Abstract
Pseudomonas aeruginosa PAO1 utilizes proline as the sole source of carbon and nitrogen via a bifunctional enzyme (the putA gene product) that has both proline dehydrogenase (EC 1.5.99.8) and pyrroline 5-carboxylate dehydrogenase (EC 1.5.1.12) activities. We characterized the pruR-putAP loci encoding the proline catabolic system of this strain. In contrast to the putA and putP (encoding proline permease) genes of other gram- negative bacteria, which are located at divergent or separate loci, Northern blotting demonstrated that the two genes form an operon in strain PAO1. While the phylogenetic lineage of the PutP protein of strain PAO1 was related to that of the origin (80% identity to the P. putida counterpart), PutA of PAO1 (PutAPAO) was rather distantly related (47% identity) to the P. putida counterpart. Moreover, unlike the PutA proteins of P. putida and enteric bacteria, PutAPAO appeared to lack a regulatory function. Upstream of the putAP operon, the divergent PA0781 gene specified a hypothetical outer membrane protein with a molecular weight of 74,202. This gene appeared to be dispensable for proline utilization as indicated by the normal growth of a knockout mutant of PA0781 on medium containing proline. The pruR (proline utilization regulator) gene immediately upstream of PA0781 encoded a transcriptional activator of the AraC/XylS protein family and mediated the proline-responsive expression of putAP. Primer extension studies identified a PruR-dependent promoter responsive to proline in the 5′-flanking region of putA. Thus, the proline utilization system of P. aeruginosa differs from that of P. putida with respect to putA structure, the organization of the putAP genes, and the regulatory mechanism of putA expression.
A proline catabolic enzyme of Sinorhizobium meliloti contributes to root colonization as well as to the establishment of a symbiotic interaction with roots (15, 16, 42). Proline catabolic enzymes also participate in the utilization of octopines and nopalines by Agrobacterium tumefaciens (5) and in the development of nodules induced by S. meliloti (15, 16, 42). Since proline is extruded along with other organic compounds from plant roots into soil at concentrations sufficient to induce a proline catabolic enzyme (1, 3, 44), root-colonizing bacteria may use proline for growth and sustenance in the rhizosphere.
Root-colonizing fluorescent pseudomonads such as Pseudomonas aeruginosa, P. fluorescens, and P. putida efficiently utilize proline as the sole source of carbon and nitrogen (25, 37, 44). In P. aeruginosa PAO1 and P. putida, proline is catabolically converted, as in other gram-negative bacteria, into glutamate by a bifunctional catabolic enzyme (encoded by the putA gene) that has proline dehydrogenase (ProDH; EC 1.5.99.8) and Δ1-pyrroline-5-carboxylate dehydrogenase (P5CDH; EC 1.5.1.12) activities. The resulting glutamate is subsequently channeled into the tricarboxylic acid cycle via 2-ketoglutarate (23, 24, 30, 44). In addition to the catalytic function, PutA proteins of Escherichia coli, Salmonella enterica serovar Typhimurium, and P. putida control their expression and that of the divergent putP gene (encoding a proline permease) by acting as a repressor in the absence of proline (2, 23, 27, 40, 44). The PutA of S. meliloti also autoregulates its synthesis (36); the putP gene of this strain has not been identified but appears to be located separately from putA (4). Proline abolishes the repressive effect of PutA on putAP expression by preventing its binding to the control sites of the promoters (2, 27, 40). A leucine zipper-like sequence of the C-terminal region has been proposed as a DNA-binding motif interacting with the control sites, and ProDH activity and PutA phosphorylation are associated with the DNA-binding activity (2, 20, 27). In contrast, A. tumefaciens, Rhodobactor capsulatus, and probably Bradyrhizobium japonicum use another mechanism to regulate putA expression. In these bacteria, the PutR protein of an Lrp-type transcriptional activator essentially controls putA expression with proline as a coinducer (4, 17, 39). Thus, gene organization and the regulatory mechanism of putA differ within the family Rhizobiaceae (36).
P. aeruginosa PAO6087 (pru::Tn5-751) has been isolated from P. aeruginosa PAO6049, a derivative of strain PAO1, as a mutant that is unable to utilize proline as a source of carbon and of nitrogen (33). Sequence analysis in this study revealed that the pru::Tn5-751 gene encodes an AraC/XylS-family regulatory protein (9, 22, 41) and that it is located close to the putAP genes. In this system, the putA and putP genes constitute an operon and PruR mediates the proline-responsive expression of this operon. The PutA protein of strain PAO1 appears to lack a repressor function and is distantly related to the P. putida counterpart in terms of its primary structure.
MATERIALS AND METHODS
Strains, plasmids, and media.
