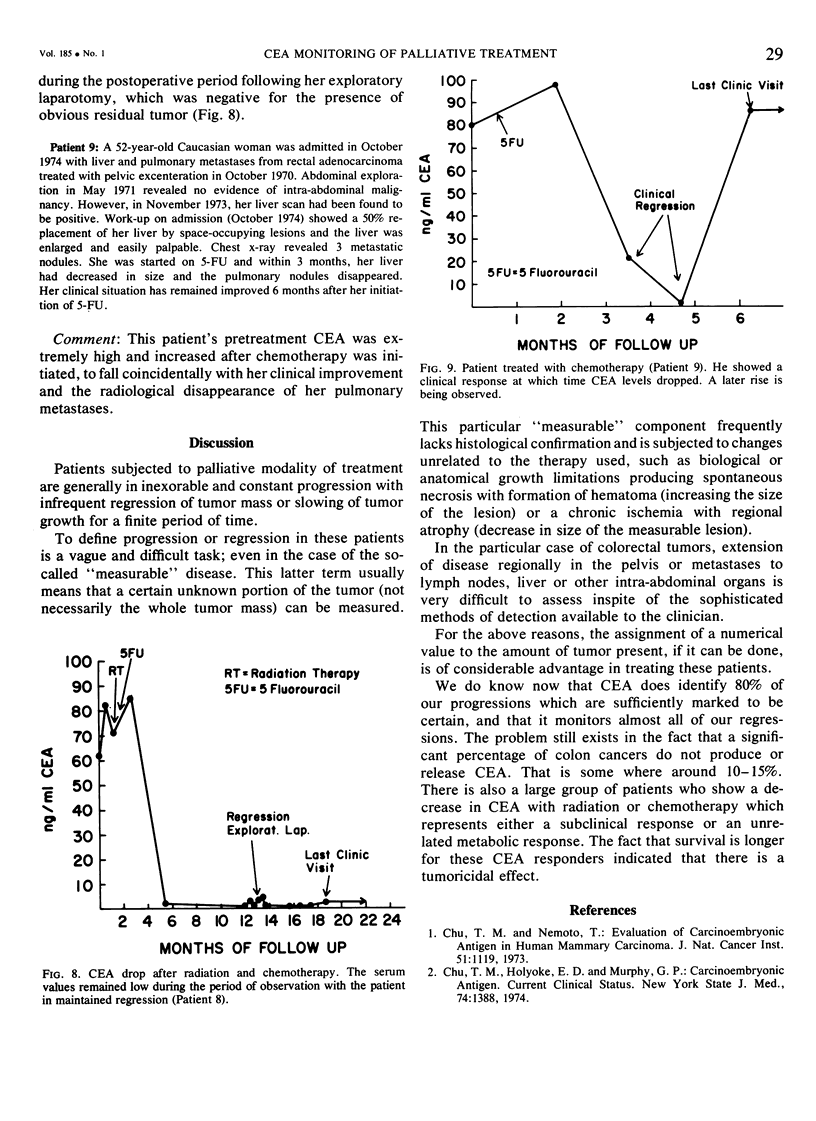
Abstract
Palliative treatment was applied to 131 cases of unresectable or palliatively resected colorectal carcinoma being monitored with serial CEA determinations. There were 84 instances of disease progression with 67 (80%) of them showing an increase in CEA above pretreatment levels or maintaining high levels, and 17 (20%) showing a fall when compared to pretreatment values or maintaining low initial values. There was a clear-cut regression of the disease in only 9 instances. In all 9, the CEA clearly dropped or maintained low valles throughout the period of regression. No patient in regression had a rise or maintained an elevated CEA level. These changes in CEA followed closely the clinical response of our patient to the use of a particular agent, although for the Nitrosourea compounds there may be a tendency to lower the CEA regardless of the patient's tumor response to the drug. This could be due to the fact that the Nitrosoureas produce a diffuse block of cellular activity, both at the nucleous and cytoplasm; while other compounds act as alkylating agents or by inhibition of enzymes involved in the metabolism of nucleic acids (i.e., 5-FU inhibiting thymidylate synthetase). In general, longer survival was found in those patients who had initially lower levels of CEA as compared to those with high initial levels. The patients with a favorable CEA response to the treatment (falling CEA or maintained low value), even in many who did not show a clinical response had a longer survival than the group with rising or stable high levels. The main value in CEA monitoring of patients resides in its correlation with the amount of disease present and then its ability to detect progression of tumor mass which is not clinically measurable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu T. M., Holyoke E. D., Murphy G. P. Carcinoembryonic antigen. Current clinical status. N Y State J Med. 1974 Jul;74(8):1388–1393. [PubMed] [Google Scholar]
- Chu T. M., Nemoto T. Evaluation of carcinoembryonic antigen in human mammary carcinoma. J Natl Cancer Inst. 1973 Oct;51(4):1119–1122. doi: 10.1093/jnci/51.4.1119. [DOI] [PubMed] [Google Scholar]
- Dhar P., Moore T., Zamcheck N., Kupchik H. Z. Carcinoembryonic antigen (CEA) in colonic cancer. Use in preoperative and postoperative diagnosis and prognosis. JAMA. 1972 Jul 3;221(1):31–35. [PubMed] [Google Scholar]
- Hansen H. J., Snyder J. J., Miller E., Vandevoorde J. P., Miller O. N., Hines L. R., Burns J. J. Carcinoembryonic antigen (CEA) assay. A laboratory adjunct in the diagnosis and management of cancer. Hum Pathol. 1974 Mar;5(2):139–147. doi: 10.1016/s0046-8177(74)80061-4. [DOI] [PubMed] [Google Scholar]
