxA, and its cognate cytoplasmic response regulator, CpxR (58). CpxA localizes to the inner membrane and contains transmembrane, cytoplasmic, and periplasmic domains (58). CpxA exhibits autokinase activity, and both kinase and phosphatase activity specific for CpxR (43). CpxR is a cytoplasmic OmpR-like transcriptional activator (12) which, when phosphorylated, binds target promoters at a consensus sequence (11, 39). As is typical for such systems, the stress-inducing signal is transferred from CpxA to CpxR through a highly conserved phosphotransferase reaction (17).
A variety of signals can activate the Cpx stress response, including overexpression of the outer membrane lipoprotein NlpE (51), overexpression of misfolded P-pilus subunits (20), and elevated pH (36), among others. Overproduction of NlpE and P-pilus subunits cause an increased level of misfolded proteins in the cell envelope, which is thought to be the main activating signal of the Cpx system (41). Active, phosphorylated CpxR upregulates the transcription of several genes which are involved in protein folding and degradation in the bacterial envelope (8, 9, 20). Examples of such Cpx-activated targets are the periplasmic protease DegP (8, 39) and the periplasmic disulfide oxidase, DsbA (6, 20, 39).
cpxA* gain-of-function mutants were characterized by their ability to suppress the toxic effects of mislocalized and misfolded proteins in the cell envelope (5). cpxA* mutants exhibit up to a 10-fold increase in expression of targets of the Cpx regulatory pathway (8). Two distinct classes of cpxA* mutations have been characterized. The first class contains point or deletion mutations in the periplasmic sensing domain of the protein, which are believed to render CpxA “signal blind” (43). The effect of this condition is constitutive activation of CpxA and thus upregulation of both the cpxRA operon and its downstream targets (41, 42). The second class of cpxA* mutants contains point mutations in the cytoplasmic domain of CpxA. An example is cpxA101*, which contains a single amino acid change from threonine to proline at position 253, located near the putative site for autophosphorylation (43). This mutant retains its autokinase and kinase functions but has lost its phosphatase activity. The result is elevated levels of active, phosphorylated CpxR, shifting the Cpx regulon to a constitutively active state (43). cpxA* mutants exhibit numerous and varied phenotypes, including resistance to amikacin (40), sensitivity to elevated temperatures (31), and tolerance to elevated pH (7).
The cpxA mutation that was first shown to inhibit F transfer by Silverman et al. (48) was later identified as a constitutively activated gain-of-function mutation (43). The decrease of F transfer and reduction of TraJ expression (48) was therefore thought to be due to constitutive activation of the Cpx regulon. We have confirmed this observation by determining that F transfer and expression of several F tra regulatory proteins are significantly lowered in a well-defined cpxA* mutant background. Our results also demonstrate that activation of the cpxAR operon in a wild-type background mimics the negative effects on F transfer which result from a cpxA* mutation, suggesting that F transfer is sensitive to cell envelope stress. The observed effects were determined to be the result of posttranscriptional reduction of the level of cytoplasmic F regulatory protein, TraJ.
MATERIALS AND METHODS
Strains and plasmids.
All strains and plasmids used in this work are described in Table 1. Standard genetic techniques were employed (46).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or descriptiona | Source or reference |
|---|---|---|
| Bacterial strains | ||
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150(Strr) relA1 flb5301 deoC1 ptsF25 rbsR | 2 |
| XK1200 | Nalr F−lacΔU124 Δ(nadA araG gal attL) | 34 |
| TR8 | MC4100 cpxA::cam | 42 |
| TR20 | MC4100 cpxA101 | This work |
| TR51 | MC4100 cpxR::spc | 42 |
| TR189 | MC4100 cpxA101 zii::Tn10 λRS88[degP-lacZ] | This work |
| TR981 | MC4100 cpxA101 zii::Tn10 λRS88[degP-lacZ] recA | This work |
| TR984 | MC4100 cpxA101 zii::Tn10 λRS88[degP-lacZ] clpP lonA | This work |
| JMR201 | MC4100 degP::Tn10 | A. E. Rizzitello and T. J. Silhavy |
| Plasmids | ||
| pOX38-Km | Kmr F tra region, Rep FIA replicon | 4 |
| pOX38-Tc | Tcr F tra region, Rep FIA replicon | 1 |
| pRS27 | Tcr 9-kb partial EcoRI F fragmentin pSC101 | 50 |
| pBAD24 | Apr PBAD cloning vector | 16 |
| pBADTraJ | traJ coding region fused to PBAD | This work |
| pBADTraM | traM coding region fused to PBAD | This work |
| pBADTraY | traY coding region fused to PBAD | This work |
| pBR322 | General cloning vector | New England Biolabs |
| pLD404 | Apr NlpE in pBR322 | 51 |
| pMC874 | KmrlacZ+ Δplac | 3 |
| pMCJ211 | KmrtraJ ΩlacZ FinP+traJ-lacZ reporter plasmid | 57 |
| pGEX-KG | IPTG-inducible GST fusion expression | Amersham Pharmacia Biotech |
| pLJ5-13 | AprT7Φ10-finP fusion in pUC19 | 19 |
| pSLF20 | finP F derivative | 24 |
Strr, streptomycin resistant; Nalr, nalidixic acid resistant; Kmr, kanamycin resistant; Tcr, tetracycline resistant; Apr, ampicillin resistant.
Media, antibiotics, and growth conditions.
All cultures were grown and maintained on Luria-Bertani (LB) medium or Trypticase soy broth (TSB) agar plates at either 30°C (cpxA* strains) or 37°C (all other strains). Liquid cultures were grown in LB medium or TSB broth at either 30 or 37°C as indicated. Antibiotics (Sigma) were used at the following concentrations in selective media: ampicillin, 25 μg/ml, kanamycin; 25 μg/ml; streptomycin, 200 μg/ml; and nalidixic acid, 20 μg/ml. cpxA* strains were grown at 30°C and were maintained on nutrient agar plates supplemented with 3 μg of amikacin/ml to prevent reversion (42).
