Abstract
The BacA protein is essential for the long-term survival of Sinorhizobium meliloti and Brucella abortus within acidic compartments in plant and animal cells, respectively. Since both the S. meliloti and B. abortus bacA mutants have an increased resistance to bleomycin, it was hypothesized that BacA was a transporter of bleomycin and bleomycin-like compounds into the bacterial cell. However, our finding that the S. meliloti bacA mutant also has an increased sensitivity to detergents, a hydrophobic dye, ethanol, and acid pH supported a model in which BacA function affects the bacterial cell envelope. In addition, an S. meliloti lpsB mutant that is defective at a stage in infection of the host similar to that found for a bacA mutant is also sensitive to the same agents, and the carbohydrate content of its lipopolysaccharide (LPS) is altered. However, analysis of crude preparations of the bacA mutant LPS suggested that, unlike that for LpsB, BacA function did not affect the carbohydrate composition of the LPS. Rather, we found that at least one function of BacA is to affect the distribution of LPS fatty acids, including a very-long-chain fatty acid thought to be unique to the α-proteobacteria, including B. abortus.
Over the last few years, evidence has been accumulating suggesting that there are certain strategies for intracellular survival that are used both by bacteria that interact with plant cells and bacteria that interact with animal cells (18). We have been investigating one of these strategies involving the BacA protein, which is essential for Sinorhizobium meliloti symbiosis and Brucella abortus pathogenesis (14, 24, 26). S. meliloti forms a symbiosis with the agriculturally important legume alfalfa and is taken up into the plant cells into a membrane-bound acidic compartment known as a symbiosome. Once inside this compartment, S. meliloti undergoes a number of changes both in its metabolism and cell envelope and differentiates into a nitrogen-fixing bacteroid (1, 35). Although B. abortus infects animal cells, it too is taken into the host cell in a membrane-bound acid compartment where it persists for an extensive period of time, resulting in a chronic infection of the host (30). In both the case of the S. meliloti-alfalfa and the B. abortus-BALB/c mouse systems, the respective bacA mutants were able to enter into the host cells normally but, unlike the wild-type strains, died shortly after entry into the membrane-bound acidic compartments (14, 24). Thus, BacA is required for persistence of bacteria within both plant and animal cells. However, the mechanism by which BacA enables the survival of bacteria within their hosts has remained elusive.
The S. meliloti bacA gene encodes a 420-amino-acid inner membrane protein, which is predicted to have seven transmembrane-spanning domains and is 82% similar (67% identical) to the B. abortus BacA protein (14, 24). The S. meliloti BacA protein is functionally interchangeable with SbmA (derived from “sensitivity to B17 microcin”) protein of Escherichia coli with which it is 79% similar (64% identical) (19). It was originally proposed that SbmA transports compounds with a bithiazole-oxazole moiety into the bacterial cell, since, in the absence of this protein, E. coli becomes resistant to exogenous microcin B17 and the glycopeptide bleomcyin, both of which possess this moiety (19, 23, 46). However, the subsequent finding that an E. coli sbmA mutant is also resistant to microcin J25 (37), which lacks this moiety, argued against this proposal (4). Since no other phenotypes have yet been identified for the E. coli sbmA mutants, the present model for the role of SbmA is that it is a transporter of peptide-like antibiotics into the cell (37). The S. meliloti and B. abortus bacA mutants similarly have an increased resistance to bleomycin; hence, it had been proposed that, in their hosts as well, BacA is required to transport an essential compound with some structural resemblance to bleomycin into the bacterial cell (19, 24). However, further analysis of the physiology of an S. meliloti bacA mutant in the Rm8002 genetic background revealed that loss of BacA also confers low-level resistance to aminoglycoside antibiotics and an increased sensitivity to ethanol and sodium dodecyl sulfate (SDS) (19, 25). In addition, the analysis of a series of site-directed mutations in the S. meliloti bacA gene provided evidence that BacA could have multiple, nonoverlapping functions (25). These experiments provided preliminary evidence for the hypothesis that the S. meliloti bacA mutant could have an altered cell envelope, and it was proposed that the inability of this mutant to survive within the host could be due to an enhanced sensitivity to one or more of the stresses encountered in the host cell (19).
Changes in the bacterial cell envelope, especially in the lipopolysaccharide (LPS), are thought to be involved in the differentiation of S. meliloti into the bacteroid state within the host cell (35). Recently an S. meliloti lpsB mutant, with a significantly altered LPS core structure, was also found to be defective at a critical stage in the alfalfa symbiosis process similar to that found for the S. meliloti bacA mutant (5), providing further support that the bacterial LPS plays an important role in survival within the host. Much of the LPS research on symbiotic bacteria has focused on Rhizobium leguminosarum. It has been proposed that the LPS of R. leguminosarum bacteroids is more hydrophobic than the LPS extracted from free-living bacteria, due to changes in the O antigen and to the accumulation of a very-long-chain fatty acid, 27-hydroxyoctacosanoic acid (27-OH-C28:0) on the lipid A (20). 27-OH-C28:0 has also been detected in the lipid A fraction from a number of α-proteobacteria, including S. meliloti and B. abortus, but appears to be absent from the LPS of enteric bacteria (2, 3, 27).
The physiological and chemical studies described in this paper support the hypothesis that BacA function affects the structure of the bacterial cell envelope and suggest that at least one of the envelope components affected is the LPS. These BacA-dependent effects on the cell envelope appear to be necessary for S. meliloti to establish the chronic intracellular infection required for a successful symbiosis.
MATERIALS AND METHODS
Bacterial growth.
All bacterial strains and plasmids used throughout this study are described in Table 1. For all experiments unless stated otherwise, S. meliloti cells were grown from frozen stocks to early stationary phase in Luria-Bertani (LB) media supplemented with 2.5 mM CaCl2 and 2.5 mM MgSO4 and 500 μg of streptomycin (Sm)/ml for 48 h at 30°C. The cultures were then washed and resuspended to the defined optical density at 600 nm (OD600) in LB media. When required, tetracycline (Tc) was added to a final concentration of 10 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Plasmid or strain | Relevant characteristics | Source or reference |
|---|---|---|
| Plasmids | ||
| pRK404 | Tcr broad-host-range control plasmid | 10 |
| pJG51A | pRK404 carrying the bacA gene | 14 |
| S. meliloti strains | ||
| Rm1021 | Wild-type strain; Smr derivative of SU47 | F. Ausubel |
| Rm8002 | Rm1021 Pho− | 26 |
| SmGF1 | Rm1021, bacA654::Spc (ΔbacA null) | This study |
| SmGC1 | Rm1021, lpsB389::TnphoA | 5 |
| SmGF2 | SmGF1, lpsB389::TnphoA | This study |
Filter disk and gradient assays.
