Abstract
Human immunodeficiency type 1 (HIV-1) continues to spread at an alarming rate. The virus may be transmitted through blood, genital secretions, and breast milk, and higher levels of systemic virus in the index case, as measured by plasma RNA viral load, have been shown to correlate with increased risk of transmitting HIV-1 both vertically and sexually. Less is known about the correlation between transmission and HIV-1 levels in breast milk or genital secretions, in part because reliable quantitative assays to detect HIV-1 in these fluids are not available. Here we show that the Gen-Probe HIV-1 viral load assay can be used to accurately quantify viral load in expressed breast milk and in cervical and vaginal samples collected on swabs. Virus could be quantified from breast milk and swab samples spiked with known amounts of virus, including HIV-1 subtypes A, C, and D. As few as 10 copies of HIV-1 RNA could be detected above background threshold levels in ≥77% of assays performed with spiked breast milk supernatants and mock swabs. In genital swab samples from HIV-1-infected women, similar levels of HIV-1 RNA were consistently detected in duplicate swabs taken from the same woman on the same clinic visit, suggesting that the RNA values from a single swab sample can be used to measure genital viral load.
Human immunodeficiency type 1 (HIV-1) infection is associated with a long, clinically asymptomatic period that is followed by immunodeficiency disease at variable times after infection. The levels of systemic virus, as measured by HIV-1 RNA in plasma, have been shown to predict the rate of disease progression (9, 12, 17, 18). Moreover, studies in the United States and Thailand have shown that maternal plasma HIV-1 RNA levels are highly correlated with the risk of infant infection (3, 5, 13, 20). Recently, it has been shown that individuals with higher plasma viral RNA levels are significantly more likely to sexually transmit HIV-1 than individuals with lower plasma viral RNA levels (19). Relatively less is known regarding the correlation between transmission and the levels of virus in other body fluids, such as breast milk and genital secretions, that may be exchanged during high-risk behaviors for HIV-1 infection. Results of a recent clinical trial found the rate of transmission from mother to infant via breast-feeding to be 16.2% (15). HIV-1 infection in infants also occurs during labor and delivery, when the infant is exposed to infected blood and cervicovaginal secretions (10). Recent studies suggest that the detection of HIV-1-infected cells and HIV-1 RNA in genital secretions correlates with vertical transmission of HIV-1 (3, 8). Moreover, most HIV-1 infections in adults worldwide have been transmitted sexually when there is exposure to infected seminal fluid and cervicovaginal secretions, as well as blood in some cases (14). Collectively, these data suggest that increased amounts of HIV-1 in mucosal secretions may increase the risk of transmission of HIV-1.
One technical impediment to the study of the role of mucosal virus in HIV-1 transmission has been the lack of a high-throughput assay to quantify HIV-1 RNA in breast milk and genital secretions that is sensitive and specific for the genetic strains of HIV-1 circulating worldwide. Currently available commercial assays for detection of HIV-1 RNA were designed for the genetic subtype of HIV-1 most common in the developed world, subtype B. Worldwide, however, there are multiple subtypes of HIV-1, and subtype B is a relatively minor strain. HIV-1 has been categorized into the larger groups of M (main), O (outlier), and most recently, N based on the relatedness of their sequences (reviewed in reference 21 and by Robertson et al. [D. L. Robertson, J. P. Anderson, J. A. Bradac, et al., Letter, Science 288:55-56, 2000]). The group M and O viruses differ by as much as 50% in their overall sequences. Group M is made up of at least 10 subtypes (A through H and J and K), and they differ by as much as 15 to 30% from each other in gag and env, respectively. As discussed previously (4, 7), the inability of most commonly used methods to quantify genetically diverse subtypes of HIV-1 has limited the utility of such assays for studies in high-risk populations found in Africa, where, according to the Joint United Nations Programme on HIV/AIDS (http://www.unaids.org), the HIV-1 pandemic is most severe. A variety of the subtype-B-based HIV-1 RNA assays have been used for analyses of samples of genital secretions (6, 11, 22); however, most of these methods were tested and optimized using plasma, and it is somewhat unclear whether they consistently quantify virus in each of these other secretions. One study suggests that several commercial HIV-1 RNA assays give comparable results when testing cervicovaginal lavage fluid specimens, although variation in detection with these methods was observed (1).
The Gen-Probe HIV-1 viral load assay is a transcription-mediated amplification (TMA)-based assay that is carried out in a single-tube, high-throughput format. We have recently shown that the Gen-Probe HIV-1 viral load assay can quantitatively detect HIV-1 in plasma collected from people infected with subtypes found in Africa and Asia (4, 7). In the present study, we show that this assay can accurately quantify HIV-1 in cervical and vaginal secretions and in breast milk.
MATERIALS AND METHODS
Spiking experiments with cell-free virus in breast milk and genital swab samples.
Primary viral isolates, representing subtypes A, C, and D, were obtained, as described previously, from blood samples provided by HIV-1-infected individuals in Nairobi, Kenya (4). Two concentrations of cell-free virus (103 and 104)—one each of subtype A, C, and D (as determined previously [4, 16])—were used for spiking experiments. We used these viruses to generate spiked breast milk supernatant samples and mock genital swab samples. HIV-1-negative whole breast milk, which had been stored at −70°C, was spun at 1,000 rpm in a Beckman GPKR centrifuge for 20 min, the lipid layer was removed with a transfer pipette, and the clear supernatant layer was pipetted into vials. The aliquots of supernatant were then spiked with virus. To generate mock samples, virus was pipetted onto a Dacron swab, 106 uninfected human (CEM × 174 or 293T) cells were added, and the spiked swab was immersed into 1 ml of freezing medium (70%RPMI 1640 with l-glutamine and 25 mM HEPES, 20% fetal bovine serum, 10% dimethyl sulfoxide). To control for inhibitors present in either the freezing medium or the Dacron swab, the same amount of virus was added in parallel to 1-ml aliquots of freezing medium alone and 1-ml aliquots of freezing medium containing a Dacron swab. Aliquots of HIV-1-negative plasma were also spiked in parallel to serve as a “gold standard” comparison.
