Abstract
The importance of the mtrCDE-encoded efflux pump in conferring chromosomally mediated penicillin resistance on certain strains of Neisseria gonorrhoeae was determined by using genetic derivatives of penicillin-sensitive strain FA19 bearing defined mutations (mtrR, penA, and penB) donated by a clinical isolate (FA6140) expressing high-level resistance to penicillin and antimicrobial hydrophobic agents (HAs). When introduced into strain FA19 by transformation, a single base pair deletion in the mtrR promoter sequence from strain FA6140 was sufficient to provide high-level resistance to HAs (e.g., erythromycin and Triton X-100) but only a twofold increase in resistance to penicillin. When subsequent mutations in penA and porIB were introduced from strain FA6140 into strain WV30 (FA19 mtrR) by transformation, resistance to penicillin increased incrementally up to a MIC of 1.0 μg/ml. Insertional inactivation of the gene (mtrD) encoding the membrane transporter component of the Mtr efflux pump in these transformant strains and in strain FA6140 decreased the MIC of penicillin by 16-fold. Genetic analyses revealed that mtrR mutations, such as the single base pair deletion in its promoter, are needed for phenotypic expression of penicillin and tetracycline resistance afforded by the penB mutation. As penB represents amino acid substitutions within the third loop of the outer membrane PorIB protein that modulate entry of penicillin and tetracycline, the results presented herein suggest that PorIB and the MtrC-MtrD-MtrE efflux pump act synergistically to confer resistance to these antibiotics.
Chromosomally mediated resistance of gonococci to certain antibiotics (e.g., erythromycin, penicillin, and tetracycline) developed in the 1970s because of changes in genes (7, 25-27) encoding penicillin-binding proteins (PBPs), a mutation termed Mtr (multiple transferable resistance) that enhanced gonococcal resistance to structurally diverse antimicrobial hydrophobic agents (HAs) (12, 15), and the replacement of the major outer membrane porin protein (Por) with a similar but antigenically distinct porin (5, 10). The molecular basis for these mutations and how they contribute to antimicrobial resistance are now better understood. Thus, insertion of an aspartic acid-encoding codon between positions 345 and 346 (3) of the wild-type gene (penA) encoding PBP2 likely arose by horizontal gene exchange between a commensal neisserial species and the gonococcus (26). This mutation reduces the binding affinity of PBP2 for penicillin, which results in decreased (fourfold) susceptibility of gonococci to penicillin (7, 25). The Mtr property is due to the action of the MtrC-MtrD-MtrE efflux pump that exports HAs by an energy-dependent process (6, 12, 13). Mutations in the coding or promoter region of a gene encoding a transcriptional repressor (mtrR) of the mtrCDE operon result in the Mtr property (12, 21, 23). These mtrR mutations, by themselves, can decrease the susceptibility of gonococci to penicillin by only twofold (12, 15, 24). However, when a coresident penA mutation is present, resistance increases 8- to 10-fold (10, 22, 24). Replacement of the PorIA-encoding gene with the PorIB-encoding allele and subsequent missense mutations that result in amino acid replacements in loop 3 of PorIB, resulting in the penB genotype, decrease influx of penicillin and tetracycline (10). In gonococcal strains harboring penA, penB, and mtrR mutations, resistance to penicillin increases nearly 66-fold. Curiously, penicillin and tetracycline resistance due to penB seems to be dependent on the presence of an mtrR mutation in the host strain (24).
In the early 1980s, an outbreak of gonorrhea occurred in the Durham, N.C., region that was caused by a strain (FA6140) for which the MICs of penicillin and tetracycline were both 4.0 μg/ml (9). On the basis of transformation studies of a penicillin-sensitive recipient (strain FA19) generated with FA6140 donor DNA, FA6140 seemed to harbor penA, mtrR, and penB mutations, as well as other mutations that increased gonococcal resistance to penicillin to a high level (22). However, these additional mutations could not be introduced into strain FA19, even when it contained penA, mtrR, and penB. Recent work by Ropp et al. (22) has detected two mutations in FA6140 that seem to be essential for high-level penicillin resistance. Thus, strain FA6140 contains a mutation (ponA1) in the gene encoding PBP1 that decreases the rate of acylation of the PBP by penicillin three- to fourfold. The second mutation is at an undetermined locus that is necessary for the ponA1 mutation to increase penicillin resistance to its final level (22). Studies with an FA19 transformant containing the penA, mtrR, and penB mutations revealed a mutation in a locus termed penC, which, like the undefined mutation in FA6140, allows the ponA1 mutation to increase penicillin resistance. Although the identity of penC is not known, penC does not appear to be present in FA6140 (22).