Construction of PBAD-Tra overexpression plasmids.
Plasmid pRS27 (Table 1) was used as the template for PCR amplification of the traJ coding region of F by using the upstream primer 5′-CCATGGATCCGATGGATCGTAT-3′ to introduce a NcoI site, and the downstream primer 5′-CTGCAGAATAATCAGAAAAGGT-3′ to introduce a PstI site. Vent polymerase (NEB) was used to generate the ca. 750-bp PCR product, which was cloned into PstI/NcoI-digested pBAD24 in frame with PBAD. Positive clones were sequenced by using the DYEnamic ET fluorescent sequencing system (Amersham Pharmacia Biotech) to confirm that the entire and correct coding region of traJ was present in the plasmid. pBADTraY was constructed by using the same techniques, with the upstream primer 5′-GGCGGATCCATGGCAAAAAGATTTGGTACACG-3′ and the downstream primer 5′-CGCGTCGACTAGAGTGTATTAAATGTTA-3′. pBADTraM was constructed by using the same techniques, with the upstream primer 5′-GGATCCATGGCTAAGGTGAACCTG-3′ and the downstream primer 5′-GAATTCTTATTCATCATCATTTTTTG-3′.
Western immunoblot analysis.
Cell pellets corresponding to optical density at 600 nm (OD600) equivalents of 0.1 were used in all immunoblot assays. Samples were boiled in sodium dodecyl sulfate (SDS) sample buffer (23) for 5 min, and the supernatants were electrophoresed on SDS-15% polyacrylamide gels by using the Bio-Rad Minigel system. Proteins were transferred to Immobilon-P membranes (Millipore) by using Towbin buffer (56). Membranes were blocked overnight at 4°C with 10% (wt/vol) skim milk (Difco) dissolved in TBST (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 0.1% [vol/vol] Tween 20 [Caledon Laboratories]). Polyclonal antisera (raised in rabbits) were diluted (anti-TraJ, 1:15,000; anti-TraM, 1:5,000; anti-TraY, 1:2,000) in the same blocking solution and then added to the blots and incubated at room temperature for 1 h. Blots were washed at room temperature (four times 15 min) with TBST. Blots were incubated with the secondary antibody (1:10,000 horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin G [Amersham Life Sciences]), washed as described above, and then developed with Renaissance Western blot Chemiluminescence Reagent Plus (NEN) and exposed to Kodak X-Omat R film.
Stability of TraJ, TraM, and TraY.
Cultures of various E. coli mutants containing the arabinose-inducible plasmids pBADTraJ/TraM/TraY were grown at 30°C in TSB supplemented with 1.0% (wt/vol) glucose and appropriate antibiotics to an OD600 of 0.6 to 0.9. Preinduction samples were collected, and these and all subsequent samples were frozen at −20°C until required. Then, 2-ml portions of the preinduction cultures were centrifuged and washed to remove antibiotics and glucose. Next, 0.1% (wt/vol) arabinose in 2 ml of fresh TSB was added to induce the expression of the tra proteins, and induction was carried out at 30°C for 50 min with agitation. The zero time sample was collected, and the induced culture was centrifuged and washed to remove arabinose. Then, 2 ml of fresh TSB containing 1.0% (wt/vol) glucose and 200 μg of rifampin (Sigma)/ml was added to prevent further expression from PBAD. Samples were collected at 15, 30, 60, and 120 min postinduction and subjected to immunoblot analysis as described above.
Northern blot analysis.
Total RNA was isolated via a modified hot phenol method as described earlier (19) from strains grown in liquid cultures at 30°C to an OD600 of 0.8 to 1.0. RNA (40 μg) was denatured, electrophoresed, and transferred to Zeta-Probe nylon membranes (Bio-Rad) as described previously (19). Blots to be probed for traJ were prehybridized at 58°C for 4 h as described elsewhere (45), except 200 μg each of boiled E. coli strain W tRNA type XX and sonicated calf thymus DNA (Sigma)/ml were added to the hybridization solution. Fresh hybridization buffer containing ca. 10 pmol of 32P-labeled FinP RNA (19) was added to the blots and incubated overnight at 58°C. Blots were washed as described previously (45) and then exposed on a Molecular Dynamics storage phosphor screen. Blots were then stripped at 70°C in 5 mM Tris-HCl (pH 8)-2 mM EDTA-0.1× Denhardt solution-0.1% SDS and prehybridized at 37°C for 4 h as described previously (19). The tRNASer-specific oligonucleotide probe JSA12 (45) and the FinP-specific probe primer A (19) were 5′ end labeled with [γ-32P]ATP (Perkin-Elmer), and ca. 10 pmol of each probe was added to the blots in fresh hybridization solution. Incubation proceeded overnight at 37°C, and washing and exposure were performed as described above. Bands corresponding to the traJ and FinP transcripts and tRNASer were quantified by using a Molecular Dynamics Phosphorimager 445 SI and ImageQuant software.
Bacterial matings.