To examine the sensitivity of the assay for low copy numbers of RNA, replicate breast milk supernatants and mock swab samples were spiked with a control included with the Gen-Probe HIV-1 viral load assay that corresponded to 10 copies of HIV-1 RNA. Unspiked breast milk supernatants and mock swab samples were assayed to test the reliability of the assay for HIV-1-negative samples.
HIV-1-positive clinical samples.
Breast milk samples collected from HIV-1-positive women in Nairobi (8, 15) were processed as described above. Supernatants were frozen in 1.5-ml aliquots, stored at −70°C in Nairobi, shipped on dry ice to Seattle, Wash., and stored at −70°C until use.
Duplicate cervical and vaginal swabs from HIV-1-positive women in Mombasa, Kenya (23), were assayed in two ways. The first swab sample was vortexed for approximately 5 s, and medium was tested directly out of the vial. The second swab sample was fractionated. A sample was fractionated as follows. (i) The sample vial containing swab and medium was vortexed for approximately 5 s, and a 300-μl aliquot of medium was removed and reserved for testing (undiluted aliquot). (ii) The swab was pressed against the inner wall of the vial and then rotated 360° to express remaining liquid, and the swab was discarded; the remaining contents of the vial, which ranged from 400 to 800 μl, were centrifuged at 1,500 rpm in a Beckman GPKR centrifuge for 5 min, and the supernatant was removed to another vial (supernatant aliquot). (iii) The entire cell pellet was resuspended in 300 μl of fresh freezing medium (cell aliquot). The three fractions were assayed independently.
Cell-associated virus mock sample panel.
To generate HIV-1-infected cells, approximately 3 × 105 MAGI-CCR5 cells (2) were seeded into a T25 flask and allowed to grow overnight. The following day, 50 μl of cell-free tissue culture supernatant, 100 μl of DEAE-dextran (1.5 mg/ml), and 850 μl of DMEM complete (Dulbecco's modified Eagle's medium [DMEM] supplemented with 10% fetal bovine serum and 100 U of penicillin, 100 μg of streptomycin, puromycin [1 μg/ml], and hygromycin [50 U/ml]) were added to the seeded cells and allowed to incubate for 2 h at 37°C and 5% CO2, and then 4 ml of DMEM complete was added. Cells were passaged for about 2 weeks to allow virus spread, at which point cytopathic effects were evident throughout the culture. Infected cells were collected and used to spike swabs.
Because swab samples from HIV-infected persons have a mixture of infected and uninfected cells, cell-associated mock swab samples were generated by spiking either 103 or 104 infected MAGI-CCR5 cells onto a Dacron swab containing approximately 103 uninfected CEM × 174 cells. The swab was then immersed into an aliquot of 1 ml of freezing medium. Cell-free virus was spiked onto swabs containing approximately 103 uninfected CEM × 174 cells in parallel, and the swabs were immersed into an aliquot of 1 ml of freezing medium. The mock swab samples were frozen overnight at −70°C and then transferred to liquid nitrogen the following day, because this is the protocol typically used for patient swabs collected in the field. Mock samples were either assayed directly out of the vials, or were fractionated as described above.
Gen-Probe HIV-1 viral load assay.
The Gen-Probe HIV-1 viral load assay (Gen-Probe Incorporated, San Diego, Calif.), which is a TMA assay, was performed in our laboratory. A description of the assay has been published previously (4, 16). Briefly, the three steps involved in the assay protocol are: (i) sample preparation and target capture, which utilizes magnetic beads to isolate target RNA; (ii) amplification by TMA; and (iii) detection of the amplified material with the hybridization protection assay, are all carried out in a single tube. This integrated approach allows processing of 200 samples in 6 to 8 h (C. Giachetti, D. Kolk, J. Dockter, J. Knowlton, R. Wang, H. Hotaling, and S. McDonough, unpublished data [presented at the 12th World AIDS Conference, Bologna, Italy, 1998]). The sample preparation and target capture step is not volume dependent (S. Bodrug, personal communication). Thus, a range of input sample volumes was used, but the assay output results were adjusted to reflect the appropriate dilution factor.
Data analysis.
SPSS (Chicago, Ill.) software (version 10.0) or STATA software (Statistical Software, College Station, Tex.) were used for all analyses. Repeated-measures analysis of variance models was used with log10-transformed outcomes to test for differences in the amount of virus detected in spiked breast milk samples compared to that detected in spiked plasma samples and to test for differences between results from the same breast milk samples tested at different dilutions. Similar models were used to test for differences between results from spiked genital swabs in freezing medium and freezing medium alone versus results from spiked plasma. Receiver operator characteristic (ROC) curves were used to determine the best threshold for the lower limit of detection of the assay. Spearman's correlation coefficient was used to compare results for vaginal and cervical swabs tested at different dilutions. The average quantity of HIV-1 RNA detected in cervical and vaginal samples tested at different dilutions was compared using the paired t test. Finally, the proportions of cervical and vaginal samples above the level of quantitation of the assay using different genital swab preparation methods were compared using McNemar's test.
RESULTS
Testing of spiked breast milk and swab samples.
To test whether cell-free HIV-1 can be detected in breast milk samples, the supernatant fraction from 8 different uninfected breast milk donors was spiked with virus that corresponded to approximately 3.0 log10 copies/ml of clade A HIV-1 RNA. These spiked samples were tested at a 500-μl volume, which is the maximum volume that can to be added during the specimen-processing step of the Gen-Probe HIV-1 viral load assay. Each sample was tested six times, and the log10-transformed results are summarized in Table 1. The average of the replicates from each donor ranged from 3.3 to 3.4 log10 copies/ml. The maximum difference between replicates from an individual donor was 3.1 log10 copies (2.1-fold), and that between donors was 3.3 log10 copies (2.7-fold), with an average of the maximum differences between donors of 2.9 log10 copies (95% confidence interval [CI], 547 to 1,131 copies). Comparable results were observed when we spiked a duplicate set of the uninfected breast milk samples with approximately 4.0 log10 copies of HIV-1 per ml (data not shown). In all cases, the results of all the assays performed on this panel of spiked breast milk samples agreed within threefold.
TABLE 1.