The early-stationary-phase cultures were resuspended to an OD600 of 0.2. For the filter disk assay, 100 μl of culture was added to 3 ml LB of soft agar (6.5 g/liter) and poured onto LB plates (25 ml). After 30 min, a paper disk (6-mm diameter; Becton Dickinson) was applied to the center of the plate and 5 μl of the agent to be tested was applied. At least three plates were prepared for each agent tested. The plate contents were incubated for 48 h at 30°C, and then the diameter of growth inhibition was recorded. For the sodium deoxycholate (DOC) and ethanol assays, the contents of 100-ml LB gradient plates (50 ml per layer) were poured into a large, round (13.5 cm in length) petri dish. The selective top layer contained either 24 mM DOC, 8% (vol/vol) ethanol, or 10% (vol/vol) ethanol, as defined in the figure legends. For the ethanol experiments, CaCl2 and/or MgSO4 was also added to both layers as defined in the figure legends. The cultures to be tested were streaked (30-μl aliquots) evenly across the plates, the plate contents were incubated for 72 h at 30°C, and then the length of the growth inhibition zone was recorded. Since the differences in the sensitivity of the strains to pH and Zn2+ were smaller than for the DOC and ethanol assays, we used larger, square plates (22.5 cm in length), employing 200 ml of LB agar per layer to enable us to test all the strains on the same plate, and 50 μl of culture was streaked across the plates. The pH gradient (pH 7.0 to 5.5) was created by adding 35 mM 2-(N-morpholino)ethanesulfonic acid (MES) to the top LB agar layer, following a previously described method (7). To create a Zn2+ gradient, 1 mM ZnSO4 was added to the top LB agar layer. For both the filter disk and gradient plates, we measured the growth inhibition zone from at least three plates for each strain and set of conditions. The results were averaged, and the error bars represent the standard deviation from the mean.
Microaerobic sensitivity assays.
The early-stationary-phase cultures were resuspended to an OD600 of 1.0 and serially diluted (10−1 to 10−4), and 5-μl aliquots were spotted onto LB plates. Growth was assessed after incubation of the plates at 30°C for 48 to 72 h under either normal or microaerobic conditions in a GasPak with a CampyPak Plus hydrogen and carbon dioxide generator envelope with palladium catalyst (Becton Dickinson).
Small-scale LPS extraction and analysis.
The early-stationary-phase cultures were washed and resuspended in LB media. One-hundred-microliter aliquots were then spread onto LB-Sm plates, the plate contents were incubated for 72 h, and then the lawn of bacteria was scraped into LB media. The OD600 of the cultures was adjusted to 0.9, and the LPS was extracted from duplicate 1.5-ml volumes using either the hot water-phenol procedure as described previously (34) or a modification of an SDS lysis method (43). The SDS lysis method was performed as described previously, except the samples were boiled for 10 min and then treated with proteinase K (final concentration of 0.6 mg/ml) at 60°C for 1 h. The LPS samples were suspended in SDS sample buffer (50 μl for the SDS lysis method and 20 μl for the hot water-phenol procedure). Aliquots (4 μl) were analyzed on NuPage 4 to 12% Bis-Tris gradient gels (Invitrogen) using the Invitrogen MES running buffer. To visualize the LPS, the gels were silver stained (Bio-Rad kit) following the standard procedure, except the oxidant in the kit was replaced with 0.7% sodium m-periodate, to enable oxidation of sugars (43).
Large-scale LPS extraction and analysis.
Cultures of the defined strains were grown to mid-exponential phase in LB medium supplemented with 2.5 mM CaCl2 and 2.5 mM MgSO4, washed, and diluted in 4 liters of LB to an of OD600 of 0.2. The cultures were grown overnight to mid-exponential phase (OD600 = 2.0), and the cells were then harvested by centrifugation at 4,000 rpm for 30 min (Sorvall RC-3B). The LPS was extracted using a large-scale hot water-phenol method as previously described (33, 34), and the carbohydrate and fatty acid analysis of the samples was performed as part of a service contract by the Complex Carbohydrate Center (Athens, Ga). In brief, the aqueous- and phenol-phase samples were hydrolyzed using 1 M methanolic HCl (freshly prepared) for 16 h at 80°C. The released sugars were derivatized with Tri-Sil and separated by gas chromatography (GC) by using a Supelco column and were analyzed by mass spectrometry (MS). myo-inositol (20 μg) was added as an internal standard. The amount of each sugar moiety was expressed as a percentage of total sample carbohydrate. The fatty acids were also analyzed by GC-MS, and the data were calculated based on the ion at m/z 175 for β-OH-C14:0 and β-OH-C18:0 fatty acids and m/z 482 for the 27-OH-C28:0 fatty acid. In all cases, equivalent masses of the aqueous- and phenol-phase samples were analyzed.
RESULTS
All our previous characterizations of S. meliloti bacA mutants were carried out in the Rm8002 strain background in which the first bacA mutant was isolated (14, 19, 25, 26). Before initiating this study, we transduced the bacA654::spc mutation into the wild-type strain Rm1021, whose genome was recently sequenced (13), since strain Rm8002 was obtained by chemical mutagenesis of Rm1021 and could have multiple mutations (26). Although Rm1021 was slightly more resistant than Rm8002 to the different conditions tested, the Rm1021 bacA mutant displayed all the previous phenotypes of the Rm8002 bacA mutant (19) (data not shown).
Mg2+ and Ca2+ increase the resistance of both the wild type and the bacA mutant to stress.