- Herrera M. A., Chu T. M., Holyoke E. D. Carcinoembryonic antigen (CEA) as a prognostic and monitoring test in clinically complete resection of colorectal carcinoma. Ann Surg. 1976 Jan;183(1):5–9. doi: 10.1097/00000658-197601000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holyoke D., Reynoso G., Chu T. M. Carcinoembryonic antigen (CEA) in patients with carcinoma of the digestive tract. Ann Surg. 1972 Oct;176(4):559–564. doi: 10.1097/00000658-197217640-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holyoke E. D., Chu T. M., Murphy G. P. CEA as a monitor of gastrointestinal malignancy. Cancer. 1975 Mar;35(3):830–836. doi: 10.1002/1097-0142(197503)35:3<830::aid-cncr2820350340>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lo Gerfo P., Krupey J., Hansen H. J. Demonstration of an antigen common to several varieties of neoplasia. N Engl J Med. 1971 Jul 15;285(3):138–141. doi: 10.1056/NEJM197107152850302. [DOI] [PubMed] [Google Scholar]
- Lo Gerfo P., Lo Gerfo F., Herter F., Barker H. G., Hansen H. J. Tumor-associated antigen in patients with carcinoma of the colon. Am J Surg. 1972 Feb;123(2):127–131. doi: 10.1016/0002-9610(72)90321-2. [DOI] [PubMed] [Google Scholar]
- McCartney W. H., Hoffer P. B. The value of carcinoembryonic antigen (CEA) as an adjunct to the radiological colon examination in the diagnosis of malignancy. Radiology. 1974 Feb;110(2):325–328. doi: 10.1148/110.2.325. [DOI] [PubMed] [Google Scholar]
- Moore T. L., Kupchik H. Z., Marcon N., Zamcheck N. Carcinoembryonic antigen assay in cancer of the colon and pancreas and other digestive tract disorders. Am J Dig Dis. 1971 Jan;16(1):1–7. doi: 10.1007/BF02233781. [DOI] [PubMed] [Google Scholar]
- Mulcare R., Lo Gerfo P. Tumor associated antigen in the chemotherapy of solid tumors. J Surg Oncol. 1972;4(5):407–417. doi: 10.1002/jso.2930040502. [DOI] [PubMed] [Google Scholar]
- Reynoso G., Chu T. M., Holyoke D., Cohen E., Nemoto T., Wang J. J., Chuang J., Guinan P., Murphy G. P. Carcinoembryonic antigen in patients with different cancers. JAMA. 1972 Apr 17;220(3):361–365. [PubMed] [Google Scholar]
- Schmall B., Cheng C. J., Fujimura S., Gersten N., Grunberger D., Weinstein I. B. Modification of proteins by 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (nsc 79037) in vitro. Cancer Res. 1973 Aug;33(8):1921–1924. [PubMed] [Google Scholar]
- Skarin A. T., Delwiche R., Zamcheck N., Lokich J. J., Frei E., 3rd Carcinoembryonic antigen: clinical correlation with chemotherapy for metastatic gastrointestinal cancer. Cancer. 1974 May;33(5):1239–1245. doi: 10.1002/1097-0142(197405)33:5<1239::aid-cncr2820330508>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Sorokin J. J., Sugarbaker P. H., Zamcheck N., Pisick M., Kupchik H. Z., Moore F. D. Serial carcinoembryonic antigen assays. Use in detection of cancer recurrence. JAMA. 1974 Apr 1;228(1):49–53. [PubMed] [Google Scholar]
- Steward A. M., Nixon D., Zamcheck N., Aisenberg A. Carcinoembryonic antigen in breast cancer patients: serum levels and disease progress. Cancer. 1974 May;33(5):1246–1252. doi: 10.1002/1097-0142(197405)33:5<1246::aid-cncr2820330509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Zamcheck N., Moore T. L., Dhar P., Kupchik H. Immunologic diagnosis and prognosis of human digestive-tract cancer: carcinoembryonic antigens. N Engl J Med. 1972 Jan 13;286(2):83–86. doi: 10.1056/NEJM197201132860207. [DOI] [PubMed] [Google Scholar]