The strains and plasmids used in this study are listed in Table 1. E. coli and P. aeruginosa PAO strains were grown in Luria-Bertani medium (35) and nutrient yeast-extract medium or minimal medium P (MMP) supplemented with 20 mM (each) carbon and nitrogen sources (10). Antibiotics were added as described when necessary (12).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant phenotypes or genotypesa | Reference or source |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| PAO1 | Wild type | 38 |
| PAO4484 | ntrC::FRT | 30 |
| PAO4502 | putA::FRT-Gm | This work |
| PAO4503 | putP::ΩSp/Sm | This work |
| PAO4517 | PA0781::ΩSp/Sm | This work |
| PAO4528 | putA::FRT | This work |
| PAO4534 | purR::FRT-Gm putA::FRT | This work |
| PAO4547 | putA::ΩSp/Sm | This work |
| PAO6087 | pruR::Tn5-751 (=pru::Tn5-751) met-9011 amiE200 strA | 33 |
| E. coli | ||
| DH5α | F−/endA1 hsdR17 (rk− mk+) supE44 thi-1 recA1 gyrA relA1 Δ(laclZYA-argF)U169 deoR [φ80dlacΔ(lacZ)M15] | Bethesda Research Laboratories |
| HB101 | supE44 hsdS20(rB− mB−) recA13 ara-14 proA2 lacY galK2 rpsL20 xyl-5 mtl-1 | 35 |
| XL1-Blue | endA1 gyrA96 thi-1 hsdR17 supE44 recA1 lac [F′, proAB laclq ZΔM15 Tn10 (Tcr)] | Stratagene |
| Plasmids | ||
| pBluescript II KS(+) | Apr, ColE1 replicon | Stratagene |
| pEX18Ap | Apr, ColE1 replicon, oriT sacB | 12 |
| pFLP2 | Apr, ColE1 replicon, Flp oriT sacB | 12 |
| pHP45ΩSp/Sm | Apr, ColE1 replicon, ΩSp/Sm | 8 |
| pME6015 | Tcr, pACYC177 and pVS1 replicons, ′lacZ | S. Heeb |
| pNIC6011 | Apr Cbr, pACYC177 and pVS1 replicons, mob | 30 |
| pNIT6012 | Tcr, pACYC177 and pVS1 replicons, mob | 30 |
| pPS858 | Apr Gmr, ColE1 replicon carrying a FRT-Gmr cassette | 12 |
| pRK2013 | Kmr, ColE1 replicon, tra (RK2) | 6 |
| pUC118 | Apr, ColE1 replicon | 43 |
| pYI358 | Apr Cbr, pNIC6011 derivative carrying a 5.9-kb ′PA0779-pruR-PA0781-putA′ region | This work |
| pYI366 | Apr Kmr, a pBluescript II KS(+) derivative carrying a 8.0-kb ′ pruR-PA0781-putA-putP′ region | This work |
| pYI367 | Apr, pEX18Ap derivative carrying the 4.3-kb EcoRI-KpnI putA fragment from pYI366 | This work |
| pYI368 | Apr Gmr, pYI367 derivative having the FRT-Gmr cassette from pPS858 | This work |
| pYI369 | Tcr, pNIT6012 derivative having the 3.8-kb KpnI-XhoI fragment of the PA0781-putA region | This work |
| pYI370 | Tor, pNIT6012 derivative carrying the putA-His6 fusion as a KpnI-XhoI fragment | This work |
| pYI378 | Tcr, pME6015 derivative carrying the putA′-′lacZ fusion | This work |
| pYI388 | Apr, pEX18Ap derivative carrying the 3.5-kb XhoI-PstI fragment containing putP | This work |
| pYI389 | Apr, pYI388 derivative carrying the FRT-Gmr cassette on putP | This work |
| pYI415 | Apr, pEX18Ap derivative carrying the 2.6-kb EcoRI fragment containing PA0781 | This work |
| pYI416 | Apr Spr Smr, pYI415 with an insertion of ΩSp/Sm at the Bbsl site on PA0781 | This work |
| pYI425 | Apr, pEX18Ap derivative carrying 2.2-kb XhoI-SalI fragment containing pruR | This work |
| pYI426 | Apr Gmr, pYI425 derivative carrying the FRT-Gmr cassette on pruR | This work |
Cloning of the pruR-putAP region.
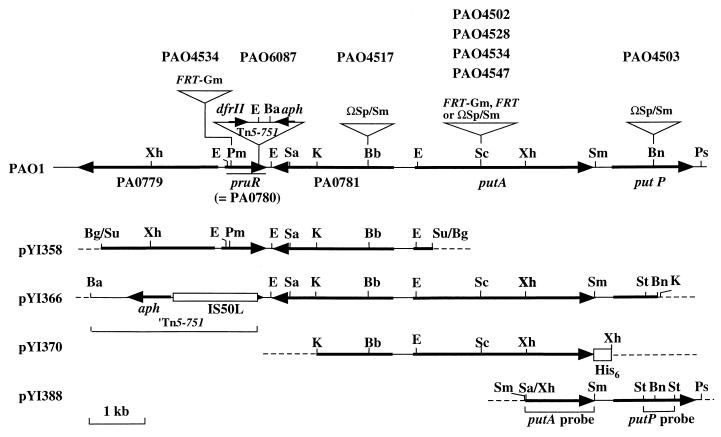
Stain PAO6087 (pru::Tn5-751) was isolated as a mutant unable to utilize proline (Pru− phenotype) as a source of carbon and nitrogen from a mutant library of P. aeruginosa PAO6049 (met-9011 amiE200 strA). This library was constructed using the transposon Tn5-751 (34). To clone an allele of pru::Tn5-751, we constructed a library of the partially digested (5- to 10-kb) Sau3AI chromosomal DNA of P. aeruginosa PAO1 by using the E. coli-Pseudomonas shuttle vector pNIC6011 in E. coli XL1-Blue as described previously (11, 28). We then conjugated library plasmids into strain PAO6087 (pru::Tn5-751) from E. coli XL1-Blue (28, 30). Among several Pru+ transconjugants selected on MMP agar supplemented with 20 mM proline, we selected one transconjugant harboring plasmid pYI358 with an insert of a 5.9-kb chromosomal DNA segment (Fig. 1) for further analysis. We cloned the PAO871-putA region downstream of pruR as a 8.0-kb aph (Kmr)-putP′ fragment into the plasmid pBluescript II KS(+) (Stratagene) from the chromosomal DNA of strain PAO6087 by using restriction endonucleases BamHI and BanIII, which cut the transposon around the center and cut putP at nucleotide position (nt) 795 (Fig. 1), respectively. The putP region was amplified by PCR using KOD Dash DNA polymerase (Toyobo Biochemicals), the chromosomal DNA of strain PAO1 as a template, and oligonucleotide primers (5′-GCATCGACGAGCTGAAGAAAGAGAACTTC-3′, nt −688 to −660 of putP; and 5′-TGAAGGCATTCTTCGAATGGCTGAGGCTG-3′, complementary to the region 193 to 221 bp downstream of putP). After digestion with both XhoI and PstI, the DNA fragment was cloned into plasmid pEX18Ap (12) between the SalI (compatible with the XhoI site) and PstI sites, to produce plasmid pYI388 (Fig. 1). The nucleotide sequence of the cloned fragment was verified by DNA sequencing.
FIG. 1.
Structure of pruR-putP loci of PAO strains and plasmids. Gene names (PA) correspond to annotation of the Pseudomonas Genome Project (www.pseudomonas.com). DNA cassettes, Tn5-751, and histidine- codon tag sequences are not drawn to scale. Thin solid and broken lines indicate chromosomal and plasmid sequences, respectively. Strains PAO4502, PAO4528, and PAO4547 carry FRT-Gm, FRT and ΩSp/Sm cassettes on putA, respectively. Strain PAO4534 has FRT and FRT-Gm inserts on putA and pruR, respectively. Only relevant restriction sites are indicated: E, EcoRI; Ba, BamHI; Bb, BbsI; Bg, BglI; Bn, BanIII; K, KpnI; Pm, PmaCI; Sa, SalI; Sc, SacI; Su, Sau3AI; Sm, SmaI; Xh, XhoI.