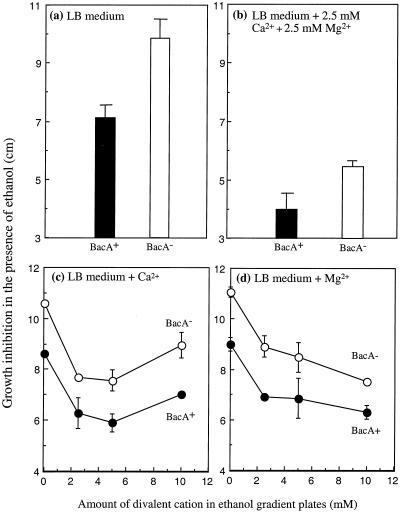
Previous experiments suggested that the inclusion of millimolar quantities of Mg2+ and Ca2+ suppresses the ethanol sensitivity phenotype of the S. meliloti Rm8002 bacA mutant (19). Since S. meliloti is usually grown in LB media supplemented with these divalent cations, we felt it important to characterize their apparent suppressive effects more carefully prior to conducting a detailed physiological analysis of the Rm1021 bacA mutant. To quantify the effect of Ca2+ and Mg2+, we used LB plates containing a gradient of ethanol and fixed amounts of divalent cations. As can be seen by comparison of Fig. 1a and b, the main effect of Ca2+ and Mg2+ was to increase the resistance of both the wild-type and bacA mutant strains to ethanol and not to suppress the ethanol sensitivity phenotype of the bacA mutant. We also observed that Ca2+ and Mg2+ could individually increase the resistance of S. meliloti to ethanol and that, although the bacA mutant is more sensitive to ethanol than the wild-type strain, the Ca2+ and Mg2+ effects on both strains were similar, suggesting that BacA was not involved in this resistance process (Fig. 1c and d, respectively). Intriguingly, we found that 10 mM Ca2+ was less effective at protecting against ethanol than were lower concentrations (Fig. 1c). The difference between Ca2+ and Mg2+ was not due to the different anions, since MgSO4 and MgCl2 showed almost identical effects at select concentrations (data not shown). Thus, the degree of protection against ethanol is dependent upon both the nature and amount of divalent cation present. Since we also found that Mg2+ and Ca2+ increased the resistance of S. meliloti to all the other conditions used in the previous characterizations of the bacA mutant (data not shown) (19), all subsequent experiments described in this paper were performed using LB agar or medium without divalent cation supplementation.
FIG. 1.
Effect of divalent cations on the sensitivity of S. meliloti to ethanol. Cultures of the wild-type strain Rm1021 and the bacA mutant were grown and exposed to gradients containing either 0 to 8% (vol/vol) (a and b) or 0 to 10% (vol/vol) (c and d) ethanol on LB plates exactly as described in Materials and Methods. Where indicated, the plates also contained CaCl2 and/or MgSO4.
The S. meliloti bacA mutant and an LPS mutant have an increased sensitivity to acid pH and zinc.
Within the plant cell, S. meliloti survives within the symbiosome and is subjected to a number of environmental insults, one of which is thought to be acid pH. It has been proposed that the pH of the S. meliloti symbiosome is somewhere in the range of 5.5 to 6.0 (29). Thus, it is possible that a bacA mutant is unable to survive in a plant cell due to an increased sensitivity to one or more of the many stresses that it experiences there. As shown in Fig. 2a, a bacA mutant is more sensitive to a gradient (pH 5.5 to 7.0) of acid pH than is the wild-type strain Rm1021. The difference observed in the growth was due to the pH change and not to the MES buffer used to alter the pH, since 50 mM MES adjusted to pH 7.0 had no effect on the growth of either strain (data not shown). In addition, mating a bacA+ vector into the S. meliloti bacA mutant increased the resistance to acid pH relative to the mutant with the control vector (data not shown). These data provide evidence that BacA is required to protect S. meliloti cells against acid pH. Thus, it is possible that the increased sensitivity of the bacA mutant to acid pH could play a role in the in planta defect of this mutant.
FIG. 2.
Effect of BacA and LpsB on the sensitivity of S. meliloti to acid pH and zinc. Cultures of the wild-type strain Rm1021 and defined isogenic mutants were grown and LB gradient plates were handled exactly as described in Materials and Methods.
Since an lpsB mutant, which has a significantly altered LPS structure, is defective at essentially the same stage in the alfalfa symbiosis (5), we tested whether it too might be more sensitive to a gradient of pH (Fig. 2a). In fact, the acid sensitivity of the lpsB mutant was even greater than for the bacA mutant (Fig. 2a). In addition, a bacA lpsB double mutant was even more sensitive to acid pH than the respective single mutants (Fig. 2a). These data indicate that an alteration in the LPS, as in the lpsB mutant, can lead to sensitivity to acid pH. They also suggest that tolerance to acid pH may play a role in the ability of S. meliloti to survive upon release into the plant cell during the alfalfa symbiosis. However, the increased sensitivity of the double mutant to acid pH suggests that LpsB and BacA protect S. meliloti by different underlying mechanisms.
It has been shown previously that certain acid-sensitive S. meliloti mutants also have an enhanced sensitivity to zinc and copper (42). Consistent with these observations, we also observed that the bacA, lpsB and bacA lpsB double mutant strains are more sensitive to a gradient of zinc sulfate than is the wild-type strain and that the sensitivities followed the same trend as for the acid pH experiment (Fig. 2b and a, respectively). These data show that BacA and LpsB are required to protect S. meliloti against zinc and confirm previous observations suggesting that bacteria have related protective mechanisms against acid pH and zinc.
In addition to low pH, S. meliloti encounters a number of other stresses within the plant such as oxidative stress (38, 39) and microaerobic conditions (9, 11). However, we did not observe any difference in growth between Rm1021 and the bacA mutant on LB plates containing filter disks supplemented with H2O2 (data not shown), nor did we find a mutation in bacA to affect the growth of S. meliloti on LB plates under microaerobic conditions in a GasPak system (data not shown).
The S. meliloti bacA mutant has an altered cell envelope.