RNA levels from replicate testing of eight uninfected donor samples spiked with virus corresponding to approximately 3.0 log10 copies/ml
| Replicate test no. | RNA level (log10 copies/ml) in breast milk supernatant specimen:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| F | G | H | I | J | K | L | M | |
| 1 | 3.28 | 3.36 | 3.41 | 3.38 | 3.32 | 3.43 | 3.32 | 3.44 |
| 2 | 3.43 | 3.33 | 3.33 | 3.37 | 3.28 | 3.51 | 3.07 | 3.44 |
| 3 | 3.17 | 3.42 | 3.42 | 3.34 | 3.34 | 3.41 | 3.30 | 3.43 |
| 4 | 3.43 | 3.38 | 3.40 | 3.31 | 3.35 | 3.31 | 3.35 | 3.32 |
| 5 | 3.38 | 3.41 | 3.45 | 3.43 | 3.33 | 3.31 | 3.40 | 3.32 |
| 6 | 3.37 | 3.35 | 3.43 | 3.39 | 3.25 | 3.46 | 3.31 | 3.44 |
| Avg | 3.33 | 3.37 | 3.39 | 3.35 | 3.32 | 3.42 | 3.26 | 3.41 |
| SD | 2.67 | 2.30 | 2.37 | 2.36 | 2.26 | 2.68 | 2.64 | 2.54 |
| % CVa | 21.49 | 8.40 | 9.59 | 10.21 | 8.66 | 18.22 | 23.46 | 13.21 |
CV, coefficient of variation.
To examine the performance of the assay across a range of nonplasma specimen volumes, uninfected breast milk supernatant was spiked with an HIV-1 standard included with the Gen-Probe HIV-1 viral load assay corresponding to an estimated 2.0 log10 copies. The spiked breast milk supernatant was made as a large stock, and aliquots of 5 to 500 μl were assayed. Uninfected plasma was spiked in parallel with a predicted 2.0 log10 copies and assayed across the same range. Results for the plasma and breast milk supernatant that were tested across this range of volumes were within twofold (data not shown). However, there was some suggestion for a modest inhibition when breast milk supernatant was spiked in parallel with plasma, and more extensive testing was performed (Table 2). In this case, three viruses were used (one each of subtype A, C, and D), and each sample type was tested four or more times at 500 μl by two technicians; aliquots of HIV-1-negative human plasma were spiked in parallel for comparison. Because viral stocks rather than commercial standards were used, the exact amounts of viral RNA added for each stock were not identical. For example, only 2.50 log10 copies of subtype D virus was added to each tube versus 3.00 log10 copies of subtype C virus. Thus, the spiked plasma, which received the same stock of virus as the breast milk supernatant, served as the control for each virus stock tested. The difference between results of spiked breast milk supernatant and spiked plasma was statistically significant (P < 0.001). Taken together, these small differences suggest that there could be a small amount of inhibition when 500 μl of breast milk is added to the assay.
TABLE 2.
RNA levels from four to eight replicates of quantitative RNA assays using spiked plasma, breastmilk supernatant, and mock swab samples
| Virus used for spiking | Avg log10 RNA level/ml in plasmaa (% CVb) | Log10 RNA level
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Breast milk supernatant
|
Freezing medium
|
Freezing medium plus:
|
|||||||
| Swab
|
Swab and cells
|
||||||||
| Avg/ml (differencec) | % CV | Avg/ml (difference) | % CV | Avg/specimen (difference) | % CV | Avg/specimen (difference) | % CV | ||
| Clade A | 3.28 (12.42) | 3.17 (−0.11) | 21.14 | 3.36 (0.08) | 21.77 | 3.25 (−0.03) | 36.94 | 3.20 (−0.08) | 33.38 |
| Clade C | 3.00 (18.67) | 2.85 (−0.15) | 41.78 | 3.04 (0.04) | 8.67 | 2.70 (−0.30) | 31.37 | 2.81 (−0.19) | 13.69 |
| Clade D | 2.50 (23.07) | 2.18 (−0.32) | 24.44 | 2.47 (−0.03) | 51.00 | 2.27 (−0.23) | 42.72 | 2.32 (−0.18) | 33.65 |
The amount of clade A, C, and D virus added was estimated based on previous assays. Previous studies showed that the Gen-Probe HIV-1 viral load assay can accurately quantify all three subtype. Thus, differences in the spiked plasma reflect small differences in the amount of viral stock added.
CV, coefficient of variation.
Log10 difference between nonplasma samples and plasma samples is shown in parentheses.
For the studies above, breast milk supernatants were used for spiking experiments. To examine whether similar results would be obtained if we spiked whole breast milk and then separated the supernatant, whole breast milk was spiked with approximately 3.0 log10 copies of a clade A HIV-1. The supernatant was then separated as described in Materials and Methods and assayed. In addition, whole breast milk from the same donation was also first separated, the supernatant fraction was spiked with the same virus, and uninfected plasma was spiked in parallel to serve as a control. The average of results from five assays was 3.14 log10 HIV-1 RNA copies in breast milk supernatant that was separated from spiked whole milk, 3.15 log10 copies in supernatant that was first separated and then spiked, and 3.28 log10 copies in the plasma control. The log10 differences between the breast milk supernatant results and the plasma results were comparable to those obtained in the experiment presented in Table 2, and there was no observable difference between the results from the two breast milk supernatant samples regardless of whether supernatant or whole milk was spiked. Thus, in this small pilot study, it appears that little to no virus is trapped in the lipids of the breast milk upon separation.
Replicate mock genital samples were also spiked with the three viruses in parallel to examine if HIV-1 RNA can be detected in genital samples collected with Dacron swabs (Table 2). All replicates for each virus subtype agreed within twofold (data not shown), and all sample types agreed within threefold compared to the corresponding plasma copy number. There was a significant difference between the results of swabs in freezing medium versus plasma (P = 0.006). However, there was not a significant difference in the amount of virus detected in plasma versus the amount detected in freezing medium alone (P = 0.8). Similar results were observed when we tested samples spiked with virus corresponding to approximately 4.0 log10 copies of HIV-1 RNA (data not shown). This may suggest that there is a modest inhibitory effect when using 500 μl of sample from swabs collected in 1 ml of freezing medium. This is not the effect of freezing medium itself; more likely, it is due to either the presence of large amounts of DNA, which may affect target capture; inhibitory material on the swab itself; or the potential for virus particles to remain trapped (and therefore inaccessible) in the swab.