Donor strains containing pOX38-Km and the recipient strain XK1200 were grown in 2 ml of LB medium at 30°C with appropriate antibiotic selection to an OD600 of 0.6 to 1.0, and 1 ml of each donor culture was pelleted by centrifugation and resuspended in 0.9 ml of fresh LB medium to remove antibiotics. Then, 0.1 ml of recipient cells were added, and mating proceeded for 45 min at 30°C. Mating mixtures were vortexed vigorously for 30 s and placed on ice to disrupt mating pairs, and 10-fold serial dilutions (1 ml) in ice-cold 1× phosphate-buffered saline were performed. Next, 10 μl of each dilution was inoculated onto selective plates to select donors (kanamycin and streptomycin) and transconjugants (kanamycin and nalidixic acid). Plates were incubated overnight at 30°C, and donor and transconjugant colonies were counted. The ratio of transconjugants to donors was used to measure the efficiency of transfer of pOX38-Km from donors to recipients.
β-Galactosidase assays.
Cultures of the various E. coli strains containing either the parental control vector pMC874 or the PtraJ-lacZ reporter plasmid pMCJ211 were grown at 30°C in LB broth supplemented with the appropriate antibiotics to an OD600 of 0.6 to 1. Then, 100 to 300 μl of each culture was assayed for β-galactosidase activity as described earlier (41), except the A420 value of each sample was determined by using a Bio-Rad Smartspec 3000. The β-galactosidase activity was calculated as described by Miller (33).
RESULTS
Expression of F-encoded proteins TraJ, TraM, and TraY in the cpxA101* background.
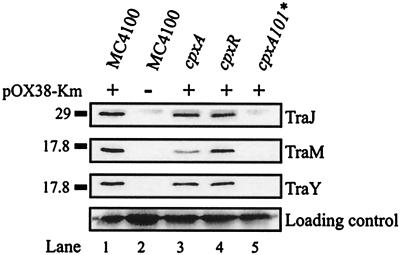
In order to determine the effect of the cpxA101* mutation on several tra regulatory proteins, immunoblot analysis of the F plasmid derivative pOX38-Km in various Cpx regulon mutants was performed. As expected, TraJ was not detectable in the cpxA101* strain TR189 (Fig. 2). Since TraJ is a positive activator of PY and indirectly of PtraM, immunoblot analysis was performed to determine whether a lack of TraJ expression had an effect on TraY or TraM levels (Fig. 2). TraY and TraM proteins were also undetectable in the cpxA101* background. In the cpxR strain TR51 and cpxA strain TR8, the levels of TraJ, TraM, and TraY were close to wild-type, although TraM levels were slightly more reduced in the cpxA background (Fig. 2). Similar results were obtained for a cpxA24* mutant which carries a “signal blind” mutation in the periplasmic domain of CpxA (data not shown).
FIG. 2.
F-encoded proteins TraJ, TraM, and TraY are not detectable in a cpxA101* background. Immunoblot analysis with polyclonal antisera directed against TraJ, TraM, and TraY was carried out. Lanes 1 and 2, E. coli MC4100 with (+) or without (−) pOX38-Km; lanes 3 to 5, cpxA (TR8), cpxR (TR51), and cpxA101* (TR189) strains containing pOX38-Km, resepctively. The positions of TraJ, TraM, and TraY are indicated on the right, and relevant molecular weight markers are indicated (in kilodaltons) on the left. The loading control indicated at the bottom of the figure is obtained from a protein that nonspecifically cross-reacts with the antiserum.
Transfer of pOX38-Km in the cpxA101* background.
The observed lack of expression of TraJ, TraM, and TraY prompted an investigation into the effect of several cpx mutations on pOX38-Km transfer. Mating assays were performed to test the ability of pOX38-Km to transfer into an F− recipient strain, XK1200, from a variety of cpx mutant strains. The cpxA and cpxR donor strains were nearly as proficient for pOX38-Km transfer as the wild-type MC4100 donor, whereas in the cpxA101* donor strain, transfer was reduced by more than 600-fold (Table 2).
TABLE 2.
Efficiency of pOX38-Km transfer from a variety of donor strains
| Donor strain/plasmid(s) | No. of transconju- gants/100 donors | % Mating efficiencya (versus wild type) |
|---|---|---|
| MC4100/pOX38-Km | 43 | 100 |
| TR8 (cpxA)/pOX38-Km | 36 | 84 |
| TR51 (cpxR)/pOX38-Km | 42 | 97 |
| TR189 (cpxA101*)/pOX38-Km | 0.06 | 0.15 |
| JMR201 (degP)/pOX38-Km | 21 | 49 |
| MC4100/pOX38-Km/pBR322 | 28 | 65 |
| MC4100/pOX38-Km/pLD404 | 12 | 28 |
That is, the ratio of transconjugants to donors in each strain divided by the transconjugant/donor ratio of F transfer from a wild-type background.
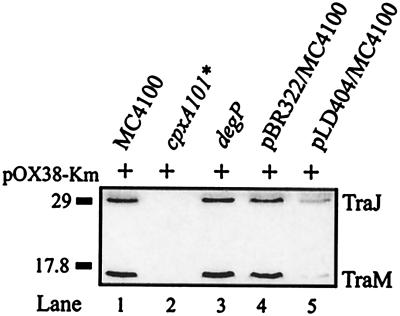
Since the periplasmic protease DegP is a member of the Cpx regulon (8, 39), the effect of a degP mutation on pOX38-Km transfer was also examined (Table 2). Conjugative transfer from the degP donor strain JMR201 was reduced by approximately twofold, whereas immunoblot analysis of the levels of TraJ and TraM expressed from pOX38-Km in the degP mutant revealed that both proteins were expressed at or near wild-type levels (Fig. 3, compare lanes 1 and 3). The degP mutation therefore had only a small effect on pOX38-Km transfer. These results suggest that DegP has no direct influence on the level of TraJ or TraM expression or on the expression of the F tra operon. A number of F tra proteins localize to the inner membrane and therefore may be affected by the Cpx regulon under conditions of stress, which could potentially lead to loss of TraJ expression and F plasmid transfer. Immunoblot analysis of TraJ and TraM expressed from F′lac plasmids carrying mutations in traA, traB, traD, and traG, all of which encode proteins that localize to the inner membrane (14), was performed. In a wild-type background, TraJ and TraM were expressed at wild-type levels from these mutant F′lac plasmids, whereas in a cpxA101* background, TraJ and TraM were undetectable by immunoblot analysis (data not shown). These results suggest that the reduction of F transfer and TraJ expression observed in the cpxA101* background was specific to TraJ and was not influenced by any potential secondary effects of constitutive activation of the Cpx regulon on tra proteins that localize to the cell envelope. Similarly, the reduction of TraJ expression was not mediated by a signal from the periplasm conducted through one of the selected inner membrane F transfer proteins.