Construction of strains and plasmids.
Knockout mutants of PA0781, putA, and putP were constructed by the method of Hoang et al. (12). DNA fragments containing a gene of interest were cloned into plasmid pEX18Ap (mob+ sacB) (12). An ΩSp/Sm interposon (8) or a DNA cassette (FRT-Gm) containing FRT and the gentamicin resistance (Gmr) gene (12) was then inserted into a target gene on the plasmids at an appropriate site (Fig. 1). Target genes on the PAO1 chromosome were replaced with the corresponding knockout genes on plasmids by recombination as described previously (12, 28, 30). We eliminated the Gmr sequence of the FRT-Gm cassette integrated into the chromosome by introducing plasmid pFLP2 carrying Flp recombinase (12) into mutant cells. The plasmids used for construction of knockout mutants are listed in Table 1.
To facilitate purification of the PutA protein so that the amino terminal could be sequenced, we constructed a putA gene tagged with six histidine codons (putA-His6). The 3.8-kb KpnI-XhoI fragment of plasmid pYI366 containing the 5′-flanking region and the ProDH domain of putA (Fig. 1) was first cloned into plasmid pNIT6012 (30). The carboxyl-terminal half of the P5CDH domain was then amplified by PCR using plasmid pYI366 (putA) (Table 1) as a template, and primers 5′-CCGGCAAGGCCCTCGACGTGTTGCA-3′ (nt 1817 to 1841, upstream of the XhoI site, of putA) and 5′-GCTGCTGTCGCTGGCCGACGCCGAGCACCACCACCACCACCACTGACTCGAG-3′ (complementary to the terminal putA coding sequence followed by six histidine codons [bold type], the TGA termination codon, and the XhoI site [underlined]). We verified the nucleotides by sequencing and then rejoined the DNA fragment to the 3′ end of putA to produce functional putA in plasmid pYI370 (Fig. 1). For fusion assays of the putA promoter, the 5′-flanking region of putA was amplified by PCR using pYI370 as the template and primers designed to add BamHI and PstI sites (underlined) at the ends: 5′-CCGGATCCGGCTCCATGGTCATGGGTCGGACTT-3′ (corresponding to nt −459 to −435 of putA) and 5′ CCCTGCAGTTTGAACATCACGCCCTCCTCTTG-3′ (complementary to nt −15 to +9 of putA). The amplified fragment was cloned into plasmid pME6015 (′lacZ) (Table 1) between the BamHI and PstI sites to produce plasmid pYI378 (putA′::′lacZ).
Determination of the Tn5-751 insertion site.
To determine the insertion site of Tn5-751 on the PAO6087 (pru::Tn5-751) chromosome by nucleotide sequencing, the junction regions between the chromosome and the transposon were cloned as follows. The chromosomal DNA of strain PAO6087 was digested with EcoRI, which cleaves the Tn5-751 sequence into aph (Kmr) and dfrII (Tpr) segments (34) (Fig. 1). The resulting EcoRI fragments containing the junctions were then ligated to the corresponding site of plasmid pUC118 (43), and the ligated DNA was transformed into E. coli DH5α (14). Plasmid DNA was isolated from Kmr and Tpr transformants, and the junction sequences in the inserts were determined using an IS50 (terminal inverted sequences of Tn5)-specific primer (5′-CGTTACCATGTTAGGAGG-3′, complementary to nt 71 to 88 of IS50).
Enzyme purification and assays.
Enzymes were prepared from cells grown in MMP supplemented with the indicated 20 mM carbon and nitrogen sources by passage through a French pressure cell (SLM Instruments) at 20,000 lb/in2. ProDH was measured as described previously (24, 25). One unit was defined as the amount of enzyme required to generate 1 μmol of product per min. To purify PutAPAO-His6 strain PAO4502 (putA::FRT-Gm) harboring plasmid pYI370 (putA-His6) was grown to on optical density at 600 nm (OD600) of 1.0 in 1 liter of MMP containing 20 mM proline and tetracycline at 50 μg/ml. After centrifugation, sedimented cells were washed with 20 mM sodium phosphate buffer (pH 7.4) containing 0.5 M NaCl, suspended in 50 ml of the same buffer containing 10 mM imidazole, and disrupted by passage through the French pressure cell. The cell extract obtained by centrifugation at 100,000 × g at 4°C for 1 h was applied to a HiTrap chelating HP column (1 ml; Amersham Pharmacia Biotech). The column was washed with 10 ml of sodium phosphate buffer containing 0.5 M NaCl and 10 mM imidazole, and then PutAPAO-His6 was eluted using a stepwise gradient of imidazole (50, 100, 150, 200, and 500 mM, each at 10 ml) in the sodium phosphate buffer. The amino-terminal sequence was determined using PutAPAO-His6 eluted with 150 mM imidazole. The activity of β-galactosidase was measured using o-nitrophenyl-β-galactopyranoside by the method of Miller (26), and is expressed as Miller units. The protein concentration was determined using a protein assay kit (Bio-Rad Laboratories) with bovine serum albumin as the standard.
Nucleotide and amino acid sequencing.
Nucleotides were sequenced using a Dye Terminator cycle-sequencing kit (Perkin-Elmer) and an ABI 311A DNA sequencer (Perkin-Elmer). Purified PutAPAO-His6 (10 pmol) was resolved by electrophoresis on sodium dodecyl sulfate-polyacrylamide gels (19) and blotted onto a polyvinylidene difluoride membrane (Millipore). The amino-terminal sequence was then determined using an HP 10000A protein sequencer (Hewlett-Packard).
Northern blotting and primer extension.