Determination of the lower limits of the Gen-Probe HIV-1 viral load assay for breast milk and swab samples.
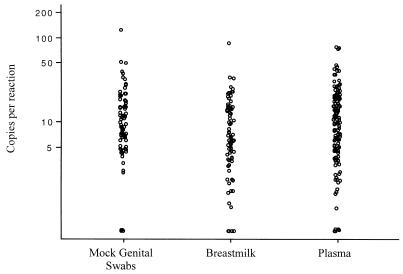
Forty-one samples of uninfected breast milk from five donors and 41 mock swab samples were used to determine the specificity of the Gen-Probe HIV-1 viral load assay for different threshold values. Similarly, 82 uninfected breast milk supernatant samples and 82 uninfected mock swab samples that were spiked with a predicted 10 copies per reaction were used to determine the sensitivity of the Gen-Probe HIV-1 viral load assay for different threshold values. Using ROC curves, the optimum lower limit of detection of the assay was defined as more than three copies per reaction. With this threshold, the sensitivity of the assay in breast milk supernatant samples is 77% (95% CI, 68 to 86%), and the specificity is 100% (95% CI, 98 to 100%). The sensitivity of the assay for swab specimens is 92% (95% CI, 95 to 98%), and the specificity is 98% (95% CI, 93 to 100%). These results are similar to previous findings using uninfected plasma. Eighty-five percent of plasma specimens spiked with a predicted 12.5 copies per reaction and <1% of uninfected plasma specimens tested in parallel yielded results above the threshold of detection (three copies per reaction) (Bodrug et al., unpublished). These data are shown in Fig. 1 for comparison.
FIG. 1.
Detection of HIV-1 RNA in mock genital swabs, breast milk, and plasma spiked with a low level of HIV-1 using the Gen-Probe HIV-1 viral load assay. Mock genital swabs were spiked with a control virus corresponding to 10 copies of RNA (n = 82). Similarly, breast milk samples from five donors were spiked with a similar amount of virus (n = 82). For comparison, the results for plasma samples spiked with 12.5 copies of HIV-1 RNA per reaction are shown (n =140). The results of the assays are displayed on a log scale. Each circle represents one result.
Examination of breast milk from HIV-1-infected women.
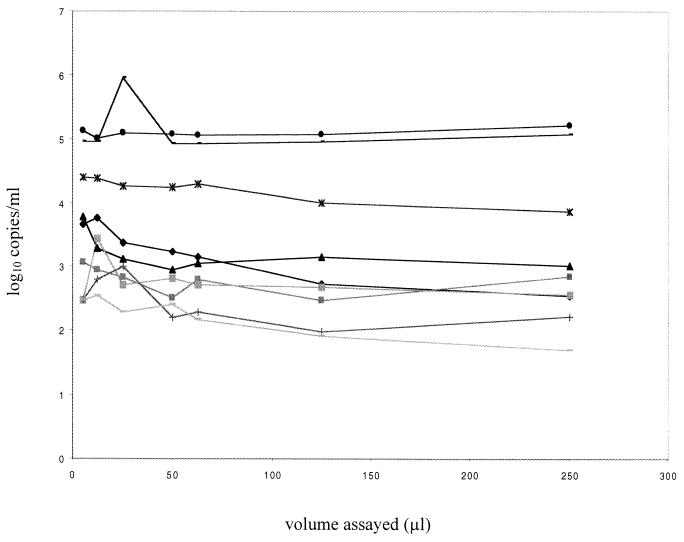
To assess the performance of the assay on clinical specimens from HIV-1-infected individuals, nine breast milk supernatant samples from HIV-positive women were assayed in stepwise dilutions. Because the available samples were limited, a range of 5 to 250 μl of breast milk supernatant from each sample was tested in the assay, and the results were adjusted to reflect the appropriate dilution factor (Fig. 2). There was not a statistically significant difference between results from the same sample tested in this range of dilutions (P = 0.3). Taken together, these data suggest RNA can be quantified in volumes of breast milk up to 250 μl, although future studies will be needed to carefully address whether there is any inhibition when testing 250- to 500-μl volumes of breast milk.
FIG. 2.
RNA copy number in clinical breast milk supernatant samples collected from HIV-1-positive women. Samples were assayed at a range of volumes from 5 to 250 μl, and the log10-transformed results are shown. Each line connected by like symbols represents the results from one sample of one woman.
Examination of cervical and vaginal swabs from HIV-1-infected women.