FIG. 3.
Cell envelope stress induced by NlpE overexpression reduces TraJ and TraM levels. Immunoblot analysis with polyclonal antisera directed against TraJ and TraM was carried out. Lanes 1 to 3, wild-type (MC4100), cpxA101* (TR189), and degP (JMR201) E. coli containing pOX38-Km, respectively; lanes 4 and 5, E. coli MC4100 containing pOX38-Km and the control vector, pBR322, or pLD404, expressing NlpE. The positions of TraJ and TraM are indicated on the right, and the relevant molecular weight marker is indicated (in kilodaltons) on the left.
Induction of cell envelope stress reduces TraJ expression and F plasmid transfer.
Overproduction of the outer membrane lipoprotein NlpE is known to activate the Cpx pathway (51). In order to determine whether the effects of the cpxA101* mutation were physiologically relevant, the effect of NlpE overproduction on TraJ and TraM levels expressed from pOX38-Km and on the conjugative transfer of the plasmid was tested. TraJ and TraM levels were significantly reduced when NlpE was overexpressed in a wild-type background but to a lesser extent than the reduction in TraJ and TraM levels evident in the cpxA101* background (Fig. 3, compare lanes 1, 2, and 5). Similarly, pOX38-Km transfer was reduced but not abolished when NlpE was overexpressed (Table 2), suggesting that the levels of TraJ and TraM were sufficient to allow for a reduced level of plasmid transfer to occur.
traJ transcription in various cpx backgrounds.
To determine whether the lack of TraJ in the constitutively activated Cpx background was caused by transcriptional or posttranscriptional events, the PtraJ-lacZ reporter plasmid pMCJ211 (57) was used to test PtraJ activity by using β-galactosidase assays in several cpx mutant strains (Fig. 4C). Compared to the wild-type strain MC4100, PtraJ activity was reduced by approximately twofold in both the cpxA and cpxR strains and approximately threefold in the cpxA101* strain.
FIG. 4.
Northern analysis and β-galactosidase assays show that traJ and FinP transcripts are expressed in both wild-type and cpxA101* E. coli. (A) Relative levels of traJ mRNA and FinP antisense RNA expressed from pOX38-Km in various backgrounds. Lanes 1 and 2, E. coli MC4100 without (−) or with (+) pOX38-Km; lanes 2 to 4, pOX38-Km in cpxA101* (TR189), cpxR (TR51), and cpxA (TR8) strains. The positions of the traJ transcript and FinP antisense RNA are indicated on the right. (B) Northern analysis to show a direct comparison of traJ mRNA levels in wild-type and cpxA101* backgrounds. Lanes 1 and 2, MC4100 without (−) and with (+) pOX38-Km; lanes 3 and 4, cpxA101* (TR189) without (−) and with (+) pOX38-Km. The position of the traJ transcript and the loading control, tRNASer, are shown on the right. (C) PtraJ activity is reduced in several cpx mutants. β-Galactosidase assays of MC4100 (lane 1), cpxA (lane 2), cpxR (lane 3), and cpxA101* (lane 4) carrying the PtraJ-lacZ reporter plasmid pMCJ211 were performed. Assays with the parental control plasmid resulted in insignificant levels of β-galactosidase activity and are not included.
Northern blot analysis was performed to detect whether the traJ transcript was expressed from pOX38-Km in the cpxA101* strain. The traJ transcript was detectable in this strain and was reduced by approximately threefold compared to the wild-type (Fig. 4A and B). Northern analysis to detect the traJ transcript in cpxA and cpxR strains (Fig. 4A) also indicated that the traJ transcript was expressed, at a level similar to the wild-type strain MC4100 containing pOX38-Km. Since expression of FinP antisense RNA is known to affect F tra operon expression (24, 55), the level of FinP was examined in several cpx mutants. Northern analysis of FinP antisense RNA expressed from pOX38-Km in wild-type, cpxA, cpxR, and cpxA101* strains revealed a wild-type level of FinP in the cpxA and cpxR strains and an elevated FinP level in the cpxA101* strain (Fig. 4A). In order to determine whether elevated FinP levels in a cpxA101* background affected TraJ expression, immunoblot analysis of the finP mutant F plasmid, pSLF20, was performed. TraJ was not detectable in the cpxA101* strain carrying pSLF20 (data not shown), indicating that elevated FinP levels in the cpxA101* strain did not affect expression of TraJ. Both inactivation (cpxA/R) and constitutive activation (cpxA101*) of the Cpx pathway appeared to have only a minor inhibitory effect on traJ transcription. Taken together, the β-galactosidase assays and Northern blot analyses suggest that the reduction of TraJ expression in the cpxA101* background was due to a posttranscriptional event and was not related to elevated FinP expression.
Stability of F-tra regulatory proteins expressed from a foreign promoter in a cpxA101* background.