While strains of gonococci expressing increased resistance to penicillin and/or other antibiotics have attracted considerable attention because of treatment failures, certain clinical isolates frequently express hypersusceptibility to penicillin and HAs (8). This phenotype is of interest because these strains often harbor penA, penB, and/or mtrR mutations that would normally provide for decreased susceptibility to these antimicrobials. We recently described two such strains that contain small deletions within their mtrC and mtrD genes, which encode the membrane fusion protein (MtrC) and the cytoplasmic membrane transporter component (MtrD) of the MtrC-MtrD-MtrE efflux system (29). Repair of these mutations by gene-specific PCR products from wild-type strain FA19 resulted in enhanced (16-fold) resistance to penicillin and HAs (29). Thus, mutations within the mtrCDE-encoded efflux pump seemed to phenotypically suppress other mutations involved in antimicrobial resistance.
In order to better define the role of the MtrC-MtrD-MtrE efflux pump in determining levels of gonococcal resistance or susceptibility to penicillin, we examined strain FA6140. We confirmed the presence of mutations in the penA, mtrR, and penB genes and demonstrated that loss of the MtrC-MtrD-MtrE efflux pump in this strain results in 16- and 4-fold decreases in the MICs of penicillin and tetracycline, respectively. This observation suggested that even though penicillin is a relatively hydrophilic antibiotic, the presence of an intact MtrC-MtrD-MtrE efflux pump is essential for chromosomally mediated penicillin resistance in gonococci that results from the presence of penA, mtrR, and penB. This decrease in penicillin resistance seemed paradoxical because the presence and expression of mtrR mutations by themselves have only a minor (twofold) impact on levels of penicillin resistance. Our results emphasize the synergistic action of chromosomal mutations in the development of penicillin resistance in gonococci even when those mutations by themselves (e.g., mtrR) provide for only a minor change in antibiotic susceptibility.
(A preliminary account of these studies was presented at the Fourteenth Meeting of the International Society for Sexually Transmitted Diseases Research during the International Congress of Sexually Transmitted Infections held in Berlin, Germany, 24 to 27 June 2001.)
MATERIALS AND METHODS
Strains of Neisseria gonorrhoeae used and growth conditions.
The strains of gonococci used in this investigation are listed in Table 1. For routine growth, they were propagated as nonpiliated, opacity-negative colony variants on GCB agar (Difco Laboratories, Detroit, Mich.) plates containing defined supplements I and II (14) at 37°C under 3.8% (vol/vol) CO2.
TABLE 1.
Genotypes and sources of gonococcal strains used in this study
| Strain | Genotype (reference) | Sourcea |
|---|---|---|
| FA19 | Wild type | P. F. Sparling |
| WV30 | mtrR171 | FA6140 DNA × FA19 |
| WV31 | mtrR171 mtrD::kan | mtrD::kan PCR × WV30 |
| WV32 | penA4 | FA6140 DNA × FA19 |
| WV33 | penA4 mtrD::kan | mtrD::kan PCR × WV32 |
| WV34 | mtrR171 penA4 | FA6140 DNA × WV32 |
| WV35 | mtrR171 mtrD::kan penA4 | mtrD::kan PCR × WV34 |
| WV36 | mtrR171 penA4 pen B | FA6140 DNA × WV34 |
| WV37 | mtrR171 mtrD::kan penA4 penB | mtrD::kan PCR × WV36 |
| FA19AB | penA penB | R. Nicholas |
| WV38 | mtrR171 penA penB | FA6140 mtrR PCR × FA19AB |
| FA140 | mtrR140 penA2 penB2 (24) | P. F. Sparling |
| WV24 | mtrR140 mtrD::kan penA2 penB2 | mtrD::kan PCR × FA140 |
| FA6140 | mtrR171 penA4 penB ponA1 | P. F. Sparling |
| WV22 | mtrR171 mtrD:: kan penA4 penB ponA1 | mtrD:: kan PCR × FA6140 |
Transformants are shown as donor DNA × recipient strain, produced as described in Materials and Methods. PCR products were gel purified prior to transformation. Gene descriptions: mtrR encodes a transcriptional repressor of MtrCDE expression; mtrD encodes the transporter component of the MtrCDE efflux system; penA is the structural gene for PBP-2; penB is an allele of porB, encoding the gonococcal porin; and ponA is the structural gene for PBP-1.