We isolated RNA from cells exponentially growing (OD600 = 0.3) in MMP containing the indicated carbon and nitrogen sources as described previously (28). Samples of RNA (50 μg) in 10% (wt/vol) glyoxal were resolved along with RNA markers (Toyobo Biochemicals) on 1.0% agarose HS (Nippon Gene) and blotted onto Hybond N+ nylon membranes (Amersham Pharmacia Biotech) using a GenVac blotter (Pharmacia LKB). Membrane transcripts of putA and putP were then detected using 1.3-kb XhoI-SmaI (a SmaI fragment excised from plasmid pYI388) and 0.5-kb StuI (isolated from pYI388) fragments (Fig. 1) labeled with fluorescein-dUTP, respectively, and the ECL detection system (version II; Amersham Pharmacia Biotech). Samples of RNA (20 μg) were annealed with oligonucleotides (5′-TAGTTGGCGCTGATGACAGGGAAGAATTC -3′, complementary to nt 55 to 83 of putA, or 5′-AGCGACATCACCAGTTGGTGATGCTCGTG-3′, complementary to nt 52 to 80 of pruR) and then labeled with 32P at the 5′ end using [γ-32P]ATP (220 Bq/nmol) (Amersham Pharmacia Biotech) and polynucleotide kinase (Takara Shuzo) for primer extension studies. A complementary strand was synthesized using avian reverse transcriptase (RAV-2; Takara Shuzo) in the presence of deoxyribonucleotides as described previously (28) and resolved on 6% denatured polyacrylamide gels along with sequence ladders generated using the BcaBEST sequencing kit (Takara Shuzo), plasmid pYI358 DNA as the template, and the 32P-end-labeled oligonucleotide primers. Radioactive DNA fragments on the gels were visualized on X-ray film.
RESULTS
Cloning and gene organization of the pru::Tn5-751 region.
P. aeruginosa PAO6087 (pru::Tn5-751) was isolated as a mutant unable to grow on proline (Pru− phenotype) by insertional mutagenesis using the transposon Tn5-751 (33, 34). To determine whether the observed Pru− phenotype of strain PAO6087 is due to a lack of ProDH/P5CDH, we assayed ProDH activity in this mutant and in the wild-type strain PAO1 grown in MMP containing 20 mM glutamate (noninducible conditions) or in 20 mM each glutamate and proline (inducible conditions). Noninduced PAO1 cells had only 0.8 U of ProDH/mg of protein, and proline induced ProDH synthesis to 41 U/mg of protein in wild-type strain PAO1, in accordance with previous results (25, 30). In contrast, little proline-inducible ProDH was synthesized in PAO6087 cells (0.7 U/mg of protein) even when cultured under inducible conditions, indicating that the insertion of Tn5-751 had impaired the structure or the regulatory gene for ProDH and P5CDH in this mutant.
To identify the pru::Tn5-751 gene, we first cloned the corresponding chromosomal DNA region of strain PAO1 as a 5.9-kb DNA fragment in plasmid pYI358 (Fig. 1) by functional complementation (Pru+) of the mutation (see Materials and Methods). Nucleotide sequencing revealed that the insert in this plasmid carries a PAO1 chromosome segment spanning from the 3′ portion of PA0779 through the 5′ portion of putA (38) (Fig. 1). The PA0779 gene encodes a putative protease with an Mr of 89,549, resembling the E. coli (41% identity, accession no. AAA16837) and human (42% identity, accession no. CAA53625) ATP-dependent protease, LA. The second gene (PA0780), which is transcribed in the direction opposite to PA0779, specifies a protein (250 residues) with an Mr of 27,587 (Fig. 1). A search of PROSIT files (www.expasy.ch/prosite) revealed significant similarity (value of 29.0) between the C-terminal region (152 to 250 residues) and the PS0121124 profile of the AraC/XylS family DNA-binding domains: this value was within the range (between 30.74 and 12.52) defined for the AraC/XylS members (41). The Helix-Turn-Helix program (7) detected a diagnostic helix-turn-helix DNA-binding motif of the AraC/XylS family (9, 22), between residues 166 and 186. The third PA0781 gene, located opposite PA0780, encodes a hypothetical outer membrane protein (687 residues; Mr, 74,202) with similarity (about 50%) to hemin receptor proteins of PAO1 (PhuR; www.pseudomonas.com) and Xylella fastidiosa (AAF83194). The last gene on plasmid pYI358 was the 5′ part of the putA gene, which is annotated as the gene for ProDH/P5CDH (www.pseudomonas.com). The putA gene (putAPAO) of strain PAO1 is referred to as pruA and pruB, which encode ProDH and P5CDH, respectively (13, 25). The absence of a functional putAPAO gene and the presence of the complete PA0780 and PA0781 genes on the cloned DNA fragment suggested that either PA0780 or PA0781 could be an allele of pru::Tn5-751.
Next, we sequenced the nucleotides to determine the precise insertion site of pru::Tn5-751. EcoRI fragments carrying the aph (Kmr) or dhrll (Tpr) genes were separately cloned along with the adjacent chromosomal segment (Fig. 1) into plasmid pUC118 as described in Materials and Methods. Sequencing the junctions between the transposon and the chromosome sequences revealed that the Tn5-751 sequence is preceded by the upstream sequence of nt 564 of PA0780 and followed by the downstream sequence of nt 556 of this gene. Thus, the 9-bp target sequence (nt 556 to 564) that had been duplicated during transposition reactions (21) flanked the transposon, establishing the insertion of Tn5-751 on PA0780 between nt 564 and 565 (Fig. 1). We designated this gene pruR (proline utilization regulator), because it encodes an AraC/XylS-type transcriptional regulator controlling putA expression as described above.
Identification of a proline-responsive promoter for putAPAO using primer extension.
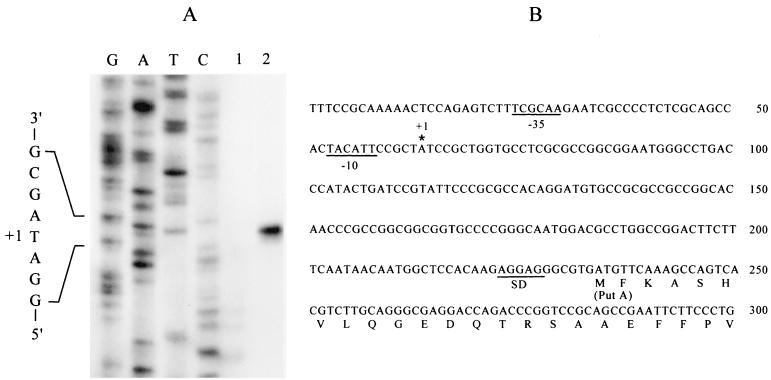
Since the Ω interposon with transcription termination signals (8) on PA0781 of strain PAO4517 (Fig. 1) did not affect proline utilization, putA would be transcribed downstream of the insertion site. We accordingly determined the 5′ end of putAPAO by primer extension using a 32P-end-labeled oligonucleotide complementary to nt 55 to 83 of putAPAO (the amino terminal of PutA was determined by sequencing, as described below) as a primer and RNA samples from PAO1 cells grown in MMP and glutamate or MMP and proline as templates. A 253-bp cDNA was synthesized only with the RNA sample from cells grown on proline (Fig. 2A). A comparison with sequencing ladders determined the 5′ end of the transcript 170 bp upstream of putAPAO (Fig. 2), allowing identification of the plausible −10 and −35 sequences of the σ70 promoter at appropriate distances (Fig. 2B). Similar primer extension studies using an oligonucleotide primer complementary to 52 to 80 of pruR did not detect pruR cDNA (data not shown), indicating very low-level expression of this gene.