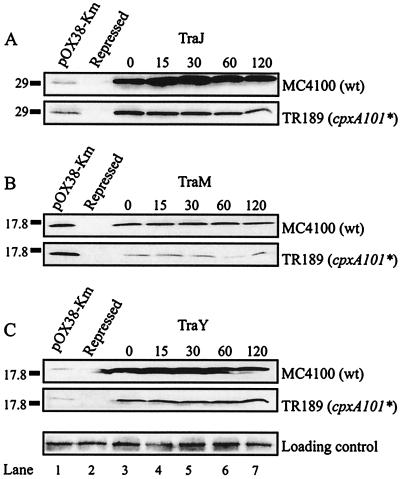
Since the traJ transcript but not the TraJ protein is detectable in the cpxA101* mutant, the fate of TraJ expressed from a foreign promoter was tested in order to further separate transcriptional from posttranscriptional effects. TraJ expression from the pBADTraJ overexpression vector was induced with arabinose for 50 min, and samples were collected at various times over a 2-h period after the 50-min induction from cultures to which rifampin and glucose were added to prevent further rounds of transcription. Bacterial lysates were subjected to SDS-polyacrylamide gel electrophoresis and immunoblot analysis to determine TraJ levels at each time point. TraJ was stable in the wild-type strain MC4100 over the duration of the experiment (Fig. 5A, compare lane 0 through lane 120). However, in the cpxA101* strain, TraJ levels began to decrease at 15 min after the addition of rifampin and steadily decreased with time. By 120 min postinduction, TraJ levels decreased by ca. 75% (Fig. 5A, compare lane 0 and lane 120). Similar results were observed with a cpxA24* mutant (data not shown). The stability of TraM expressed from the pBADTraM overexpression vector was assessed in the same manner (Fig. 5B). The level of TraM was significantly reduced in the cpxA101* strain, but TraM was stable over time (Fig. 5B, compare lane 0 through lane 120). The stability of TraY, expressed from the pBADTraY expression plasmid, was also examined (Fig. 5C). The level of TraY was slightly reduced in the cpxA101* strain compared to the wild-type strain, but TraY was stable over time in both strains (Fig. 5C, compare lane 0 and lane 120). Examination of the stability of an unrelated protein, glutathione S-transferase (GST), revealed that it was stable in both wild-type and cpxA101* backgrounds (data not shown). Together, these data suggest that the decreased level of TraJ in the cpxA101* strain was a specific phenomenon.
FIG. 5.
Stability of TraJ, TraM, and TraY expressed from a foreign promoter in cpxA101*. (A) Immunoblot analysis with polyclonal antiserum to detect TraJ expressed from the pBADTraJ overexpression vector. Lane 1, TraJ expressed from pOX38-Km in MC4100; lane 2, TraJ expressed from pBADTraJ under repressed conditions; lanes 3 to 7, TraJ expressed from pBADTraJ after induction by arabinose and subsequent inhibition of further rounds of transcription by the addition of glucose and rifampin. The number above each lane indicates the time (in minutes) at which each sample was taken after the addition of rifampin and glucose. The host strains tested are shown on the right, and the relevant molecular weight marker is shown (in kilodaltons) on the left. The loading control indicated at the bottom of the figure is obtained from a protein that nonspecifically cross-reacts with the antiserum. (B and C) Stability of TraM and TraY, respectively, expressed from the pBADTraM and pBADTraY overexpression vectors were examined as described in panel A.
Effect of recA and clpP lonA mutations on TraJ expression in the cpxA101* strain.
Deletion of several proteases in the cpxA101* strain was performed to determine whether they were involved in degradation of TraJ. Immunoblot analysis of pOX38-Tc in cpxA101* recA and cpxA101* clpP lonA backgrounds showed that TraJ was undetectable (Fig. 6, lanes 4 and 5). These results suggest that none of these proteases was directly involved in reducing TraJ levels expressed from F in the cpxA101* strain.
FIG. 6.
recA and clpP lonA mutations do not rescue TraJ expression in the cpxA101* mutant. Immunoblot analysis with polyclonal antiserum directed against TraJ. Lanes 1 and 2, E. coli MC4100 without (−) or with (+) pOX38-Km; lanes 3 to 5, pOX38-Tc in cpxA101* (TR189), cpxA101* recA (TR981), and cpxA101* clpP lonA (TR984) strains. The position of TraJ is indicated on the right, and the position of the relevant molecular weight marker is shown (in kilodaltons) on the left. The loading control indicated at the bottom of the figure is obtained from a protein that nonspecifically cross-reacts with the antiserum.
DISCUSSION
The results presented in this work reveal that the decreased level of F plasmid positive regulatory protein, TraJ, in a cpxA101* gain-of-function mutant (48) resulted from a specific posttranscriptional event. Not only was F TraJ undetectable in cpxA101* but TraJ expressed from a foreign promoter in this mutant was not stable. This result is somewhat unexpected, considering that the typical targets of the activated Cpx regulon are subject to regulation at the level of transcription (6, 8, 41). It is also unexpected because the activated Cpx pathway typically affects the folding and stability of envelope proteins (reviewed in reference 41), although evidence exists for the influence of the Cpx regulon on the function and stability of the cytoplasmic enzyme, acetohydroxyacid synthase I (54). However, the specific posttranscriptional reduction of the cytoplasmic regulatory protein, TraJ, appears to be unique.