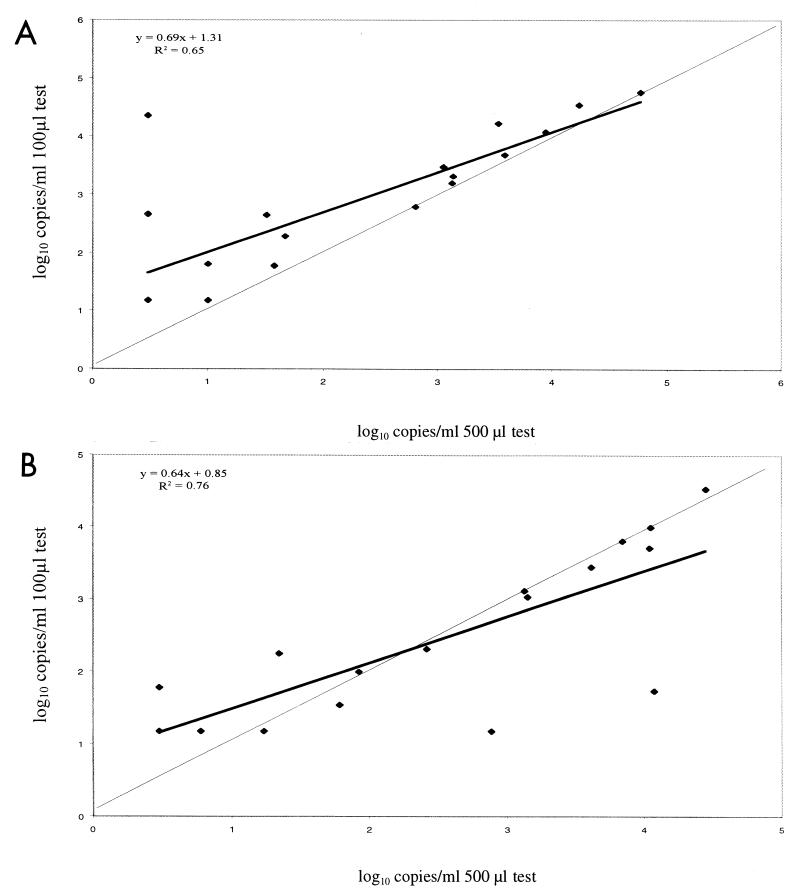
Cervical and vaginal swab samples taken from 21 HIV-1-positive women were analyzed at two concentrations. Each swab was tested in 500- and 100-μl volumes, and the results comparing the two tests are summarized in Fig. 3. For cervical swab samples, the adjusted results from the 100-μl test correlated with the results from the 500-μl test (r2 = 0.65). For samples that had quantifiable results at both testing volumes, the 100-μl-test results were as much as 10-fold higher than the corresponding 500-μl-test result, and the average of the100-μl-test results were 1.3 logs higher than those from the 500-μl tests. In general, vaginal swab samples from the same women contained fewer copies of HIV-1 RNA than did cervical swab samples. The results from vaginal samples tested at the two dilutions were more closely correlated than their cervical counterparts (r2 = 0.76). Twelve vaginal samples tested at or below the threshold of detection in 100-μl volumes, and 10 tested at or below the threshold of detection in 500-μl volumes, compared to 6 and 7 in 100- and 500-μl volumes, respectively, for the comparable group of cervical swabs.
FIG. 3.
Comparison of the results of testing 500- and 100-μl specimen volumes of the same swab sample. The thick line represents the observed correlation between the results from each volume, and the thin line represents a perfect correlation. (A) Cervical swabs; (B) vaginal swabs.
To examine how the assay performs using 100 versus 200 μl of specimen volume, 67 cervical and 109 vaginal samples from HIV-1-positive women were analyzed at test volumes of 100 and 200 μl. For the cervical samples, the mean log10 at the 200-μl volume was 3.67, and that at the 100-μl volume was 3.69, and this small difference was not statistically significant (P = 0.7). The correlation between log10 copies per milliliter for 100- and 200-μl volumes was 0.94. In addition, 36 of 67 (54%) of the results from the 200-μl test were lower than the results from the 100-μl test, and 31 of 67 (46%) were higher. For the vaginal samples, the mean log10 was 3.48 at the 200-μl volume and was 3.53 at the100-μl volume, and this small difference was not statistically significant (P = 0.2). The correlation between log10 copies per milliliter for 100- and 200-μl volumes was 0.92. Additionally, 56 of 109 (51%) of the results from the 200-μl test were lower than the results from the 100-μl test and 53 of 109 (49%) were higher. The 100- and 200-μl tests yielded similar numbers of samples that were below the limit of detection (three copies per reaction; 30 and 15 copies/ml for 100- and 200-μl sample input volumes, respectively). With this group of specimens, one sample was at or below the threshold of three copies per reaction when tested at 200 μl but above the threshold of three copies per reaction when tested at 100 μl.
Comparative analysis of cell-free and cell-associated RNA in genital swab specimens.
Cervical and vaginal swabs collected from 21 HIV-1-positive women were thawed and fractionated. Each fraction (the undiluted or untouched aliquot that was reserved prior to fractionation, the supernatant fraction, and the resuspended cell pellet fraction) was assayed independently, and the results are summarized in Table 3. We were able to quantify HIV-1 RNA in 11 of 21 (52%) cervical undiluted aliquots and 11 of 21 (52%) vaginal undiluted aliquots, and HIV-1 RNA above the level of quantitation in a vaginal sample was strongly predictive of HIV-1 RNA in a cervical sample from the same subject. HIV-1 RNA was detected in various amounts in both the cell-free and the cell pellet fractions. For the most part, a patient's swab sample was either consistently positive (subjects 1 to 3 and subjects 5 to 8 in Table 3) or consistently negative (subjects 15 to 21 in Table 3) in each of the three fractions, with the exception of samples with viral levels near the threshold for detection in this assay (subjects 9 to 14 in Table 3).
TABLE 3.
RNA levels from assays of fractionated clinical and cervical and vaginal swab samplesa
| Subject no. | RNA level (log10 copies/swab) in fraction
|
|||||
|---|---|---|---|---|---|---|
| Cervical
|
Vaginal
|
|||||
| Neat | Supernatant | Cell pellet | Neat | Supernatant | Cell pellet | |
| 1 | 4.10 | 4.24 | 3.65 | 3.72 | 3.99 | 3.66 |
| 2 | 4.05 | 3.98 | 3.32 | 3.93 | 3.96 | 3.39 |
| 3 | 3.92 | 3.91 | 3.10 | 3.97 | 3.94 | 3.40 |
| 4 | 3.85 | 3.86 | 3.15 | 3.27 | 2.86 | BL |
| 5 | 3.83 | 4.06 | 3.42 | 3.01 | 3.00 | 2.41 |
| 6 | 3.23 | 3.54 | 3.45 | 4.21 | 3.99 | 4.51 |
| 7 | 3.03 | 3.20 | 2.96 | 3.22 | 3.34 | 3.70 |
| 8 | 2.89 | 3.14 | 2.42 | 3.85 | 3.80 | 3.50 |
| 9 | 2.61 | 3.10 | 1.88 | 1.90 | 2.19 | BL |
| 10 | 2.31 | 2.30 | BL | 2.64 | 2.47 | 2.11 |
| 11 | 2.18 | 2.43 | 2.67 | BL | BL | BL |
| 12 | BLb | 2.43 | 1.60 | 2.04 | BL | BL |
| 13 | BL | 2.31 | BL | BL | BL | BL |
| 14 | BL | 2.06 | 1.60 | BL | BL | BL |
| 15 | BL | BL | BL | BL | BL | BL |
| 16 | BL | BL | BL | BL | BL | BL |
| 17 | BL | BL | BL | BL | BL | BL |
| 18 | BL | BL | BL | BL | BL | BL |
| 19 | BL | BL | BL | BL | BL | BL |
| 20 | BL | BL | BL | BL | BL | BL |
| 21 | BL | BL | BL | BL | BL | BL |
Aliquots (100 μl) of each fraction were assayed. Results are adjusted to reflect the dilution factor.