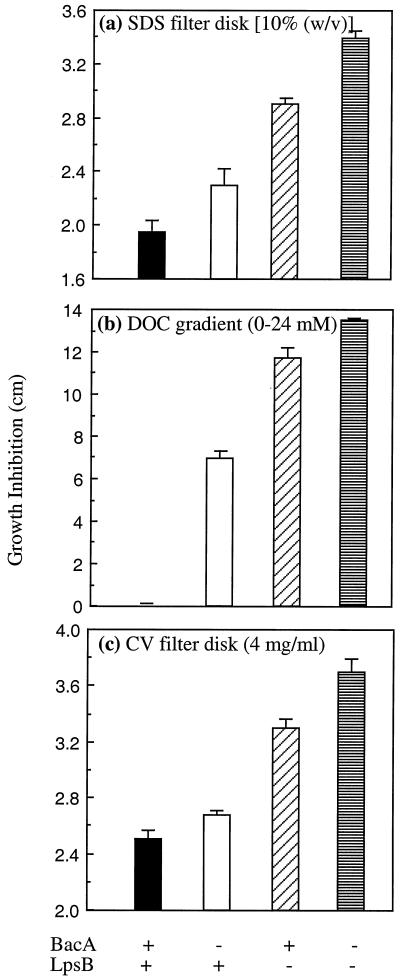
Our observation that the S. meliloti bacA mutant is more sensitive to acid pH further supported the proposal that BacA, rather than being involved in the transport of an essential compound from the host, could be involved in protecting the bacterial cell against an environmental stress encountered in the host (19). However, the mechanism by which BacA could perform such a function has remained elusive. Preliminary evidence presented previously suggested that BacA could be affecting the cell envelope, since an S. meliloti Rm8002 bacA mutant is more sensitive to sodium dodecyl sulfate (SDS) (19, 25), but our subsequent discovery that strain Rm8002 had an altered phospholipid head group composition compared with that of Rm1021 (O. Geiger and F. Martinez-Morales, personal communication) led us to question whether a bacA mutation alone could increase detergent sensitivity. However, when we investigated the effect of a bacA mutation in the wild-type strain Rm1021, we found that it increased the sensitivity to SDS and DOC on LB filter disks and gradient plates, respectively (Fig. 3a and b, respectively). In addition, the presence of a bacA+ plasmid in the Rm1021 bacA mutant also decreased SDS and DOC sensitivity relative to the derivative carrying just the control vector (data not shown). An altered sensitivity to detergents is usually an indicator of a change in the bacterial cell envelope. In particular, bacterial mutants with defects in their LPS have an increased sensitivity to DOC (5, 22). Thus, it seemed that the increased sensitivity of the S. meliloti bacA mutant to detergents could be due, at least in part, to an altered LPS. The S. meliloti lpsB mutant is also more sensitive to SDS and DOC but is considerably more sensitive than the bacA mutant (Fig. 3a and b). In addition, a double bacA lpsB mutant has an even greater sensitivity to detergents than either mutant alone (Fig. 3a and b), an observation consistent with the above conclusion that BacA and LpsB exert their effects by different mechanisms.
FIG. 3.
Effect of BacA on the sensitivity of S. meliloti to cell envelope agents. Cultures of the wild-type strain Rm1021 and defined isogenic mutants were grown and filter disk and gradient assays were performed on LB plates exactly as described in Materials and Methods. For the filter disk experiments, a 5-μl aliquot of the defined stock was applied to the disks. CV, crystal violet.
The hydrophobic dye crystal violet has also been used as an indicator of alterations in the cell envelope such as those caused by changes in the LPS (16), and consistent with this, we also found that the S. meliloti lpsB mutant is more sensitive to this agent (Fig. 3c). As shown in Fig. 3c, a bacA mutant is also more sensitive to crystal violet than is the wild-type strain. Mating in a bacA+ vector but not a control vector also increased the resistance of the bacA mutant to crystal violet (data not shown). Thus, this observation provides further evidence that bacA mutants have alterations in their cell envelope, possibly in the LPS.
LPS is made of three components: the hydrophobic lipid A, the more hydrophilic carbohydrate core, and O antigen (31). The LPS from an lpsB mutant has been previously characterized by DOC-polyacrylamide gel electrophoresis and GC-MS and has been shown to have a significantly altered core structure but to still have O antigen attached (5). The fact that BacA function makes lpsB mutants, who have a LPS core very different from that of a wild-type strain, more resistant to detergents is noteworthy. If the BacA protein is required for LPS modification, then either it affects the lipid A and O-antigen moieties, which are common to the LPS of both lpsB+ and lpsB strains, or it is capable of affecting both the wild type and the highly divergent lpsB LPS core.
A deficiency of BacA does not affect the LPS in a manner similar to that of a deficiency of LpsB.
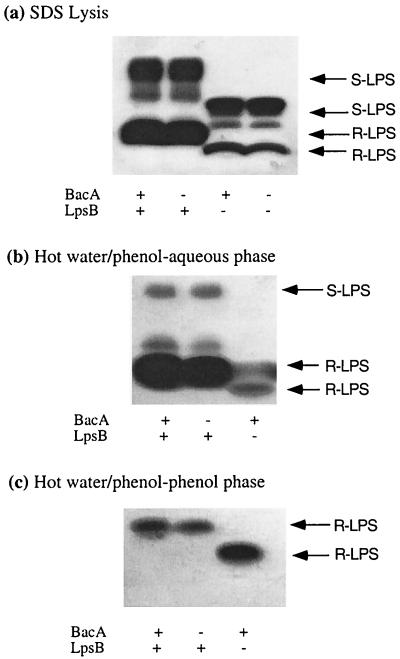
As a first step in investigating the possibility that the bacA mutant has an altered LPS, we prepared crude LPS samples from wild-type, bacA, and lpsB strains by SDS lysis and analyzed these by gradient gels and silver staining (Fig. 4a). A similar approach was initially used to detect the LPS alterations of lpsB mutants (5, 8, 28). The previous studies of lpsB mutants were carried out using cultures grown in tryptone-yeast medium supplemented with millimolar quantities of Ca2+. However, since our detergent sensitivity experiments were carried out on LB agar plates and since Ca2+ affects the phenotypes of the bacA mutant (19) (see above), we analyzed LPS from S. meliloti strains grown on LB plates without Ca2+ supplementation for 72 h. Consistent with previous observations drawn using this method of analysis (5), the LPS of the lpsB mutant is significantly different from that of the wild-type strain (Fig. 4a), with both the rough (lower-molecular-weight) and smooth (higher-molecular-weight) forms migrating faster than the wild-type forms. In contrast, there was no detectable difference between the LPS of the bacA mutant and that of the wild-type strain. A double bacA lpsB mutant also gave an LPS profile identical to that of the lpsB mutant (Fig. 4a). Thus, even though the symbiotic deficiency of a bacA mutant occurs at the same stage as it does for an lpsB mutant, which has a dramatically altered LPS, using this method of analysis, we were not able to detect any alterations in the LPS caused by the bacA mutation.