The necessity for a functional Cpx regulon to correctly assemble P-pili in uropathogenic E. coli is hypothesized to reflect the requirement of the bacterium to sense and respond to envelope stress caused by physiological changes induced by the host response (18). Although correct folding and translocation of F pilin subunits to the inner membrane and assembly of the conjugative pilus is a requirement for F transfer (14, 27, 52), a fully functional Cpx regulon is not required for this process. Both cpxA and cpxR mutants displayed nearly wild-type levels of pOX38-Km transfer and normal expression of the F tra regulatory proteins TraJ, TraM, and TraY. Conversely, constitutive activation of the Cpx response regulator, CpxR, in a cpxA101* mutant (43) drastically reduced F transfer. Activation of the wild-type Cpx pathway induced by overproduction of the outer membrane lipoprotein NlpE (51) also resulted in decreased F plasmid transfer and TraJ and TraM expression. Although the effect of overproduction of NlpE on F transfer was not as severe as constitutive activation of the cpxRA operon in the cpxA101* strain, the results support the notion that activation of the wild-type CpxAR pathway by envelope stress downregulates TraJ expression and F transfer. Interestingly, the cpxR mutant (TR51) containing pOX38-Km became essentially nonviable when the NlpE-overexpression plasmid pLD404 was introduced into this strain (data not shown), suggesting that overexpression of NlpE induces envelope stress in E. coli that cannot be effectively combated in the absence of a functional Cpx pathway. Prevention of piliation and F transfer is desirable during times of actual or perceived stress, since this process requires a considerable investment in energy and metabolic resources and extensive alteration of the cell envelope (14, 59).
Examples of negative regulation of Cpx regulon targets include downregulation of expression of genes for motility (10, 22, 26) and chemotaxis (22) by active, phosphorylated CpxR. Expression of mRNA from the motAB-cheAW operon and swarming ability were shown to be reduced in a cpxA* strain in a CpxR-dependent manner (10). Phosphorylated CpxR was also shown to bind to its consensus recognition sequence found in the promoter region of the motAB-cheAW operon (11). However, these authors did not demonstrate whether this phenomenon was a function of the wild-type Cpx pathway. Our results provide evidence of another pathway that is downregulated by constitutive Cpx regulon expression. Further, we have shown that overproduction of NlpE (51) has the same effect on F transfer as constitutive activation of the Cpx pathway. No consensus CpxR binding site is present in the promoter region of traJ (14, 39), supporting the idea that TraJ reduction in a cpxA101* background is not controlled by transcriptional regulation. Both pilus and flagellum expression involve elaboration of extracytoplasmic protein appendages, which requires the secretion of protein subunits through the cell envelope (26, 52). Such processes may be inhibited when the Cpx pathway detects cell envelope stress.
TraJ expressed from pOX38-Km in a wild-type strain was found to be stable well into stationary phase and detectable in significant amounts in cultures grown for up to 24 h (15). However, the traJ transcript is unstable, short-lived, and found in only very low abundance (24). A low, basal level of traJ mRNA may therefore be sufficient to allow for enough stable TraJ to accumulate and exert positive activation on PY. Our results reveal that PtraJ activity was reduced by only two- to threefold in cpxA, cpxR, and cpxA101* backgrounds. Similarly, the level of traJ mRNA expressed from pOX38-Km was reduced by approximately threefold in a cpxA101* background, although in cpxA and cpxR strains traJ mRNA was found at a level very close to that expressed from pOX38-Km in wild-type MC4100. However, TraJ levels were observed to be vastly different in these strains, with TraJ being detectable in both cpxA and cpxR backgrounds at nearly wild-type levels but completely absent in a cpxA101* strain. Similarly, the cpxA101* strain exhibited severely reduced pOX38-Km transfer, whereas cpxA and cpxR strains exhibited only moderately reduced plasmid transfer efficiency. These results suggest that the decreased PtraJ transcription exhibited by all of the cpx mutants was not responsible for the lack of detectable TraJ and reduced F transfer in the cpxA101* strain. Although we cannot rule out minor transcriptional effects caused by the cpxA101* mutation, the data generally support a posttranscriptional level of control of TraJ in the cpxA101* mutant.
Examination of F TraJ, TraM, and TraY expressed from PBAD demonstrated that TraJ was unstable in the cpxA101* background. Interestingly, expression of all three proteins was reduced in the cpxA101* strain. These results suggest that a general reduction of expression from PBAD, or general mRNA instability, may occur in the cpxA101* strain. Alternatively, a reduction of the copy number of the PBAD overexpression vectors in this strain may lead to lower levels of expressed proteins, although no obvious difference in plasmid levels was evident (data not shown). The reduction of the level of TraM expressed from PBAD was markedly greater than the reduction of TraJ and TraY levels expressed from the same promoter. Several factors, such as alterations in local superhelical density or translational efficiency, might influence TraM expression and stability, which could account for this difference (47, 49).
Expression of FinP antisense RNA, part of the FinOP fertility inhibition system (Fig. 1) (reviewed in reference 14), affects F traJ transcription (21, 24) and is influenced by host-encoded factors such as Dam methylation and RNase E degradation of FinP (19, 55). No detectable increase in TraJ expression from the finP plasmid pSLF20 occurred in the cpxA101* mutant strain (data not shown), suggesting that the decreased TraJ level in a cpxA101* background is not influenced by FinP transcription. Recent microarray data (unpublished results) revealed that rne, which encodes RNase E, was downregulated in a cpxA101* strain; this could account for the increase in FinP expression.
Since TraJ is a cytoplasmic protein (14), the observed lack of accumulation of TraJ is most likely not directly attributable to the periplasmic protein folding and degradation pathways typically involved in response to cell envelope stress (6, 8). Constitutive expression of the Cpx regulon in the cpxA101* mutant may simply mimic the induction of cell envelope stress, resulting in a reduction of expression of F tra regulatory proteins. Reduced TraJ and TraM levels caused by NlpE overexpression in a wild-type background supports this idea. A cytoplasmic protein degradation pathway may be triggered by the Cpx regulon when envelope stress is induced and/or when the Cpx regulon is constitutively activated. Inner membrane transfer proteins such as TraA, TraB, TraD, and TraG might affect TraJ and TraM expression in a cpxA101* background by transducing the stress signal through the membrane and interacting with components of the Cpx regulon.