BL, below the limit of quantitation.
HIV-1 RNA detected in the supernatant fraction could be either from lysed virions or from cells that lysed prior to centrifugation and separation. To determine how much of the RNA detected in the supernatant fraction could be attributed to infected cell lysis during storage and processing, infected cells that had been washed of virus were added to swabs previously seeded with uninfected cells. Cell-free tissue culture supernatant was also spiked onto an additional set of swabs to test whether virus may be trapped in the cell pellet fraction. Samples were thawed and fractionated 1 week after being frozen. Each fraction was assayed independently, and the results are summarized in Table 4. We were able to detect more than 5.0 log10 copies of HIV-1 RNA in the supernatant fraction of swabs spiked with washed infected cells and then frozen, thawed, and fractionated, indicating that lysis of infected cells occurred during the freeze and thaw required to process the samples. For the mock samples that were spiked with cell-free virus, all copies detected were in the supernatant fraction, and none were detected in the cellular fraction.
TABLE 4.
RNA levels from four samples that were spiked and then fractionateda
| Spiked sample (n = 4) | Avg log10 RNA level in fraction (copies/swab)
|
||
|---|---|---|---|
| Neat | Supernatant | Cell pellet | |
| Infected cells | >5.00b | >5.00b | 4.68 |
| Cell-free virus | 3.45 | 3.42 | 0 |
Aliquots (200 μl) of each fraction were assayed independently.
Result above the linear range of the assay.
Comparative analysis of successive swabs collected from the same woman.
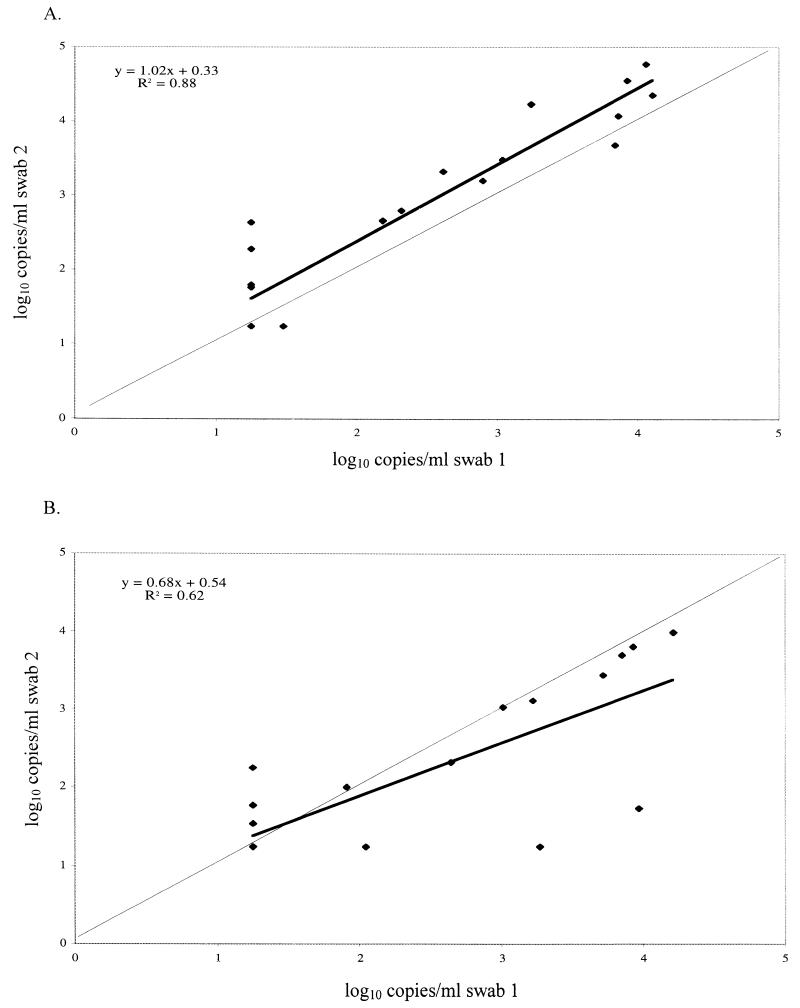
One issue with using swabs as a collection method for clinical studies is the potential variability in the quantity of sample obtained on each swab. To examine how RNA levels in one swab compare with RNA levels in a second swab from the same person by the same clinician, we compared the results from the two 100-μl tests performed on the duplicate cervical and vaginal specimens collected from the 21 HIV-1-positive women described above, and the results are summarized in Fig. 4. We found RNA levels at or above the limit of detection in 11 of 21 (52%) of the first cervical swabs and 15 of 21 (71%) of the second cervical swabs, with a correlation of 0.88. For the first and second vaginal swabs, RNA levels in 11 of 21 (52%) and 12 of 21 (57%) were above the limit of detection, with a correlation of 0.62. Although the numbers are too small to thoroughly evaluate statistically, this small pilot study suggests that testing of a single genital swab sample from an HIV-1-infected subject can provide a reliable measure of HIV-1 viral load.
FIG. 4.
Comparison of results of two successive swabs collected from the same woman on the same visit. The thick line represents the observed correlation between the results of the two swabs, and the thin line represents a perfect correlation. (A) Cervical swabs; (B) vaginal swabs.
DISCUSSION
The Gen-Probe HIV-1 viral load assay was developed to quantify the number of HIV-1 RNA copies in a plasma-based sample. This assay can accurately quantify different subtypes of HIV-1, making it useful for studies of HIV-1 in the countries most affected by the pandemic (4, 7). Here we show that this assay can also be adapted to quantify HIV-1 RNA in breast milk and genital secretions. Thus, the Gen-Probe HIV-1 viral load assay will be useful for studies that are designed to assess the role of mucosal virus in transmission, as well as studies that examine the correlates of HIV-1 genital or breast milk virus shedding.