FIG. 4.
Effect of BacA and LpsB on the S. meliloti LPS. Cultures of the wild-type strain Rm1021 and the defined isogenic mutants were grown on LB plates and LPS extractions were performed exactly as described in Materials and Methods by using either an SDS lysis (a) or hot water-phenol method (b and c). S-LPS and R-LPS refer to smooth and rough LPS, respectively.
During a previous study of a series of bacterial mutants defective in their LPS, the type and yield of the LPS were found to be dependent upon the LPS extraction method (36). Thus, we also extracted LPS from wild-type S. meliloti, bacA, and lpsB strains using the hot water-phenol method (34) and analyzed the LPS in the aqueous and phenol phases using SDS gradient gels (Fig. 4b and c, respectively). The amount of LPS extracted into each phase is dependent upon the composition of the LPS. Analysis of the LPS extracted into the aqueous phase showed that, although the LPS profiles looked slightly different from how it looked with the SDS lysis method, the LPS profile of the bacA mutant was not significantly different from that of the wild-type strain (Fig. 4b). In contrast, the LPS profile of the lpsB mutant was dramatically affected, and under these conditions, we did not extract any smooth LPS into the aqueous phase (Fig. 4b). Since we know from the SDS lysis method (Fig. 4a) that the lpsB mutant produces smooth LPS, and since in a previous study using cells grown in tryptone-yeast-Ca2+ smooth LPS was detected from this mutant in the aqueous phase (5), this finding suggests that the growth conditions can have a profound effect on the composition of the LPS. Analysis of the phenol phases from all three strains showed that the rough LPS from all three strains was present (smooth LPS is too hydrophilic to be detected in the phenol phase) (Fig. 4c). These data suggest that, if the bacA mutant has an altered LPS, then this change is not detectable by these methods, even using cells grown under conditions where the bacA mutant is more sensitive to agents that affect the cell envelope (Fig. 3).
The S. meliloti bacA mutant has an altered distribution of LPS fatty acids.
Although we were unable to detect an LPS alteration in the bacA mutant using polyacrylamide gel electrophoresis, it was still possible that a more subtle change had occurred that could not be detected using this low-resolution method. We therefore prepared large-scale crude LPS samples from Rm1021 and the bacA mutant using a hot water-phenol extraction procedure and determined the carbohydrate content of the aqueous and phenol layers by GC-MS. The majority of the carbohydrate from both strains was extracted into the aqueous phase (Table 2), suggesting that the LPS is predominately present in the aqueous phase and that the bacA mutation was not affecting the partitioning of the LPS between the aqueous and phenol phases. The virtually identical sugar composition of the LPS from both strains that partitioned into the aqueous phase indicated that, unlike that of LpsB (5), BacA function does not affect the sugar composition of the LPS (Table 2). Since the carbohydrate content of the phenol phase was so low, no accurate determination of the sugar composition has been obtained thus far (data not shown). Thus, our data suggest that BacA is not having a dramatic effect on the carbohydrate composition of the core and O-antigen moieties of the LPS under our growth conditions.
TABLE 2.
Carbohydrate composition of the aqueous phase
| Sugar | % of total carbohydrate in aqueous phasea
|
|
|---|---|---|
| Rm1021 | bacA654::Spc | |
| Rhamnose | 4.8 | 6.1 |
| Galacturonic acid | 6.8 | 6.1 |
| Mannose | 2.3 | 1.5 |
| Galactose | 1.8 | 1.8 |
| Glucose | 72.5 | 72.7 |
| N-Acetyl glucosamine | 5.0 | 4.5 |
| 3-Deoxy-d-manno-2-octulosonic acidb | 6.8 | 7.3 |
The aqueous phases from Rm1021 and bacA mutant contained 54 and 63% carbohydrate, respectively, whereas the phenol phases contained 4 and 5% carbohydrate, respectively.
Known as Kdo.
In contrast, GC-MS analysis of the β-OH fatty acids in the material from both phases revealed that the bacA mutation has a marked effect on their relative amount and on the phase partitioning of the LPS (Table 3). The aqueous phase from the bacA mutant had a reduced amount of β-OH-C14:0 and 27-OH-C28:0 (52.5 and 31% less than the aqueous phase of the wild-type strain, respectively), whereas it had an increased amount of β-OH-C18:0 (51% more than the aqueous phase of the wild-type strain). The phenol phases of both strains contained substantially less fatty acids than the aqueous phases (Table 3). Interestingly, the phenol phase of the wild-type strain contained 51.5% more β-OH-C18:0 than did the corresponding phase of the bacA mutant. These data suggest that the S. meliloti bacA mutant has an alteration in its LPS fatty acids, and this finding could help rationalize the cell envelope defect of this mutant.
TABLE 3.
Fatty acid analysis of aqueous and phenol phases
| Strain | Phase | Results for peak areasa
|
||
|---|---|---|---|---|
| β-OH-C14:0 | β-OH-C18:0 | 27-OH-C28:0 | ||
| Rm1021 | Aqueous | 2,682 | 2,100 | 4,986 |
| bacA654::Spc | Aqueous | 1,272 | 4,294 | 3,428 |
| Rm1021 | Phenol | 177 | 1,805 | Tr |
| bacA654::Spc | Phenol | 106 | 875 | Tr |
Equivalent masses of the water and phenol phases were analyzed. Results are in arbitrary units.