Preliminary microarray analysis revealed that several proteases, including RecA (25) and ClpP (28), were upregulated in the cpxA101* strain carrying pOX38-Km. The observation that filamentous growth, characteristic of cells experiencing induction of the SOS response, occurs in cpxA* mutants (38), coupled with our evidence of increased recA transcription in cpxA101* carrying pOX38-Km, prompted an examination of the potential involvement of RecA in destabilizing TraJ. Examination of TraJ expressed from F-derivative plasmid pOX38-Tc in a cpxA101* recA mutant background revealed that TraJ was not detectable, suggesting that the RecA proteolytic pathway was not involved in destabilizing TraJ. The stability of λ prophages in TR189, as well as other varied cpx mutants (43), also suggests that the SOS pathway is not active in cpxA* mutants. Similarly, a cpxA101* clpP lonA strain carrying pOX38-Tc exhibited undetectable levels of TraJ, implying that the ClpP and LonA proteases were not involved in the reduction of TraJ expression in the cpxA101* mutant. Further work to examine the involvement of other E. coli cytoplasmic proteases in reducing F TraJ levels in the cpxA101* mutant is planned. Continued examination of the mechanisms involved in reducing F plasmid transfer in cpxA* mutants should provide insight into new physiological roles for the Cpx envelope stress response.
Acknowledgments
Michael J. Gubbins is the recipient of Alberta Heritage Foundation for Medical Research and Canadian Institutes of Health Research Studentships. This work was supported by the CIHR (L.S.F.) and AHFMR (T.L.R.).
REFERENCES
- 1.Anthony, K. G., C. Sherburne, R. Sherburne, and L. S. Frost. 1994. The role of the pilus in recipient cell recognition during bacterial conjugation mediated by F-like plasmids. Mol. Microbiol. 13:939-953. [DOI] [PubMed] [Google Scholar]
- 2.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 3.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandler, M., and D. J. Galas. 1983. IS1-mediated tandem duplication of plasmid pBR322. Dependence on recA and on DNA polymerase I. J. Mol. Biol. 165:183-190. [DOI] [PubMed] [Google Scholar]
- 5.Cosma, C. L., P. N. Danese, J. H. Carlson, T. J. Silhavy, and W. B. Snyder. 1995. Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol. Microbiol. 18:491-505. [DOI] [PubMed] [Google Scholar]
- 6.Danese, P. N., and T. J. Silhavy. 1997. The sigma(E) and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 7.Danese, P. N., and T. J. Silhavy. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese, P. N., W. B. Snyder, C. L. Cosma, L. J. Davis, and T. J. Silhavy. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387-398. [DOI] [PubMed] [Google Scholar]
- 9.Dartigalongue, C., and S. Raina. 1998. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 17:3968-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean, G. E., R. M. Macnab, J. Stader, P. Matsumura, and C. Burks. 1984. Gene sequence and predicted amino acid sequence of the motA protein, a membrane-associated protein required for flagellar rotation in Escherichia coli. J. Bacteriol. 159:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Wulf, P., O. Kwon, and E. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181:6772-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong, J., S. Iuchi, H. S. Kwan, Z. Lu, and E. C. Lin. 1993. The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene 136:227-230. [DOI] [PubMed] [Google Scholar]
- 13.Finnegan, D., and N. Willetts. 1972. The nature of the transfer inhibitor of several F-like plasmids. Mol. Gen. Genet. 119:57-66. [DOI] [PubMed] [Google Scholar]
- 14.Frost, L. S., K. Ippen-Ihler, and R. A. Skurray. 1994. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol. Rev. 58:162-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost, L. S., and J. Manchak. 1998. F-phenocopies: characterization of expression of the F transfer region in stationary phase. Microbiology 144:2579-2587. [DOI] [PubMed] [Google Scholar]
- 16.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. ASM Press, Washington, D.C.
- 18.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerome, L. J., T. van Biesen, and L. S. Frost. 1999. Degradation of FinP antisense RNA from F-like plasmids: the RNA-binding protein, FinO, protects FinP from ribonuclease E. J. Mol. Biol. 285:1457-1473. [DOI] [PubMed] [Google Scholar]
- 20.Jones, C. H., P. N. Danese, J. S. Pinkner, T. J. Silhavy, and S. J. Hultgren. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16:6394-6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koraimann, G., and G. Hogenauer. 1989. A stable core region of the tra operon mRNA of plasmid R1-19. Nucleic Acids Res. 17:1283-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kundu, T. K., S. Kusano, and A. Ishihama. 1997. Promoter selectivity of Escherichia coli RNA polymerase sigmaF holoenzyme involved in transcription of flagellar and chemotaxis genes. J. Bacteriol. 179:4264-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. H., L. S. Frost, and W. Paranchych. 1992. FinOP repression of the F plasmid involves extension of the half-life of FinP antisense RNA by FinO. Mol. Gen. Genet. 235:131-139. [DOI] [PubMed] [Google Scholar]
- 25.Little, J. W., S. H. Edmiston, L. Z. Pacelli, and D. W. Mount. 1980. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc. Natl. Acad. Sci. USA 77:3225-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macnab, R. B. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 27.Majdalani, N., and K. Ippen-Ihler. 1996. Membrane insertion of the F-pilin subunit is Sec independent but requires leader peptidase B and the proton motive force. J. Bacteriol. 178:3742-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurizi, M. R., W. P. Clark, S. H. Kim, and S. Gottesman. 1990. ClpP represents a unique family of serine proteases. J. Biol. Chem. 265:12546-12552. [PubMed] [Google Scholar]