Antibiotic susceptibility testing.
The antibiotic dilution agar procedure described previously (24) was used to determine the MICs of antimicrobials (erythromycin, nafcillin, penicillin, tetracycline, and Triton X-100 [TX-100]) for the test strains. MICs were recorded after 48 h of incubation, as described above, and the reported values are representative of at least three determinations. All of the antimicrobials were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Preparation of DNA, PCR, DNA sequencing, genetic transformation, and RT-PCR.
Chromosomal DNA from agar-grown gonococci was isolated as described previously (17). PCR amplification of specific genes was performed essentially as described by Hagman et al. (12), with gene-specific oligonucleotide primers as follows: mtrR, KH9#3 and CEL#1; mtrD::kan, mtrD#1 and mtrD#2. Primer sequences are as reported by Veal et al. (29). The penA and porB genes were amplified with the following primers: MO-Porin5′ (5′-CGGGATCCGCCGTCTGAAATGAAAAAATCCCTGATTGCCCTG-3′), MO-Porin3′ (5′-CGGAATTCGCCGTCTGAATATGGATAGATTCGTCATTCCCGC-3′), PenA-up (5′-GGAATTCTTCAGACGGCGAAGTAAAAATGTTGATTAAAAGCG-3′), and PenA-down (5′-GAGAGAATTCTTAAGACGGTGTTTTGACGG-3′). The products were analyzed by agarose gel electrophoresis, purified, and subjected to automated DNA sequencing or used in genetic transformation experiments.
Total RNA was prepared from gonococci by the method of Biran et al. (2). Gene expression was quantified by reverse transcriptase (RT) PCR (1) with first-strand cDNA synthesized from total RNA with Superscript II RT (Gibco BRL).
Transformation of piliated gonococci with purified PCR products or chromosomal DNA was performed essentially as described by Gunn and Stein (11); chromosomal DNA was typically used at 1 μg/ml. Transformants were selected on GCB agar plates containing the described antibiotic at a concentration of at least twice the MIC for the recipient strain.
Construction of FA19 transformant strains.
We constructed a penA transformant of strain FA19 with chromosomal DNA from strain FA6140 by selecting transformants on GCB agar plates containing 0.03 μg of penicillin per ml. Representative transformants were screened for cross-resistance to HAs to rule out transformants bearing the mtrR mutation from the donor strain. The penA gene from a representative transformant (WV32) was PCR amplified and subjected to DNA sequencing, which confirmed the presence of the Asp-345a insertion characteristic of penA genes from chromosomally mediated penicillin-resistant strains. We transferred the mtrR mutation from FA6140 into WV32 (FA19 penA) by transformation with FA6140 chromosomal DNA and selection on GCB agar plates containing 1,000 μg of TX-100 per ml. We introduced the penB mutation from strain FA6140 into WV34 (FA19 penA mtrR) by selecting transformants on GCB agar plates containing 0.25 μg of penicillin per ml. The porB gene from a representative transformant (WV36) was also PCR amplified and subjected to automated DNA sequencing to confirm the presence of the penB gene.
RESULTS AND DISCUSSION
Presence of an mtrR mutation in strain FA6140.
The high-level penicillin-resistant strain FA6140 (MIC = 4 μg/ml) was previously postulated by Faruki et al. (9) to contain an mtrR mutation, as it exhibits high-level resistance to the HAs TX-100 and erythromycin (Table 2). In order to determine the nature of this presumed mutation, a PCR product of the mtrR-coding and upstream regions was subjected to DNA sequencing. The results (not shown) revealed that it possessed a wild-type mtrR-coding sequence but had a single base pair deletion within the 13-bp inverted repeat sequence within the mtrR promoter. This mutation has been previously documented by us (12) to be sufficient for high levels of TX-100 and erythromycin resistance. Indeed, transformation of HA-susceptible strain FA19 with FA6140 chromosomal DNA generated transformants, such as strain WV30 (Table 2), that expressed the erythromycin and TX-100 resistance property of strain FA6140 and was found by DNA sequencing to contain the single base pair deletion (data not presented) within the mtrR promoter. The transformant strain also expressed a twofold increase in resistance to penicillin relative to that of recipient strain FA19 (Table 2).