FIG. 2.
Primer extension analysis of the putAPAO transcript (A) and structure of the putAPAO promoter region (B). (A) The complementary DNA strand was synthesized from RNA samples extracted from PAO1 cells grown in MMP supplemented with 20 mM glutamate (lane 1) or 20 mM proline (lane 2) and a 32P-end-labeled oligonucleotide primer corresponding to the putA sequence from positions +55 to +83. Sequence ladders (lanes G, A, T, and C) were generated using the same primer and plasmid pYI358 as template. (B) The first six amino acid residues were determined by amino acid sequencing. The 5′ end of the putAPAO transcript (+1) is identified by an asterisk. Inferred −35 and −10 sequences resembling consensus −35 and −10 sequences of σ70 promoters are underlined. SD, Shine-Dalgarno sequence.
The putAP operon.
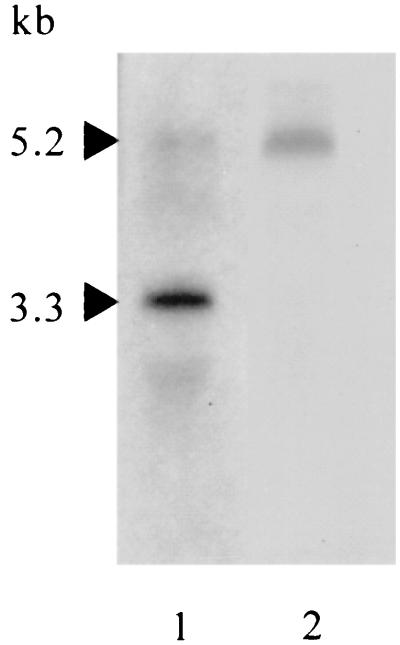
The relatively long intergenic space (312 bp) between putAPAO and putPPAO (Fig. 1) and the potential rho-independent transcription terminator with a stem (10 bp)-loop (2 bp) structure (−29.3 kcal/mol) located 10 bp downstream of putAPAO suggested that the putAPPAO genes are transcribed by either mechanism: transcription from the putAPAO promoter as a single unit that is attenuated by the stem-loop to reduce putPPAO expression or separately from the putAPAO and putPPAO promoters. To establish the transcription mechanism of putAPAOPPAO we analyzed the putAPAO and putPPAO transcripts by Northern blotting using the RNA samples prepared as described above. Probing with the putAPAO sequence (nt 1894 to 3195 from the translation initiation codon) detected major and minor transcripts of 3.3 and 5.2 kb, respectively (Fig. 3), in RNA samples from PAO1 cells grown in MMP plus proline. The latter was also detected as a unique transcript when probed with the putPPAO sequence (nt 569 to 1076), indicating that the putAPAO promoter transcribes putPPAO. This was confirmed by a genetic approach. Plasmid pYI366 (putA+) (Fig. 1) complemented the Pru+ phenotype of strain PAO4502 (putA::FRT-Gm) (Fig. 1) but not that of strain PAO4547 (putA::ΩSp/Sm) (Fig. 1), in which transcription terminators of an Ω interposon in putA prevent downstream transcription. None of the transcripts was detected in the RNA sample from PAO1 cells grown in MMP with glutamate (data not shown).
FIG. 3.
Northern blots of putAP transcripts. RNA was extracted from PAO1 cells grown in MMP with 20 mM proline, as described in the legend to Fig. 2, blotted onto Hybond N+ nylon membrane, and then probed with 1.3-kb putAPAO (lane 1) or 0.5-kb putPPAO (lane 2) regions isolated from plasmid pYI388 as SmaI or StuI fragments (Fig. 1). The molecular sizes of transcripts were determined by comparison with RNA markers and are indicated by arrowheads.
Structural features of PutA.
The amino terminal inferred for PutAPAO corresponds to around residue 70 of the PutA proteins of P. putida and E. coli, and the predicted PutAPAO coding frame has another seven possible translation initiation sites (four ATG and three GTG codons) in the 5′-flanking region (463 bp). To purify PutAPAO for amino-terminal sequencing, we constructed plasmid pYI370 carrying the putAPAO gene tagged with six histidine codons (putAPAO-His6) (Fig. 1). When introduced into strain PAO4502 (putA::FRT-Gm), this fusion gene restored the Pru+ phenotype of the mutant, confirming that PutAPAO-His6 was formed as an active enzyme. We accordingly purified PutAPAO-His6 from this strain by using a Ni2+-chelate column and determined the amino-terminal sequence. The first seven amino acids determined (M-F-K-A-S-H-V) perfectly matched the amino-terminal sequence assigned to PutAPAO (Fig. 2B). Although PutAPAO has substantial similarity to known PutA proteins of other bacteria, in contrast to PutP (PutPPAO) of this strain (see below), PutAPAO was no more similar to PutA of P. putida (47% identity) and was distantly related to all known bacterial PutA proteins (44 to 47% identity). Moreover, PutAPAO (1,060 residues) was also truncated by approximately 250 residues at the carboxyl-terminal region compared with its counterparts in enteric bacteria (1,321 residues) and P. putida (1,315 residues).
PutAPAO has no autorepressor function.
To examine whether PutAPAO autoregulates its expression, we constructed the putA null mutant, PAO4502 (putA::FRT-Gm), by introducing a FRT-Gmr cassette (12) into this gene at the SacI site (Fig. 1) and introduced plasmid pYI378 carrying a putAPAO′::′lacZ fusion into this mutant. We did not detect β-galactosidase activity of the fusion in wild-type strain PAO1 cultured in MMP plus glutamate, but proline (i.e., growth in MMP plus proline or MMP plus glutamate and proline) induced fusion expression to high levels (Table 2). In agreement with the results of the ProDH and P5CDH assays (25), this proline-inducible expression was not affected by the presence of either succinate, a catabolite repressor for P. aeruginosa, or ammonia (Table 2). The fusion levels were comparable between the wild-type strain PAO1 and the ntrC mutant PAO4484 (ntrC::FRT) (data not shown). Thus, putAPAO expression appeared to be independent of both carbon and nitrogen regulation.