The threshold for detection was defined for the various sample types using ROC curves. This value was more than three copies per reaction for swabs and breast milk supernatant samples. The sensitivity of detection for 10 copies of HIV-1 RNA was 92% for mock swab samples and 77% for breast milk samples, and the specificity was 98% for swab samples and 100% for breast milk supernatant samples when this threshold was applied as the lower limit of detection in the assay. Both the threshold for detection and the sensitivity to detect low copy numbers above this threshold were similar to that observed when examining plasma. For the Gen-Probe HIV-1 viral load assay, 25 copies per reaction is considered the limit for accurate quantitation of HIV-1 RNA in plasma, because the results of more than 95% of tests were above this threshold when 25 copies were tested. Here we show that even at 10 copies of HIV-1 RNA, the assay has a high sensitivity. Thus, when considering results from swab samples and breast milk, where the HIV-1 RNA copy is generally 10 to 100 times lower than that in plasma (Rousseau et al., unpublished), it may be appropriate to include results less than 25 copies to maximize the available data. Values in this range are certainly more variable than results with higher copy number, and at 10 copies, 20 to 22% of values will be considered negative when they may contain some HIV-1 RNA. However, we found that >50% of the observed values were within twofold of the expected result, and 73 to 83% were within threefold of the input copy number when 10 copies were tested repeatedly in this assay. Thus, results in this range still provide a reasonably reliable measure of HIV-1 RNA.
A combination of analyses of breast milk and genital swabs from HIV-1-infected women and experiments with mock samples spiked with known amounts of virus suggest that there could be a modest inhibition of the assay when maximal volumes (500 μl) are tested, particularly for swabs. This effect led to a less-than-twofold reduction in HIV-1 RNA copies per milliliter in these samples relative to spiked plasma samples, and additional experiments would be needed to determine if this inhibition is a consistent problem with 500-μl volumes. One concern is that swab samples contain various amounts of mucus, and this mucus may retard the migration of the magnetic beads through the magnetic field during the nucleic acid isolation step of the assay. It is possible that lipids in breast milk could have similar effects. In addition, high amounts of DNA from lysed cells could also compromise magnetic separation of the RNA. The results described here suggest that up to 200 to 250 μl of breast milk and swab samples can be tested in this assay without evidence of inhibition. For example, the assay results were linear when 5 to 250 μl of breast milk from infected women was tested. There was no difference between the results of tests using 100- versus 200-μl swab samples stored in freezing medium. Until more-exhaustive testing with 500-μl volumes is performed, our data suggest that sample volumes of ≤250 μl of breast milk or swab samples should be tested in this assay.
HIV-1 RNA could be recovered from swab samples spiked with known amounts of virus, as well as with swabs seeded with HIV-1-infected cells. We found that it was not possible to separately quantify HIV-1 RNA that was cell associated versus cell free on swabs, because of cell lysis during storage and/or processing. This limitation is not something specific to the HIV-1 RNA assay used here; rather, it is a limitation caused by freezing samples, and it would be expected to be a concern regardless of the method used to detect HIV-1 RNA.
Unlike plasma or breast milk samples, it is not possible to consistently assay a particular volume of genital secretions. Various strategies have been used to sample genital fluids, including diluting the sample in a lavage, collecting a saturating amount of fluid on a Sno-strip (Akorn, Inc., Buffalo Grove, Ill.), or sampling a particular surface area on a swab. In the third case, the goal is to consistently sample from each woman a representative portion of the total area to which a partner is exposed. This method permits separate examination of the vaginal and cervical compartments. However, there are few data to address the accuracy of such sampling techniques and, specifically, whether there is significant variation in the HIV-RNA levels from swab to swab, even at a single time point. Here we show that the HIV-1 RNA levels from duplicate swabs taken from the same woman on the same day correlate well; similarly, duplicate swabs from women with HIV-1 RNA copies below the limit of quantification gave consistent results. This suggests that HIV-1 RNA levels from a swab can be used to assess cervical and vaginal viral load.
Rapid and sensitive methods for detecting HIV-1 RNA from a broad range of specimen types will be an important tool for conducting larger clinical studies that examine levels of HIV-1 RNA in bodily fluids other than plasma. Our data suggest that the Gen-Probe HIV-1 viral load assay will provide a useful tool for analyses of viral load in mucosal secretions.
Acknowledgments
We thank Chia Wang, Grace John, Ruth Nduati, Dorothy Mbori-Ngacha, and Ludo Lavreys for providing samples from HIV-1-infected women.
This work was supported by NIH grant AI 38518. J.O. is supported by an Elizabeth Glaser Pediatric AIDS Foundation Scientist award.