TABLE 2.
Insertional inactivation of MtrD eliminates the intermediate- and high-level chromosomally mediated penicillin resistance of strains FA140 and FA6140a
| Strain | mtrR | mtrD | pen | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|---|---|
| TET | ERY | PEN | NAF | TX-100 | ||||
| FA19 | + | + | − | 0.25 | 0.25 | 0.015 | 0.25 | 250 |
| WV30 | mtrR171 | + | − | 0.25 | 2.0 | 0.03 | 1.0 | >16,000 |
| WV31 | mtrR171 | mtrD::kan | − | 0.25 | 0.06 | 0.015 | 0.03 | 15 |
| FA140 | mtrR140 | + | penA2, penB2 | 1.0 | 2.0 | 1.0 | 8.0 | >16,000 |
| WV24 | mtrR140 | mtrD::kan | penA2, penB2 | 0.25 | 0.06 | 0.06 | 0.125 | 31 |
| FA6140 | mtrR171 | + | penA4, penB, ponA1 | 4.0 | 2.0 | 4.0 | 32 | >16,000 |
| WV22 | mtrR171 | mtrD::kan | penA4, penB, ponA1 | 1.0 | 0.06 | 0.25 | 1.0 | 31 |
MIC of TET (tetracycline), ERY (erythromycin), PEN (penicillin G), NAF (nafcillin), and TX-100 are representative values for three or more determinations. +, wild type; −, no mutation in pen genes (see Table 1, foonote a).
Insertional inactivation of mtrD increases gonococcal susceptibility to penicillin.
We confirmed the role of the MtrC-MtrD-MtrE efflux pump in determining the HA and penicillin resistance properties of transformant strain WV30 by introducing the insertionally inactivated mtrD sequence (mtrD::kan) described previously by Hagman et al. (13). A selected kanamycin-resistant transformant (WV31) was found to contain an insertionally inactivated mtrD gene (data not presented) and displayed hypersusceptibility to HAs and a twofold decrease in the MIC of penicillin (Table 2). Thus, while the mtrR mutation from strain FA6140 by itself was needed for high levels of HA resistance, it could only contribute a twofold increase in resistance to penicillin.
We next examined the role of the MtrC-MtrD-MtrE efflux pump in conferring high-level penicillin resistance on strain FA6140 and intermediate-level penicillin resistance on strain FA140 (Table 2). Like FA6140, FA140 contains the single base pair deletion in the mtrR promoter sequence (29). FA140 also contains the penA and penB mutations needed for enhanced resistance to penicillin (10, 24). Transformants of these strains bearing the mtrD::kan sequence were generated (WV22 and WV24), and these transformants expressed hypersusceptibility to HAs and showed 16- and 4-fold decreases in the MICs of penicillin and tetracycline, respectively (Table 2). Thus, in the presence of the other mutations (e.g., penA, penB, ponA1, and penC) needed for chromosomally mediated resistance of gonococci to penicillin, the fold decrease in the MIC of penicillin due to loss of the MtrC-MtrD-MtrE efflux pump was greater than in the absence of these mutations.
penB-mediated penicillin resistance is affected by loss of MtrC-MtrD-MtrE function.
Since the fold decreases in the MICs of penicillin were identical in transformants of strains FA140 and FA6140 bearing mtrD::kan, we asked whether their common penA or penB mutation or both would be phenotypically suppressed by the mtrD::kan mutation. For this purpose, we constructed several strains as described in Materials and Methods. A penA transformant of strain FA19 (WV32) expressed a fourfold increase in penicillin but had wild-type levels of HA resistance (Table 3). Sequencing of the penA gene of this strain revealed the presence of the Asp-345a codon insertion that is characteristic of altered penA alleles (data not shown). We next transferred the mtrR mutation from FA6140 into WV32 (FA19 penA); a representative transformant, WV34, displayed resistance to HAs characteristic of strain FA6140 and resistance to penicillin that was twofold higher than that of the penA recipient (MICs of penicillin were 0.25 and 0.125 μg/ml, respectively; Table 3). We then introduced the penB mutation from strain FA6140 into WV34 (FA19 penA mtrR). The porB gene from a representative transformant (WV36) was PCR amplified and subjected to DNA sequencing to ensure correct introduction of the porB allele from strain FA6140, which is responsible for the penB property (22). Analysis of this sequence confirmed that the porA gene of strain FA19 had been replaced with the porB allele of strain FA6140; this porB sequence contained missense mutations at positions 120 and 121 (data not shown) that are thought to impart the penB phenotype (10).