- 29.McEwen, J., L. Sambucetti, and P. M. Silverman. 1983. Synthesis of outer membrane proteins in cpxA cpxB mutants of Escherichia coli K-12. J. Bacteriol. 154:375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen, J., and P. Silverman. 1980. Chromosomal mutations of Escherichia coli that alter expression of conjugative plasmid functions. Proc. Natl. Acad. Sci. USA 77:513-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEwen, J., and P. Silverman. 1980. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine syntheses. J. Bacteriol. 144:68-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwen, J., and P. M. Silverman. 1982. Mutations in genes cpxA and cpxB alter the protein composition of Escherichia coli inner and outer membranes. J. Bacteriol. 151:1553-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. 1972. Experiments in molecular genetics, p. 201-205. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Moore, D., B. A. Sowa, and K. Ippen-Ihler. 1981. Location of an F-pilin pool in the inner membrane. J. Bacteriol. 146:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullineaux, P., and N. Willetts. 1985. Promoters in the transfer region of plasmid F. Basic Life Sci. 30:605-614. [DOI] [PubMed] [Google Scholar]
- 36.Nakayama, S., and H. Watanabe. 1995. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 177:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penfold, S. S., J. Simon, and L. S. Frost. 1996. Regulation of the expression of the traM gene of the F sex factor of Escherichia coli. Mol. Microbiol. 20:549-558. [DOI] [PubMed] [Google Scholar]
- 38.Pogliano, J., J. M. Dong, P. De Wulf, D. Furlong, D. Boyd, R. Losick, K. Pogliano, and E. C. Lin. 1998. Aberrant cell division and random FtsZ ring positioning in Escherichia coli cpxA* mutants. J. Bacteriol. 180:3486-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pogliano, J., A. S. Lynch, D. Belin, E. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169-1182. [DOI] [PubMed] [Google Scholar]
- 40.Rainwater, S., and P. M. Silverman. 1990. The Cpx proteins of Escherichia coli K-12: evidence that cpxA, ecfB, ssd, and eup mutations all identify the same gene. J. Bacteriol. 172:2456-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raivio, T. L., M. W. Laird, J. C. Joly, and T. J. Silhavy. 2000. Tethering of CpxP to the inner membrane prevents spheroplast induction of the cpx envelope stress response. Mol. Microbiol. 37:1186-1197. [DOI] [PubMed] [Google Scholar]
- 42.Raivio, T. L., D. L. Popkin, and T. J. Silhavy. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 181:5263-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ecf sigma factors. Annu. Rev. Microbiol. 55:591-624. [DOI] [PubMed] [Google Scholar]
- 45.Sandercock, J. R., and L. S. Frost. 1998. Analysis of the major domains of the F fertility inhibition protein, FinO. Mol. Gen. Genet. 259:622-629. [DOI] [PubMed] [Google Scholar]
- 46.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with Gene Fusions. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 47.Silverman, P. M., S. Rother, and H. Gaudin. 1991. Arc and Sfr functions of the Escherichia coli K-12 arcA gene product are genetically and physiologically separable. J. Bacteriol. 173:5648-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverman, P. M., L. Tran, R. Harris, and H. M. Gaudin. 1993. Accumulation of the F plasmid TraJ protein in cpx mutants of Escherichia coli. J. Bacteriol. 175:921-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman, P. M., E. Wickersham, and R. Harris. 1991. Regulation of the F plasmid traY promoter in Escherichia coli by host and plasmid factors. J. Mol. Biol. 218:119-128. [DOI] [PubMed] [Google Scholar]
- 50.Skurray, R. A., H. Nagaishi, and A. J. Clark. 1978. Construction and BamHI analysis of chimeric plasmids containing EcoRI DNA fragments of the F sex factor. Plasmid 1:174-186. [DOI] [PubMed] [Google Scholar]
- 51.Snyder, W. B., L. J. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sowa, B. A., D. Moore, and K. Ippen-Ihler. 1983. Physiology of F-pilin synthesis and utilization. J. Bacteriol. 153:962-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strohmaier, H., R. Noiges, S. Kotschan, G. Sawers, G. Hogenauer, E. L. Zechner, and G. Koraimann. 1998. Signal transduction and bacterial conjugation: characterization of the role of ArcA in regulating conjugative transfer of the resistance plasmid R1. J. Mol. Biol. 277:309-316. [DOI] [PubMed] [Google Scholar]
- 54.Sutton, A., T. Newman, J. McEwen, P. M. Silverman, and M. Freundlich. 1982. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in acetohydroxyacid synthase I function in vivo. J. Bacteriol. 151:976-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torreblanca, J., S. Marques, and J. Casadesus. 1999. Synthesis of FinP RNA by plasmids F and pSLT is regulated by DNA adenine methylation. Genetics 152:31-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Biesen, T., and L. S. Frost. 1994. The FinO protein of IncF plasmids binds FinP antisense RNA and its target, traJ mRNA, and promotes duplex formation. Mol. Microbiol. 14:427-436. [DOI] [PubMed] [Google Scholar]
- 58.Weber, R. F., and P. M. Silverman. 1988. The cpx proteins of Escherichia coli K12: structure of the cpxA polypeptide as an inner membrane component. J. Mol. Biol. 203:467-478. [DOI] [PubMed] [Google Scholar]
- 59.Wilkins, B. M., and L. S. Frost. 2001. Mechanisms of gene exchange between bacteria, p. 355-400. In M. Sussman (ed.), Molecular medical microbiology. Academic Press, London, England.
- 60.Yoshioka, Y., H. Ohtsubo, and E. Ohtsubo. 1987. Repressor gene finO in plasmids R100 and F: constitutive transfer of plasmid F is caused by insertion of IS3 into F finO. J. Bacteriol. 169:619-623. [DOI] [PMC free article] [PubMed] [Google Scholar]