DISCUSSION
These data extend the previous physiological characterizations of the S. meliloti bacA mutant (19) and provide further support for a model in which BacA function affects the cell envelope. From a site-directed mutagenesis study of the S. meliloti bacA gene, it was suggested that BacA could have multiple, nonoverlapping functions (25). The data presented in this paper are consistent with a model in which at least one role of BacA is to affect the nature of the LPS fatty acid modifications. The S. meliloti bacA mutant had a significant reduction in the amount of 27-OH-C28:0 compared with the wild-type strain. Thus, the reduced level of 27-OH-C28:0 in the S. meliloti bacA mutant could contribute to the sensitivity of this strain to detergents, a hydrophobic dye, ethanol, and acid and to the inability to survive within the plant cell. This hypothesis is consistent with the observation that the level of 27-OH-C28:0 is higher in the LPS of R. leguminosarum bacteroids than in the LPS of free-living bacteria (20). Although there have been no previous reports of which we are aware stating that specific changes in the LPS fatty acids affect acid resistance, changes in phospholipid fatty acids have been found to affect acid resistance in other bacterial systems. For example, cyclopropane fatty acids protect E. coli against acid pH (6). It has been shown previously that a Pseudomonas putida mutant sensitive to organic solvents had alterations in its LPS fatty acids (21). Hence, changes in the LPS fatty acids could account for the ethanol sensitivity phenotype of the S. meliloti bacA mutant. Indeed, alterations in the LPS fatty acids could also account for the low-level resistance of the S. meliloti bacA mutant to aminoglycoside antibiotics, since cell envelope alterations have previously been shown to reduce their uptake and confer low-level resistance against these agents (41).
The discovery that the S. meliloti bacA mutant has altered LPS fatty acids raises the issue how an inner membrane protein affects a component of the outer membrane. The complete resistance of E. coli sbmA mutants to exogenous, but not endogenously synthesized, microcins stimulated the formation of a model in which SbmA and BacA were hypothesized to be involved in the transport of peptide-like compounds into the bacterial cell (19, 23, 37). However, it is difficult to account for the alteration in LPS fatty acids and the multiple phenotypes of the S. meliloti bacA mutant if this was the sole function of BacA. In contrast to the bacA mutation in S. meliloti, the sbmA mutation in E. coli did not affect resistance to ethanol, aminoglycoside antibiotics, or SDS (19). It is unlikely that SbmA and BacA have totally different functions, since the proteins are functionally interchangeable (19). Instead, there are several hypotheses that can rationalize the differences. E. coli may possess another system that can compensate for the loss of SbmA function in the sbmA mutant and prevent us from observing any of the phenotypes other than microcin and bleomycin resistance. In addition, regulation of expression of BacA and SbmA is different (19) and sbmA is poorly transcribed under standard growth conditions (23). Hence, the low level of SbmA may affect sensitivity to microcin and bleomycin, but higher levels may be required for other functions. Alternatively, BacA and SbmA may transport similar but not identical substrates due to species differences. The cell envelopes of enteric bacteria are significantly different from those of the α-proteobacteria, and the former lacks 27-OH-C28:0 (2). Thus, since BacA function affects the level of 27-OH-C28:0 and since this is absent in enteric bacteria, SbmA could also affect LPS fatty acids, but changes in these may not necessarily have the same physiological outcome. Unlike E. coli, B. abortus LPS does possess 27-OH-C28:0 (27), and a preliminary physiological characterization of the B. abortus bacA mutant suggests that its phenotypes have commonalities with the phenotypes of the S. meliloti bacA mutant (R. M. Roop II, unpublished data). Hence, it is possible that the B. abortus bacA mutant is defective in the chronic phase of infection due to alterations in LPS fatty acids, including 27-OH-C28:0. Since B. abortus also resides within an acidic compartment in the host, it would be particularly interesting to determine if a bacA mutation affects acid sensitivity.
In addition to their acid sensitivity, the S. meliloti bacA and lpsB mutants are more sensitive to Zn2+. This is in agreement with previous reports that suggest the existence of overlap between protective mechanisms against acid and zinc in S. meliloti (32, 42). Since Zn2+ has been shown to interact with the fatty acids of purified LPS, affecting their fluidity (45), the increased sensitivity of the S. meliloti bacA mutant to zinc could be rationalized by the alteration in LPS fatty acids. In addition, since the effect of the lpsB mutation on the LPS fatty acids of S. meliloti has not been determined, the increased sensitivity of the S. meliloti lpsB mutant to zinc may also suggest that this mutant has altered LPS fatty acids. It was found that alterations in the fluidity of bacterial LPS affected the effectiveness of the hosts' innate immune response (45). Thus, it is possible that a lipid A alteration in the LPS of the bacA and lpsB mutants could induce an innate immune response in the plant not normally observed for the wild-type strain. This is interesting, given that the lpsB mutant has a greatly increased sensitivity to cationic peptides (5), which are thought to form part of the innate immune response.
In gram-negative bacteria, the divalent cations Mg2+ and Ca2+ are thought to have important roles in stabilizing the cell envelope by interacting with the LPS (15, 40). Preliminary experiments conducted previously suggested that BacA function was suppressed in the presence of these divalent cations (19). However, our results suggest that, rather than suppressing the requirement for BacA, Mg2+ and Ca2+ increase the resistance of S. meliloti to stress and thereby increase the amount of stress required to observe the phenotypes of the S. meliloti bacA mutant. This could have important consequences, since changes in the levels of divalent cations, in particular Ca2+, are thought to occur throughout the alfalfa symbiosis. For example, early in the symbiosis, bacterial Nod factors are thought to induce Ca2+ spiking in the plant host (12). However, since Ca2+ spiking occurs early in the symbiosis, it is unlikely that S. meliloti would experience this fluctuation, suggesting that it would not be relevant to the bacA phenotype. There is an increasing amount of evidence suggesting that the membrane-bound compartment in which S. meliloti resides within the plant cell has some similarities to the environment within pathogen-infected macrophages (18). It is thought that macrophages are low in divalent cations and that many genes required for survival of bacterial pathogens in macrophages are induced in vitro by low levels of Ca2+ and Mg2+ (15, 44). Consistent with this, expression of the S. meliloti dctA gene, which is also required for survival within the plant cell, is induced in free-living bacteria in media low in Ca2+ (1). Thus, if the levels of Ca2+ were higher during the early stages of the symbiosis, then S. meliloti would be less dependent upon BacA for protection. However, if upon release into the plant cell, the levels of these divalent cations is reduced, then BacA would become more important for protection of S. meliloti against stress. This rationale could help account for why the bacA mutant is not defective at the early stages in the symbiosis (14). We found that Ca2+ was more effective than Mg2+ at lower concentrations in conferring protection to S. meliloti against stress. However, when the Ca2+ level was increased, it eventually became less effective. At lower concentrations, these divalent cations protect S. meliloti by stabilizing the LPS (15, 40), but at higher concentrations, they increase the permeability of the bacterial cell envelope and this forms the basis of DNA transformation protocols (17). Since Ca2+ is more effective than Mg2+ in DNA transformation (17), it seems likely that high concentrations of Ca2+ are more effective at increasing the permeability of the cell envelope and thereby sensitizing the bacteria towards stress. Thus, the degree of protection afforded is dependent upon the amount and nature of the divalent cation.