TABLE 3.
MtrD mutations eliminate the contribution of mtr and penB, but not that of penA, to penicillin and nafcillin resistancea
| Strain | mtrR | mtrD | pen | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|---|---|
| TET | ERY | PEN | NAF | TX-100 | ||||
| FA19 | + | + | − | 0.25 | 0.25 | 0.015 | 0.25 | 250 |
| WV30 | mtrR171 | + | − | 0.25 | 2.0 | 0.03 | 1.0 | >16,000 |
| WV31 | mtrR171 | mtrD::kan | − | 0.25 | 0.06 | 0.015 | 0.03 | 15 |
| WV32 | + | + | penA4 | 0.25 | 0.25 | 0.125 | 4.0 | 250 |
| WV33 | + | mtrD::kan | penA4 | 0.25 | 0.06 | 0.125 | 0.25 | 15 |
| WV34 | mtrR171 | + | penA4 | 0.25 | 2.0 | 0.25 | 16 | >16,000 |
| WV35 | mtrR171 | mtrD::kan | penA4 | 0.25 | 0.06 | 0.125 | 0.25 | 15 |
| WV36 | mtrR171 | + | penA4, penB | 1.0 | 2.0 | 1.0 | 32 | >16,000 |
| WV37 | mtrR171 | mtrD::kan | penA4, penB | 0.25 | 0.06 | 0.06 | 0.25 | 31 |
With transformants in hand of strain FA19 containing single and various combinations of resistance determinants derived from FA6140, i.e., mtrR alone (WV30), penA alone (WV32), penA and mtrR (WV34), and mtrR, penA, and penB (WV36), we asked which strains would be most severely affected in their resistance to penicillin following the introduction of mtrD::kan. As shown in Table 3, insertional inactivation of mtrD in WV32 (FA19 penA), resulting in WV33, had no impact on its level of penicillin resistance, whereas the same mutation in WV30 (FA19 mtrR) and WV34 (FA19 mtrR penA), resulting in strains WV31 and WV35, respectively, decreased penicillin resistance by twofold. In contrast, inactivation of mtrD in WV36 (FA19 mtrR penA penB) resulted in a 16-fold decrease in penicillin resistance, as shown for strain WV37 (Table 3). This fold decrease in penicillin resistance was identical to that observed when mtrD was inactivated in strains FA140 and FA6140 (Table 2).
Compared to other substrates of the MtrC-MtrD-MtrE efflux pump, penicillin is a relatively hydrophilic compound; therefore, we asked if the same decrease in resistance to a more hydrophobic β-lactam would be observed upon loss of the MtrC-MtrD-MtrE pump. Since nafcillin represents a more hydrophobic β-lactam and is better recognized by the AcrA-AcrB-TolC efflux pump of Salmonella enterica serovar Typhimurium (20), we determined its MIC for our parental and test strains. On a weight basis, nafcillin was less active than penicillin against all of the test strains (Table 3). However, the results demonstrated that nafcillin is a better substrate for the MtrC-MtrD-MtrE efflux system than is penicillin, as inactivation of mtrD and/or mtrR had a more profound effect upon nafcillin susceptibility, even in the FA19 penA strain (WV32) (Table 3). Thus, inactivation of mtrD in penA mtr penB mutant strains (i.e., FA140, FA6140, and WV36) resulted in an even greater fold decrease in nafcillin resistance, 32- to 64-fold, than the 16-fold decrease observed for penicillin resistance (WV22, WV24, and WV37; Tables 2 and 3). It was interesting that acquisition of the penB mutation by WV34 (FA19 penA mtrR) only increased nafcillin resistance twofold (see strain WV36), which was much lower than the fold increase in penicillin resistance (Table 3). The small increase in resistance upon transfer of penB presumably results from the decreased use of porins for entry of nafcillin due to its hydrophobicity; thus, porin alterations in gonococci likely have less of an effect on nafcillin susceptibility.
mtrR mutations are required for penB-mediated penicillin resistance in gonococci.
Sparling and coworkers (24) first reported that penB transformants of strain FA19 could only be recovered when the recipient strain contained a resident mutation in the gene now termed mtrR. Since MtrR is a transcriptional regulator that depresses the expression of mtrCDE (12), we hypothesized that in strains such as FA140 and FA6140, overexpression of mtrCDE, due to loss of MtrR, acts synergistically with the decreased antibiotic permeation due to penB (10) to provide increased resistance to penicillin. An alternative hypothesis, while not mutually exclusive, is that MtrR also regulates porB gene expression.