TABLE 2.
Regulation of pruA′-′lacZ fusion by PutA and PruRa
| Enzyme source | β-Galactosidase activity (102 Miller units)b
|
||||
|---|---|---|---|---|---|
| Glu | Pro | Glu + Pro | Pro + Suc | Pro + NH4 | |
| PAO1 (wild type) | <0.1 | 62 ± 1 | 49 ± 2 | 66 ± 2 | 59 ± 2 |
| PAO4502 (putA::FRT-Gm) | <0.1 | NGc | 96 ± 6 | NG | NG |
| PAO6087 (pruR::Tn5-751) | <0.1 | NG | <0.1 | NG | NG |
| PAO4534 (pruR::FRT-Gm putA::FRT) | <0.1 | NG | <0.1 | NG | NG |
Cells harboring pYI378 (putAPAO′::′lacZ) were grown in MMP medium supplemented with 20 mM carbon and nitrogen sources.
Activities of β-galactosidase were measured in cell extracts prepared as described previously (26, 28) and are represented as averages of three measurements with standard errors. Carbon and nitrogen sources added to MMP medium: Glu, glutamate; Pro, proline; Suc, succinate.
NG, no growth or very poor growth (see Fig. 4).
The amount of β-galactosidase activity was negligible in strain PAO4502 (putA::FRT-Gm)/pYI378 (putA′::′lacZ) cells cultured in MMP with glutamate (Table 2), indicating that PutAPAO does not repress its expression. When strain PAO4502 was cultured in MMP plus glutamate and proline, the fusion level in PAO4502 doubled compared with that in the wild-type strain PAO1 (Table 2). This increased expression was completely abolished by a pruR mutation, as in strain PAO4534 (putA::FRT pruR::FRT-Gm) (Table 2). When the fusion plasmid was harbored in the pruR mutant (PAO6087), the induction effect of proline on fusion expression was almost completely abolished (Table 2), indicating that PruR primarily controls the proline-responsive expression of putAPAO at the level of transcription.
Functions of PA0781 and putP in proline utilization.
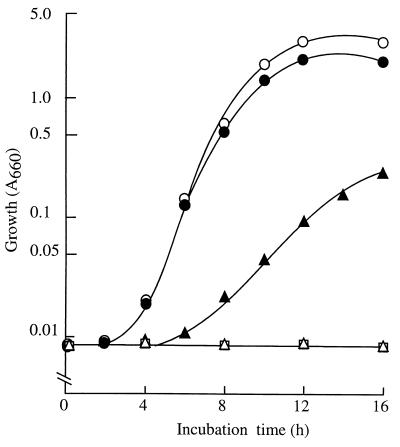
The putPPAO gene is located 312 bp downstream of putAPAO (Fig. 1). PutPPAO is highly homologous to the PutP proteins of P. fluorescens (84% identity), P. putida (80% identity), and E. coli (77% identity). To examine the possible role of PA0781, which encodes a putative outer membrane protein, and the contribution of putPPAO to proline transport, we constructed knockout mutants PAO4517 (PAO781::ΩSp/Sm) and PAO4503 (putP::ΩSp/Sm) by inserting an ΩSp/Sm cassette (8) into PA0781 (at the Bbsl site) and putP (at the BanIII site), respectively (Fig. 1). Strain PAO4517 (PA0781::ΩSp/Sm) proliferated normally on proline (Fig. 4). In contrast, the growth of strain PAO4503 (putP::ΩSp/Sm) on proline was significantly handicapped (Fig. 4). Thus, PA0781 appears to be irrelevant to proline utilization and PutPPAO is the major proline transporter for proline utilization in strain PAO1, as it is in enteric bacteria (23). Proline supporting the residual growth of the putP mutant (Fig. 4) might be taken up by basal levels of ubiquitous osmoregulated proline/glycine betaine transporters. In fact, strain PAO1 has the PA5370 and PA4343 genes, which are homologous to the osmoregulated proline transport proP and proU genes of E. coli, respectively, as well as PA3236, an orthologue of the osmoprotection proline/glycine-betaine periplasmic binding protein genes (www.pseudomonas.com).
FIG. 4.
The growth of putAP and pruR mutants in proline medium is defective. Cultures were grown in MMP with 20 mM proline as carbon and nitrogen sources. Cell growth was measured as described previously (30) and is expressed as absorbance at 660 nm (A660). Open circles, wild-type strain PAO1; solid circles, PAO4517 (PA0781); open triangles, PAO6087 (pru::Tn5-751); solid triangles, PAO4503 (putP::ΩSp/Sm); open squares, PAO4528 (putA::FRT).
DISCUSSION
The characteristic features of the pruR-putAPAOPPAO system of P. aeruginosa PAO1 disclosed in this study include the structure of ProDH/P5DH (the putAPAO product), the organization of the putAPAOPPAO genes, and regulation of the putAPAO promoter by a transcriptional activator of the AraC/XylS family. Compared with PutA proteins of enteric bacteria and of P. putida, PutAPAO is truncated at both amino and carboxyl terminals. PutA of B. japonicum, which is supposed to be regulated by an Lrp-type transcriptional activator (4), also lacks these regions. Different roles of the terminal regions in repressor function have been suggested. Ling et al. (20) have postulated that a leucine zipper (bZIP)-like sequence (having four conserved leucine residues at intervals of 6 amino acids) in the carboxyl-terminal region of the E. coli and S. enterica serovar Typhimurium PutA proteins constitutes a possible DNA-binding motif, although this putative bZIP sequence does not have adjacent basic regions that interact with target DNA (18, 32). Similar sequences were also located at the corresponding regions of PutA proteins from P. putida, R. capsulatus, A. tumefaciens, and S. meliloti. However, the PutA proteins of these bacteria have distinct regulatory properties. PutA proteins of the enteric bacteria, P. putida, and S. meliloti are powerful repressors of putA expression (36, 44). In contrast, expression of putA is increased in putA mutants of A. tumefaciens and R. capsulatus, but only when exogenous proline and functional PutR are present (4, 17). Cho and Winans (4) proposed that the increased expression of putA in a putA mutant of A. tumefaciens could be a consequence of elevated cellular concentrations of proline due to the absence of the proline catabolic enzyme; elevated proline levels could in turn exert an induction effect on putA expression through the PutR function. The situation may be analogous in a putAPAO background, where putAPAO′::′lacZ expression is increased only when both exogenous proline and functional pruR are present (Table 2). Since the PutA of A. tumefaciens lacks the amino-terminal sequence of 68 amino acids, the authors have postulated involvement of this deleted region in the regulation. However, although the PutA of S. meliloti has a shorter (by 2 amino acids) amino terminal than does the PutA of A. tumefaciens, it has apparent repressor properties (36). Thus, the present data seem to exclude the involvement of the amino- and carboxyl-terminal domains in the regulatory function of PutA, although a substitution(s) or small deletion(s) in the carboxyl-terminal regions of the R. capsulatus and A. tumefaciens PutA proteins might abolish the repressor function. Alternatively, PutAPAO and PutA proteins without the repressor function might not undergo autophosphorylation, which is associated with the repressor activity (31). The location of phosphorylation sites and a kinase domain on PutA remain unknown.