In summary, the findings of this paper provide further support for BacA function affecting the cell envelope, and in particular, they pinpoint at least one of these changes, involving an alteration in the distribution of LPS fatty acids. Since the structure of the S. meliloti LPS has not yet been determined, additional work will be required to determine the location of the fatty acids whose distribution is affected by BacA function. In addition, since phospholipids and lipoproteins also have fatty acid modifications, our future goal will be to determine whether these other components are also affected in the bacA mutant. It is important that we understand more about the function of the S. meliloti BacA protein, since, in addition to improving our understanding of the symbiosis process, it should also give us insights into the role of this protein in the pathogenesis of B. abortus.
Acknowledgments
We thank Russ Carlson and Parastoo Azadi of the Complex Carbohydrate Center for performing the carbohydrate and fatty acid analysis of our samples. Thanks also to Brad Reuhs for many helpful discussions regarding the LPS analysis and Bryan Bellaire for his Brucella insights. In addition, thanks to the Walker lab members, both past and present, for many helpful discussions and critical reading of the manuscript.
A Public Health Service grant (GM31030) awarded to G. C. Walker from the National Institute of General Medicinal Science supported this work. The work of Carlson and Azadi was supported in part by the U.S. Department of Energy-funded (DE-FG09-93ER-20097) Center for Plant and Microbial Complex Carbohydrates.
REFERENCES
- 1.Batista, S., S. Castro, O. M. Aguilar, and G. Martinez-Drets. 1992. Induction of C4-dicarboxylate transport genes by external stimuli in Rhizobium meliloti. Can. J. Microbiol. 38:51-55. [DOI] [PubMed] [Google Scholar]
- 2.Bhat, U. R., R. W. Carlson, M. Busch, and H. Mayer. 1991. Distribution and phylogenetic significance of 27-hydroxy-octacosanoic acid in lipopolysaccharides from bacteria belonging to the alpha-2 subgroup of Proteobacteria. Int. J. Syst. Bacteriol. 41:213-217. [DOI] [PubMed] [Google Scholar]
- 3.Bhat, U. R., H. Mayer, A. Yokota, R. I. Hollingsworth, and R. W. Carlson. 1991. Occurrence of lipid A variants with 27-hydroxyoctacosanoic acid in lipopolysaccharides from members of the family Rhizobiaceae. J. Bacteriol. 173:2155-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blond, A., J. Peduzzi, C. Goulard, M. J. Chiuchiolo, M. Barthelemy, Y. Prigent, R. A. Salomon, R. N. Faria, F. Moreno, and S. Rebuffat. 1999. The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Eur. J. Biochem. 259:747-755. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, G. R. O., B. Reuhs, and G. C. Walker. 2002. Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core. Proc. Natl. Acad. Sci. USA 99:3938-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, Y. Y., and J. E. Cronan, Jr. 1999. Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol. Microbiol. 33:249-259. [DOI] [PubMed] [Google Scholar]
- 7.Charles, T. C., and E. W. Nester. 1993. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 175:6614-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clover, R. H., J. Kieber, and E. R. Signer. 1989. Lipopolysaccharide mutants of Rhizobium meliloti are not defective in symbiosis. J. Bacteriol. 171:3961-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David, M., M. L. Daveran, J. Batut, A. Dedieu, O. Domergue, J. Ghai, C. Hertig, P. Boistard, and D. Kahn. 1988. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell 54:671-683. [DOI] [PubMed] [Google Scholar]
- 10.Ditta, G., T. Schmidhauser, E. Yakobson, P. Lu, X.-W. Liang, D. R. Finlay, D. Guiney, and D. R. Helinski. 1985. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13:149-153. [DOI] [PubMed] [Google Scholar]
- 11.Ditta, G., E. Virts, A. Palomares, and C. H. Kim. 1987. The nifA gene of Rhizobium meliloti is oxygen regulated. J. Bacteriol. 169:3217-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erhardt, D. W., R. Wais, and S. R. Long. 1996. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85:673-681. [DOI] [PubMed] [Google Scholar]
- 13.Galibert, F., T. M. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 14.Glazebrook, J., A. Ichige, and G. C. Walker. 1993. A Rhizobium meliloti homolog of the Escherichia coli peptide-antibiotic transport protein SbmA is essential for bacteroid development. Genes Dev. 7:1485-1497. [DOI] [PubMed] [Google Scholar]
- 15.Groisman, E. A., J. Kayser, and F. C. Soncini. 1997. Regulation of polymyxin resistance and adaptation to low-Mg2+ environments. J. Bacteriol. 179:7040-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafsson, P., K. Nordström, and S. Normark. 1973. Outer penetration barrier of Escherichia coli K-12: kinetics of the uptake of gentian violet by wild type and envelope mutants. J. Bacteriol. 116:893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1987. Mechanisms of DNA transformation, p. 1177-1183. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, M. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 2. American Society for Microbiology, Washington, D.C.