To test these ideas, we first examined the penicillin resistance of a penA penB transformant strain of FA19 containing the wild-type mtrR gene. This strain (FA19AB; Table 4) does not demonstrate any increase in penicillin resistance versus that of the parental FA19 penA strain; however, acquisition of an mtrR mutation (leading to increased TX-100 resistance) resulted in an eightfold increase in penicillin resistance (Table 4), which confirms that an mtrR mutation is required for the ability of penB to increase penicillin resistance. Therefore, we determined if the synergy observed between the mtrR and penB mutations with respect to penicillin resistance is due to regulation of porB expression by MtrR. RT-PCR with RNA extracted from FA19AB (FA19 penA penB) and WV38 (FA19 penA mtrR penB) demonstrated that expression of porB was the same in both strains, while that of mtrC, which is known to be regulated by MtrR (12), was enhanced (data not presented). These data indicated that MtrR does not regulate porB expression. Thus, the synergistic effect seen upon addition of the mtrR mutation to a strain possessing penB is not due to transcriptional regulation.
TABLE 4.
An mtrR mutation is required for expression of penB-mediated penicillin and nafcillin resistancea
| Strain | mtrR | pen | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|---|
| TET | PEN | NAF | TX-100 | |||
| FA19 | + | − | 0.25 | 0.015 | 0.25 | 250 |
| FA19AB | + | penA4, penB | 0.25 | 0.06 | 1.0 | 250 |
| WV38 | mtrR171 | penA4, penB | 1.0 | 0.5 | 8.0 | >16,000 |
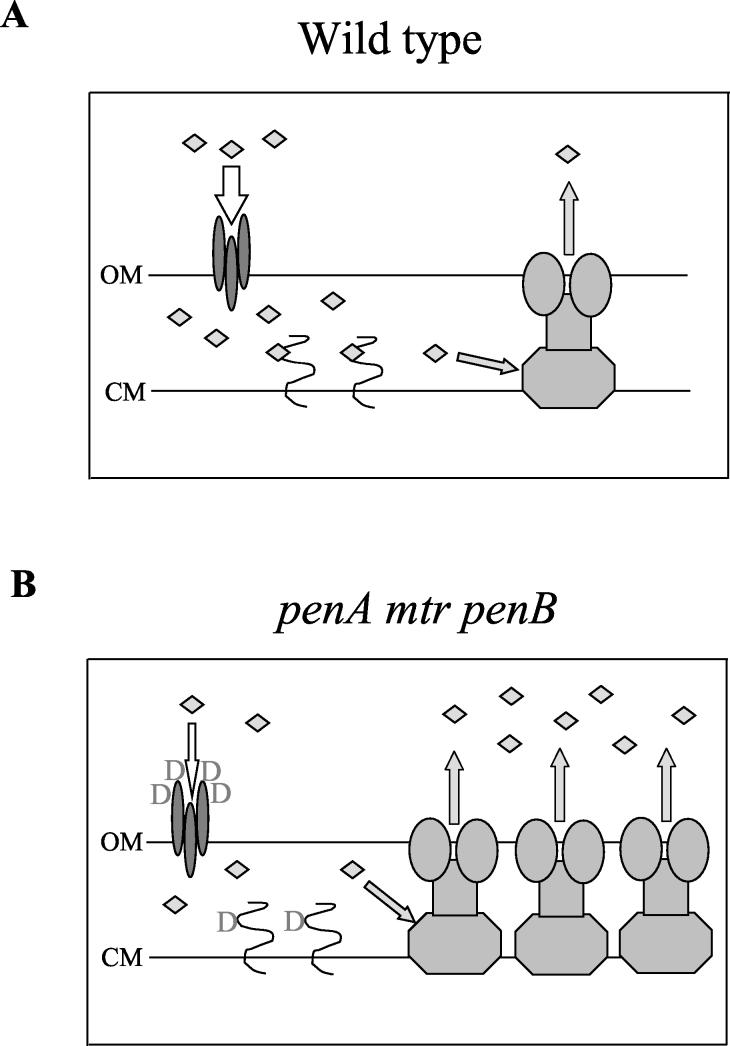
Conclusions. The evidence presented herein clearly demonstrates that the MtrC-MtrD-MtrE efflux system and PenB alteration of outer membrane permeability act synergistically to increase resistance to penicillin and tetracycline in N. gonorrhoeae. In contrast, efflux systems that only export to the periplasm of gram-negative bacteria, such as the tetracycline transporter of Escherichia coli, do not show synergistic activity with outer membrane permeability alterations (28). This report directly shows the requirement of the tripartite efflux pump MtrC-MtrD-MtrE, which exports to the extracellular medium, for expression of penicillin resistance in gonococci, demonstrating the synergy proposed by Nikaido (18, 19). This synergy is not due to transcriptional control of the porin PorIB; rather, it is likely due to a highly effective combination of efflux and reduced influx (Fig. 1). Interestingly, the proposed decrease in penicillin influx due to the penB mutation (10) is not sufficient to confer increased resistance without the concomitant overexpression of MtrCDE provided by mutations in mtrR. This explains the inability to select for the penB-mediated increase in penicillin resistance in the absence of mtrR mutations (24) and confirms the suggestion of Gill et al. (10) that the reduction in penicillin entry is not large. A small reduction in permeability to penicillin might be preferred by the bacterium, as this would presumably