REFERENCES
- 1.Bremer, J., M. Nowicki, S. Beckner, D. Brambilla, M. Cronin, S. Herman, A. Kovacs, P. Reichelderfer, et al. 2000. Comparison of two amplification technologies for detection and quantitation of human immunodeficiency virus type 1 RNA in the female genital tract. J. Clin. Microbiol. 38:2665-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chackerian, B., E. M. Long, P. A. Luciw, and J. Overbaugh. 1997. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J. Virol. 71:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuachoowong, R., N. Shaffer, W. Siriwasin, P. Chaisilwattana, N. L. Young, P. A. Mock, S. Chearskul, N. Waranawat, T. Chaowanachan, J. Karon, R. J. Simonds, T. D. Mastro, et al. 2000. Short-course antenatal zidovudine reduces both cervicovaginal human immunodeficiency virus type 1 RNA levels and risk of perinatal transmission. J. Infect. Dis. 181:99-106. [DOI] [PubMed] [Google Scholar]
- 4.Emery, S., S. Bodrug, B. A. Richardson, C. Giachetti, M. A. Bott, D. Panteleeff, L. L. Jagodzinski, N. L. Michael, R. Nduati, J. Bwayo, J. K. Kreiss, and J. Overbaugh. 2000. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J. Clin. Microbiol. 38:2688-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia, P. M., L. A. Kalish, J. Pitt, H. Minkoff, T. C. Quinn, S. K. Burchett, J. Kornegay, B. Jackson, J. Moye, C. Hanson, C. Zorrilla, J. F. Lew, et al. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N. Engl. J. Med. 341:394-402. [DOI] [PubMed] [Google Scholar]
- 6.Iversen, A. K., A. R. Larsen, T. Jensen, L. Fugger, U. Balslev, S. Wahl, J. Gerstoft, J. I. Mullins, and P. Skinhoj. 1998. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J. Infect. Dis. 177:1214-1220. [DOI] [PubMed] [Google Scholar]
- 7.Jagodzinski, L. L., D. L. Wiggins, J. L. McManis, S. Emery, J. Overbaugh, M. Robb, S. Bodrug, and N. L. Michael. 2000. Use of calibrated viral load standards for group M subtypes of human immunodeficiency virus type 1 to assess the performance of viral RNA quantitation tests. J. Clin. Microbiol. 38:1247-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John, G. C., R. W. Nduati, D. A. Mbori-Ngacha, B. A. Richardson, D. Panteleeff, A. Mwatha, J. Overbaugh, J. Bwayo, J. O. Ndinya-Achola, and J. K. Kreiss. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J. Infect. Dis. 183:206-212. [DOI] [PubMed] [Google Scholar]
- 9.Katzenstein, D. A., S. M. Hammer, M. D. Hughes, H. Gundacker, J. B. Jackson, S. Fiscus, S. Rasheed, T. Elbeik, R. Reichman, R. Japour, T. C. Merigan, and M. S. Hirsch. 1996. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. N. Engl. J. Med. 335:1091-1098. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn, L., E. J. Abrams, P. B. Matheson, P. A. Thomas, G. Lambert, M. Bamji, B. Greenberg, R. W. Steketee, D. M. Thea, et al. 1997. Timing of maternal-infant HIV transmission: associations between intrapartum factors and early polymerase chain reaction results. AIDS 11:429-435. [DOI] [PubMed] [Google Scholar]
- 11.Loussert-Ajaka, I., L. Mandelbrot, M. C. Delmas, H. Bastian, J. L. Benifla, I. Farfara, I. de Vincenzi, S. Matheron, F. Simon, and F. Brun-Vezinet. 1997. HIV-1 detection in cervicovaginal secretions during pregnancy. AIDS 11:1575-1581. [DOI] [PubMed] [Google Scholar]
- 12.Mellors, J. W., L. A. Kingsley, C. R. Rinaldo, Jr., J. A. Todd, B. S. Hoo, R. P. Kokka, and P. Gupta. 1995. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann. Intern. Med. 122:573-579. [DOI] [PubMed] [Google Scholar]
- 13.Mofenson, L. M., J. S. Lambert, E. R. Stiehm, J. Bethel, W. A. Meyer III, J. Whitehouse, J. Moye, Jr., P. Reichelderfer, D. R. Harris, M. G. Fowler, B. J. Mathieson, G. J. Nemo, et al. 1999. Risk factors for perinatal transmission of human immunodeficiency virus type1 in women treated with zidovudine. N. Engl. J. Med. 341:385-393. [DOI] [PubMed] [Google Scholar]
- 14.Mostad, S. B., and J. K. Kreiss. 1996. Shedding of HIV-1 in the genital tract. AIDS 10:1305-1315. [DOI] [PubMed] [Google Scholar]
- 15.Nduati, R., G. John, D. Mbori-Ngacha, B. Richardson, J. Overbaugh, A. Mwatha, J. Ndinya-Achola, J. Bwayo, F. E. Onyango, J. Hughes, and J. Kreiss. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167-1174. [DOI] [PubMed] [Google Scholar]
- 16.Neilson, J., G. John, J. K. Carr, P. Lewis, J. K. Kreiss, S. Jackson, R. W. Nduati, D. Mbori-Ngacha, D. Panteleeff, S. Bodrug, C. Giachetti, M. Bott, B. A. Richardson, J. Bwayo, J. Ndinya-Achola, and J. Overbaugh. 1999. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J. Virol. 73:4393-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien, T. R., W. A. Blattner, D. Waters, E. Eyster, M. W. Hilgartner, A. R. Cohen, N. Luban, A. Hatzakis, L. M. Aledort, P. S. Rosenberg, W. J. Miley, B. L. Kroner, and J. J. Goedert. 1996. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA 276:105-110. [PubMed] [Google Scholar]
- 18.O'Brien, W. A., P. M. Hartigan, D. Martin, J. Esinhart, A. Hill, S. Benoit, M. Rubin, M. S. Simberkoff, J. D. Hamilton, and the Veterans Affairs Cooperative Study Group on AIDS. 1996. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N. Engl. J. Med. 334:426-431. [DOI] [PubMed] [Google Scholar]
- 19.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer, N., A. Roongpisuthipong, W. Siriwasin, T. Chotpitayasunondh, S. Chearskul, N. L. Young, B. Parekh, P. A. Mock, C. Bhadrakom, P. Chinayon, M. L. Kalish, S. K. Phillips, T. C. Granade, S. Subbarao, B. G. Weniger, T. D. Mastro, et al. 1999. Maternal virus load and perinatal human immunodeficiency virus type 1 subtype E transmission, Thailand. J. Infect. Dis. 179:590-599. [DOI] [PubMed] [Google Scholar]
- 21.Subbarao, S., and G. Schochetman. 1996. Genetic variability of HIV-1. AIDS 10(Suppl. A):S13-S23. [DOI] [PubMed] [Google Scholar]
- 22.Uvin, S. C., and A. M. Caliendo. 1997. Cervicovaginal human immunodeficiency virus secretion and plasma viral load in human immunodeficiency virus-seropositive women. Obstet. Gynecol. 90:739-743. [DOI] [PubMed] [Google Scholar]
- 23.Wang, C. C., R. S. McClelland, M. Reilly, J. Overbaugh, S. R. Emery, K. Mandaliya, B. Chohan, J. Ndinya-Achola, J. Bwayo, and J. K. Kreiss. 2001. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J. Infect. Dis. 183:1017-1022. [DOI] [PubMed] [Google Scholar]