- 18.Hentschel, U., M. Steinert, and J. Hacker. 2000. Common molecular mechanisms of symbiosis and pathogenesis. Trends Microbiol. 8:226-231. [DOI] [PubMed] [Google Scholar]
- 19.Ichige, A., and G. C. Walker. 1997. Genetic analysis of the Rhizobium meliloti bacA gene: functional interchangeability with the Escherichia coli sbmA gene and phenotypes of mutants. J. Bacteriol. 179:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannenberg, E. L., and R. W. Carlson. 2001. Lipid A and O-chain modifications cause Rhizobium lipopolysaccharides to become hydrophobic during bacteroid development. Mol. Microbiol. 39:379-391. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi, H., H. Takami, H. Hisako, K. Kobata, R. Usami, and K. Horikoshi. 1999. Outer membrane changes in a toluene-sensitive mutant of toluene-tolerant Pseudomonas putida IH-2000. J. Bacteriol. 181:4493-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagares, A., G. Caetano-Anolles, K. Niehaus, J. Lorenzen, H. D. Ljunggren, A. Puhler, and G. Favelukes. 1992. A Rhizobium meliloti lipopolysaccharide mutant altered in competitiveness for nodulation of alfalfa. J. Bacteriol. 174:5941-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laviña, M., A. P. Pugsley, and F. Moreno. 1986. Identification, mapping, cloning and characterization of a gene (sbmA) required for microcin B17 action on Escherichia coli K12. J. Gen. Microbiol. 132:1685-1693. [DOI] [PubMed] [Google Scholar]
- 24.LeVier, K., R. W. Phillips, V. K. Grippe, R. M. Roop II, and G. C. Walker. 2000. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science 287:2492-2493. [DOI] [PubMed] [Google Scholar]
- 25.LeVier, K., and G. C. Walker. 2001. Genetic analysis of the Sinorhizobium meliloti BacA protein: differential effects of mutations on phenotypes. J. Bacteriol. 183:6444-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long, S., S. McCune, and G. C. Walker. 1988. Symbiotic loci of Rhizobium meliloti identified by random TnphoA mutagenesis. J. Bacteriol. 170:4257-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno, E., E. Stackebrandt, M. Dorsch, J. Wolters, M. Busch, and H. Mayer. 1990. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J. Bacteriol. 172:3569-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niehaus, K., A. Lagares, and A. Puhler. 1998. A Sinorhizobium meliloti lipopolysaccharide mutant induces effective nodules on the host plant Medicago sativa (alfalfa) but fails to establish a symbiosis with Medicago truncatula. Mol. Plant-Microbe Interact. 11:906-914. [Google Scholar]
- 29.Perez-Galdona, R., and M. L. Kahn. 1994. Effects of organic acid and low pH on Rhizobium meliloti 104A14. Microbiology 140:1231-1235. [DOI] [PubMed] [Google Scholar]
- 30.Piazarro-Cerda, J., E. Moreno, and J.-P. Gorvel. 1999. Brucella abortus invasion and survival within professional and nonprofessional phagocytes. Adv. Cell Biol. Mol. Biol. Membr. Organelles 6:201-232. [Google Scholar]
- 31.Price, N. P. 1999. Carbohydrate determinants of Rhizobium-legume symbioses. Carbohydr. Res. 317:1-9. [DOI] [PubMed] [Google Scholar]
- 32.Reeve, W. G., R. P. Tiwari, C. M. Wong, M. J. Dilworth, and A. R. Glenn. 1998. The transcriptional regulator gene phrR in Sinorhizobium meliloti WSM419 is regulated by low pH and other stresses. Microbiology 144:3335-3342. [DOI] [PubMed] [Google Scholar]
- 33.Reuhs, B. L., R. W. Carlson, and J. S. Kim. 1993. Rhizobium fredii and Rhizobium meliloti produce 3-deoxy-d-manno-2-octulosonic acid-containing polysaccharides that are structurally analogous to group II K antigens (capsular polysaccharides) found in Escherichia coli. J. Bacteriol. 175:3570-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuhs, B. L., J. S. Kim, A. Badgett, and R. W. Carlson. 1994. Production of cell-associated polysaccharides of Rhizobium fredii USDA205 is modulated by apigenin and host root extract. Mol. Plant-Microbe Interact. 7:240-247. [DOI] [PubMed] [Google Scholar]
- 35.Reuhs, B. L., S. B. Stephens, D. P. Geller, J. S. Kim, J. Glenn, J. Przytycki, and T. Ojanen-Reuhs. 1999. Epitope identification for a panel of anti-Sinorhizobium meliloti monoclonal antibodies and application to the analysis of K antigens and lipopolysaccharides from bacteroids. Appl. Environ. Microbiol. 65:5186-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridley, B. L., B. S. Jeyaretnam, and R. W. Carlson. 2000. The type and yield of lipopolysaccharide from symbiotically deficient Rhizobium lipopolysaccharide mutants vary depending upon the extraction method. Glycobiology 10:1013-1023. [DOI] [PubMed] [Google Scholar]
- 37.Salomón, R. A., and R. N. Farías. 1995. The peptide antibiotic microcin 25 is imported through the TonB pathway and the SbmA protein. J. Bacteriol. 177:3323-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos, R., D. Herouart, A. Puppo, and D. Touati. 2000. Critical protective role of bacterial superoxide dismutase in rhizobium-legume symbiosis. Mol. Microbiol. 38:750-759. [DOI] [PubMed] [Google Scholar]
- 39.Santos, R., D. Herouart, S. Sigaud, D. Touati, and A. Puppo. 2001. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant-Microbe Interact. 14:86-89. [DOI] [PubMed] [Google Scholar]
- 40.Snyder, S., D. Kim, and T. J. McIntosh. 1999. Lipolysaccharide bilayer structure: effect of chemotype, core mutations, divalent cations and temperature. Biochemistry 38:10758-10767. [DOI] [PubMed] [Google Scholar]
- 41.Taber, H. W., J. P. Mueller, P. F. Miller, and A. S. Arrow. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 51:439-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiwari, R. P., W. G. Reeve, M. J. Dilworth, and A. R. Glenn. 1996. An essential role for actA in acid tolerance of Rhizobium meliloti. Microbiology 142:601-610. [DOI] [PubMed] [Google Scholar]
- 43.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 44.Vescovi, E. G., Y. M. Ayala, E. Di Cera, and E. A. Groisman. 1997. Characterization of the bacterial sensor protein PhoQ. Evidence for distinct binding sites for Mg2+ and Ca2+. J. Biol. Chem. 272:1440-1443. [DOI] [PubMed] [Google Scholar]
- 45.Wellinghausen, N., A. B. Schromm, U. Seydel, K. Brandenburg, J. Luhm, H. Kirchner, and L. Rink. 1996. Zinc enhances lipopolysaccharide-induced monokine secretion. J. Immunol. 157:3139-3145. [PubMed] [Google Scholar]
- 46.Yorgey, P., J. Lee, J. Kordel, E. Vivas, P. Warner, D. Jebaratnam, and R. Kolter. 1994. Posttranslational modifications in microcin B17 define an additional class of DNA gyrase inhibitor. Proc. Natl. Acad. Sci. USA 91:4519-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]