translate into less inhibition of nutrient entry via the porin as well (19). Finally, it is important to note that chromosomally mediated penicillin and tetracycline resistance in gonococci continues to be manifested in clinical isolates (4, 16). Therefore, the finding that the MtrCDE system is required for clinically significant levels of penicillin resistance, even in strains with other mutations providing resistance (penA, penB, ponA1, and penC), and the contribution of mtrR mutations to penB-mediated tetracycline resistance emphasize the contribution of efflux to bacterial resistance to treatment with antibiotics. In addition, gonococcal strains that contain penicillin resistance determinants but are phenotypically penicillin sensitive because of mutations in mtrCDE would provide a pool of resistance genes for donation to other strains, thereby maintaining the capacity for penicillin resistance in the population.
FIG. 1.
Model for the synergistic action of the MtrCDE efflux pump with penicillin resistance determinants. (A) Penicillin action in wild-type strains. In wild-type strains, penicillin, which is represented by diamonds, crosses the outer membrane (OM) via the trimeric porin (19) and reaches a substantial concentration in the periplasmic space, where it binds to PBP2, the lethal target in gonococci (27). Some amount of penicillin is exported by the MtrCDE efflux system (12). (B) Penicillin resistance due to penA, mtrR, and penB resistance determinants. In strains bearing the penA, mtrR, and penB mutations, influx of penicillin through the porin is reduced because of the replacement of two aspartate residues in loop 3 of the porin (10), binding of penicillin to PBP2 is reduced because of the additional aspartate residue present at codon 345A in PBP2 (3), and efflux of penicillin is increased because of overexpression of MtrCDE as a result of the mutation affecting mtrR (12). CM, cytoplasmic membrane; D, aspartate.
Acknowledgments
We thank P. F. Sparling for providing strain FA6140, P. Ropp and M. Olesky for primer design, and M. Olesky for invaluable work in constructing the FA19 penA penB strain.
This work was supported by grants AI-21150 (W.M.S.) and AI-36901 (R.A.N.) from the National Institutes of Health. W.M.S. was supported by a Senior Research Career Scientist Award from the Veterans Affairs Medical Research Service.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 2.Biran, D., N. Brot, H. Weissbach, and E. Z. Ron. 1995. Heat shock-dependent transcriptional activation of the metA gene of Escherichia coli. J. Bacteriol. 177:1374-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brannigan, J. A., I. A. Tirodimos, Q.-Y. Zhang, C. G. Dowson, and B. G. Spratt. 1990. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 4:913-919. [DOI] [PubMed] [Google Scholar]
- 4.CDC Sexually Transmitted Diseases Surveillance. 2000. Supplement: Gonococcal Isolate Surveillance Project (GISP) annual report—1999. U.S. Department of Health and Human Services, Public Health Service, Atlanta, Ga.