Statistical analysis of the putP genes and the control regions among S. enterica serovar Typhimurium and E. coli strains has suggested horizontal transfer of a segment of putP and a cluster of control sites to a particular group of these bacteria from an unrelated, unknown source (29). Sequence diversity is also evident among PutA proteins of the family Rhizobiaceae, where PutA of B. japonicum are only distantly related to its A. tumefaciens and S. meliloti counterparts, which belong to the same family. While the phylogenetic relationships of PutPPAO and other PutP proteins are relevant to that of the origin, PutAPAO appears to be located at a phylogenetic position unrelated to that of the origin. The putA genes of these bacteria are regulated by different mechanisms. The putA gene of S. meliloti is autoregulated, as are the putA genes of enteric bacteria and P. putida, whereas the putA genes of A. tumefaciens and B. japonicum are controlled by the Lrp-type transcriptional activator encoded by the adjacent putR gene (4, 17, 36). Our identification of the pruR gene (encoding an AraC/XylS-type transcriptional activator) provides evidence that the regulatory mechanism of the proline catabolic genes is also totally different even within the same genus.
The putAP genes of P. putida and enteric bacteria are divergently located, and they share an intergenic promoter region controlled by PutA and proline (23, 44). On the other hand, the putP genes of A. tumefaciens, B. japonicum, S. meliloti, and R. capsulatus have not been identified but appear to be separated from putA. Thus, putAPAOPPAO is the first operon of the proline catabolic genes to be demonstrated by Northern blotting (Fig. 3). Transcripts from the putAPAO promoter appear to be prevented by the stem-loop (−29.3 kcal/mol) at 10 bp downstream of putA, and only a small fraction of these transcripts can progress into putPPAO over the attenuation signal (Fig. 3). These different amounts of transcripts allow the required high levels of expression of the catabolic enzyme PutAPAO and the low levels of expression of the membrane protein PutPPAO, which is cytotoxic at high levels.
The putA and putP promoters of Klebsiella pneumoniae and K. aerogenes are controlled by carbon and nitrogen sources through cAMP-Crp and Nac, respectively (23). In accordance with involvement of the PruR of the AraC/XylS family, which activates σ70-dependent promoters (Fig. 2B), the putAPAO promoter is not controlled by nitrogen (Table 2) and does not require the NtrC function. The absence of nitrogen control has also been demonstrated with the putA promoter of R. capsulatus, which requires PutR, an Lrp-type transcriptional activator, for σ70 promoters (17). Expression of the putAPAOPPAO operon also appears not to be subject to catabolite repression by succinate (25) (Table 2). The independence of the putAPAO promoter from carbon and nitrogen control implies the importance of proline as a nutrient for P. aeruginosa PAO1. The diversity of the structure, organization, and regulatory mechanism of the proline catabolic genes between P. aeruginosa and P. putida suggests that different genetic processes have been involved in the development of proline catabolism systems in these closely related species.
Acknowledgments
We are grateful to D. Haas for advice regarding the manuscript and for providing strain PAO6087. We also thank M. Gut-Rella, S. Heeb, and H. Schweizer for the gifts of strain PAO6087, plasmid pME6015, and plasmids pEX18 and pPS858, respectively.
This study was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports and Sciences (grant 14360060) to Y.I. Y.N. is a domestic research fellow supported by the Japan Science and Technology Corporation.