- 5.Danielsson, D., H. Faruki, D. Dyer, and P. F. Sparling. 1986. Recombination near the antibiotic resistance locus penB results in antigenic variation of gonococcal outer membrane protein I. Infect. Immun. 52:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delahay, R. M., B. D. Robertson, J. T. Balthazar, W. M. Shafer, and C. Ison. 1997. Involvement of the gonococcal MtrE protein in the resistance of Neisseria gonorrhoeae to toxic hydrophobic compounds. Microbiology 143:2127-2133. [DOI] [PubMed] [Google Scholar]
- 7.Dowson, C. G., A. E. Jephcott, K. R. Gough, and B. G. Spratt. 1989. Penicillin-binding protein 2 genes of non-β-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 3:35-41. [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein, B. I., and P. F. Sparling. 1975. Mutations to increased antibiotic sensitivity in naturally-occurring gonococci. Nature 271:242-244. [DOI] [PubMed] [Google Scholar]
- 9.Faruki, H., R. N. Kohmescher, W. P. McKinney, and P. F. Sparling. 1985. A community-based outbreak of infection with penicillin-resistant Neisseria gonorrhoeae not producing penicillinase (chromosomally-mediated resistance). N. Engl. J. Med. 313:607-611. [DOI] [PubMed] [Google Scholar]
- 10.Gill, M. J., S. Simjee, K. Al-Hattawi, B. D. Robertson, C. S. F. Easmon, and C. A. Ison. 1998. Gonococcal resistance to β-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob. Agents Chemother. 42:2799-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn, J. S., and D. C. Stein. 1996. Use of a non-selective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol. Gen. Genet. 251:509-517. [DOI] [PubMed] [Google Scholar]
- 12.Hagman, K. E., W. Pan, B. G. Spratt, J. T. Balthazar, R. C. Judd, and W. M. Shafer. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial agents is modulated by the mtrRCDE efflux system. Microbiology 141:611-622. [DOI] [PubMed] [Google Scholar]
- 13.Hagman, K. E., C. E. Lucas, J. T. Balthazar, L. Snyder, M. Nilles, R. C. Judd, and W. M. Shafer. 1997. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143:2117-2126. [DOI] [PubMed] [Google Scholar]
- 14.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maness, M. J., and P. F. Sparling. 1973. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J. Infect. Dis. 128:321-330. [DOI] [PubMed] [Google Scholar]
- 16.Mavroidi, A., L. S. Tzouvelekis, K. P. Kyriakis, H. Avgerinou, M. Danilidou, and E. Tzelepi. 2001. Multidrug-resistant strains of Neisseria gonorrhoeae in Greece. Antimicrob. Agents Chemother. 45:2651-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAllister, C. F., and D. S. Stephens. 1993. Analysis in Neisseria meningitidis and other Neisseria species of genes homologous to the FKBP immunophilin family. Mol. Microbiol. 10:13-24. [DOI] [PubMed] [Google Scholar]
- 18.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikaido, H. 2001. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin. Cell Dev. Biol. 12:215-223. [DOI] [PubMed] [Google Scholar]
- 20.Nikaido, H., M. Basina, V. Nguyen, and E. Y. Rosenberg. 1998. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J. Bacteriol. 180:4686-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan, W., and B. G. Spratt. 1994. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol. Microbiol. 11:769-775. [DOI] [PubMed] [Google Scholar]
- 22.Ropp, P. A., M. Hu, M. Olesky, and R. A. Nicholas. 2002. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafer, W. M., J. T. Balthazar, K. E. Hagman, and S. A. Morse. 1995. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology 141:907-911. [DOI] [PubMed] [Google Scholar]
- 24.Sparling, P. F., F. A. Sarubbi, Jr., and E. Blackman. 1975. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J. Bacteriol. 124:740-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spratt, B. G. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332:173-176. [DOI] [PubMed] [Google Scholar]
- 26.Spratt, B. G., L. D. Bowler, Q. Y. Zhang, J. Zhou, and J. M. Smith. 1992. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J. Mol. Evol. 34:115-125. [DOI] [PubMed] [Google Scholar]
- 27.Spratt, B. G. 1994. Resistance to antibiotics mediated by target alterations. Science 264:388-393. [DOI] [PubMed] [Google Scholar]
- 28.Thanassi, D. G., G. S. B. Suh, and H. Nikaido. 1995. Role of outer membrane barrier in efflux-mediated tetracycline resistance of Escherichia coli. J. Bacteriol. 177:998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veal, W. L., A. Yellen, J. T. Balthazar, W. Pan, B. G. Spratt, and W. M. Shafer. 1998. Loss-of-function mutations in the mtr efflux system of Neisseria gonorrhoeae. Microbiology 144:621-627. [DOI] [PubMed] [Google Scholar]