REFERENCES
- 1.Barber, D. A., and J. K. Martin. 1976. The release of organic substances by cereal roots into the soil. New Physiol. 76:68-80. [Google Scholar]
- 2.Becker, D. F., and E. A. Thomas. 2001. Redox properties of the PutA protein from Escherichia coli and the influence of the flavin redox state on PutA-DNA interactions. Biochemistry 40:4714-4721. [DOI] [PubMed] [Google Scholar]
- 3.Chabound, A. 1983. Isolation, purification and chemical composition of maize root cap slime. Plant Soil 73:395-402. [Google Scholar]
- 4.Cho, K., and S. C. Winans. 1996. The putA gene of Agrobacterium tumefaciens is transcriptionally activated in response to proline by an Lrp-like protein and is not autoregulated. Mol. Microbiol. 22:1025-1033. [DOI] [PubMed] [Google Scholar]
- 5.Cho, K., C. Fuqua, and S. C. Winans. 1997. Transcriptional regulation and locations of Agrobacterium tumefaciens genes required for complete catabolism of octopine. J. Bacteriol. 179:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comai, L., C. Schilling-Cordaro, A. Mergia, and C. M. Houck. 1983. A new technique for genetic engineering of Agrobacterium Ti plasmid. Plasmid 10:21-30. [DOI] [PubMed] [Google Scholar]
- 7.Dodd, I. B., and J. B. Egan. 1990. Improved detection of helix-turn-helix DNA-binding motif in protein sequence. Nucleic Acids Res. 18:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vivo insertional mutagenesis of Gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 9.Gallegos, M. T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. Arac/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas, D., B. W. Holloway, A. Schamböck, and T. Leisinger. 1977. The genetic organization of arginine biosynthesis in Pseudomonas aeruginosa. Mol. Gen. Genet. 154:7-22. [DOI] [PubMed] [Google Scholar]
- 11.Heeb, S., Y. Itoh, T. Nishijyo, U. Schnider, C. Keel, J. Wade, U. Walsh, F. O'Gara, and D. Haas. 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol. Plant-Microbe Interact. 13:232-237. [DOI] [PubMed] [Google Scholar]
- 12.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequence: application for isolation of unmarked Pseudomonas mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 13.Holloway, B. W., U. Römling, and B. Tümmler. 1994. Genomic mapping of Pseudomonas aeruginosa PAO. Microbiology 140:2907-2929. [DOI] [PubMed] [Google Scholar]
- 14.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 15.Jiménez-Zurdo, J. I., P. van Dillewijn, M. J. Soto, M. R. de Felipe, J. Olivares, and N. Toro. 1995. Characterization of a Rhizobium meliloti proline dehydrogenase mutant altered in nodulation efficiency and competitiveness on alfalfa roots. Mol. Plant-Microbe Interact. 8:492-498. [DOI] [PubMed] [Google Scholar]
- 16.Jiménez-Zurdo, J. I., F. M. Garia-Rodriguez, and N. Toro. 1997. The Rhizobium meliloti putA gene: its role in the establishment of the symbiotic interaction with alfalfa. Mol. Microbiol. 23:85-93. [DOI] [PubMed] [Google Scholar]
- 17.Keuntje, B., B. Masepohl, and W. Klipp. 1995. Expression of the putA gene encoding proline dehydrogenase from Rhodobacter capsulatus is independent of NtrC regulation but requires an Lrp-like activator protein. J. Bacteriol. 177:6432-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klug, A. 1995. Gene regulatory proteins and their interaction with DNA. Ann. N. Y. Acad. Sci. 758:143-160. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Ling, M., S. W. Allen, and J. M. Wood. 1994. Sequence analysis identifies the proline dehydrogenase and Δ1-pyrroline-5-carboxylate dehydrogenase domains of the multifunctional Escherichia coli PutA protein. J. Mol. Biol. 243:950-956. [DOI] [PubMed] [Google Scholar]
- 21.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, R. G., and J. L. Rosner. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132-137. [DOI] [PubMed] [Google Scholar]
- 23.McFall, E., and E. B. Newman. 1996. Amino acids and carbon sources, p. 358-379. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 24.Meile, L., and T. Leisinger. 1982. Purification and properties of the bifunctional proline dehydrogenase/1- pyrroline-5-carboxylate dehydrogenase from Pseudomonas aeruginosa. Eur. J. Biochem. 129:67-75. [DOI] [PubMed] [Google Scholar]
- 25.Meile, L., L. Soldati, and T. Leisinger. 1982. Regulation of proline catabolism in Pseudomonas aeruginosa PAO. Arch. Microbiol. 132:189-193. [DOI] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Muro-Pastor, A. M., and S. Maloy. 1995. Proline dehydrogenase activity of the transcriptional repressor PutA is required for induction of the put operon by proline. J. Biol. Chem. 270:9819-9827. [DOI] [PubMed] [Google Scholar]
- 28.Nakada, Y., Y. Jiang, T. Nishijyo, Y. Itoh, and C. D. Lu. 2001. Molecular characterization and regulation of the aguBA operon, responsible for agmatine utilization in Pseudomonas aeruginosa PAO1. J. Bacteriol. 183:6517-6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson, K., and R. K. Selander. 1992. Evolutionary genetics of the proline permease gene (putP) and the control region of the proline utilization operon in populations of Salmonella and Escherichia coli. J. Bacteriol. 174:6886-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishijyo, T., D. Haas, and Y. Itoh. 2001. The CbrA-CbrB two-component regulatory system controls the utilization of multiple carbon and nitrogen sources in Pseudomonas aeruginosa. Mol. Microbiol. 40:917-931. [DOI] [PubMed] [Google Scholar]
- 31.Ostrovsky, P. C., and S. Maloy. 1995. Protein phosphorylation on serine, threonine, and tyrosine residues modulates membrane-protein interaction and transcriptional regulation in Salmonella typhimurium. Genes Dev. 9:2034-2041. [DOI] [PubMed] [Google Scholar]
- 32.Pabo, C. O., and R. T. Sauer. 1992. Transcription factors: structural families and principles of DNA recognition. Annu. Rev. Biochem. 61:1053-1095. [DOI] [PubMed] [Google Scholar]
- 33.Rella, M. 1984. Transposon insertion mutagenesis of the Pseudomonas aeruginosa genome. Ph.D. dissertation (ETH no. 7601), Swiss Federal Institute of Technology, Zurich.
- 34.Rella, M., A. Mercenier, and D. Haas. 1985. Transposon insertion mutagenesis of Pseudomonas aeruginosa with a Tn5 derivative: application to physical mapping of the arc gene cluster. Gene 33:293-303. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Soto, M. J., J. I. Jiménez-Zurdo, P. van Dillewijn, and N. Toro. 2000. Sinorhizobium meliloti putA gene regulation: a new model within the family Rhizobiaceae. J. Bacteriol. 182:1935-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanier, R. Y., N. J. Palleroni, and M. Doudoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 38.Stover, C. V., et al. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 39.Straub, P. F., P. H. Reynolds, S. Althomsons, V. Mett, Y. Zhu, G. Shearer, and D. H. Kohl. 1996. Isolation, DNA sequence analysis, and mutagenesis of a proline dehydrogenase gene (putA) from Bradyrhizobium japonicum. Appl. Environ. Microbiol. 62:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surber, M. W., and S. Maloy. 1999. Regulation of flavin dehydrogenase compartmentalization: requirements for PutA-membrane association in Salmonella typhimurium. Biochim. Biophys. Acta 1421:5-18. [DOI] [PubMed] [Google Scholar]
- 41.Tobes, R., and J. L. Ramos. 2002. AraC-XylS database: a family of positive transcriptional regulators in bacteria. Nucleic Acids Res. 30:318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dillewijn, P., M. J. Soto, P. J. Villadas, and N. Toro. 2001. Construction and environmental release of a Sinorhizobium meliloti strain genetically modified to be more competitive for alfalfa nodulation. Appl. Environ. Microbiol. 67:3860-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 44.Vílchez, S., L. Molina, C. Ramos, and J. L. Ramos. 2000. Proline catabolism by Pseudomonas putida: cloning, characterization, and expression of the put genes in the presence of root exudates. J. Bacteriol. 182:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]