Abstract
It has recently been reported that all but one of the 102 known serotypes of the genus Rhinovirus segregate into two genetic clusters (C. Savolainen, S. Blomqvist, M. N. Mulders, and T. Hovi, J. Gen. Virol. 83:333-340, 2002). The only exception is human rhinovirus 87 (HRV87). Here we demonstrate that HRV87 is genetically and antigenically highly similar to enterovirus 68 (EV68) and is related to EV70, the other member of human enterovirus group D. The partial nucleotide sequences of the 5′ untranslated region, capsid regions VP4/VP2 and VP1, and the 3D RNA polymerase gene of the HRV87 prototype strain F02-3607 Corn showed 97.3, 97.8, 95.2, and 95.9% identity to the corresponding regions of EV68 prototype strain Fermon. The amino acid identities were 100 and 98.1% for the products of the two capsid regions and 97.9% for 3D RNA polymerase. Antigenic cross-reaction between HRV87 and EV68 was indicated by microneutralization with monotypic antisera. Phylogenetic analysis showed definite clustering of HRV87 and EV68 with EV70 for all sequences examined. Both HRV87 and EV68 were shown to be acid sensitive by two different assays, while EV70 was acid resistant, which is typical of enteroviruses. The cytopathic effect induced by HRV87 or EV68 was inhibited by monoclonal antibodies to the decay-accelerating factor known to be the receptor of EV70. We conclude that HRV87 and EV68 are strains of the same picornavirus serotype presenting features of both rhinoviruses and enteroviruses.
The family Picornaviridae contains two large and important genera of common human pathogens, Enterovirus and Rhinovirus. In structural and genetic properties, these two genera are very much alike. However, rhinoviruses, which multiply mainly in the nasal epithelium, differ from enteroviruses, infectious agents of the alimentary tract, by their sensitivity to acid and by their growth at a lower optimal temperature. The genus Enterovirus contains 64 serotypes pathogenic to humans, which have been distinguished by the neutralizing antibodies against them (17). There may still be uncharacterized serotypes, as some clinical enterovirus isolates are not typeable by existing antisera and show genetic segregation indicative of an independent serotype (22). Nucleotide analysis of the RNA genomes of different human enterovirus (HEV) serotypes has provided new insight into the classification of enteroviruses (23), resulting in the division of these viruses into four main genetic clusters, designated HEV species A to D. Poliovirus serotypes 1 to 3 are genetically related to HEV-C but are classified as a species of their own (15). The genus Rhinovirus contains 102 serotypes, which are numbered from 1 to 100 (8, 11, 12). Serotype 1 contains two subtypes, 1A and 1B. More recently, a strain referred to as the Hanks strain has been proposed to represent a new serotype (2). We have generated partial capsid sequences of all human rhinovirus (HRV) prototypes, and with the exception of HRV87, all prototypes segregated into two previously established genetic clusters, HRV-A and HRV-B. HRV87 was found to cluster together with a representative of HEV-D, enterovirus 70 (EV70) (26). Through further analysis, we found that HRV87 showed striking nucleotide identity with the partial sequence (obtained from GenBank) of the other member of HEV-D, EV68. This prompted further investigations on the relationship of HRV87 to the viruses of the HEV-D cluster. In this study, we examined (i) the nucleotide sequences of the 5′ untranslated regions (UTRs), two separate capsid regions, and the 3D RNA polymerase genes of HRV87 and two lines of EV68; (ii) the antigenic characteristics of both HRV87 and EV68; (iii) their acid sensitivities; and (iv) their receptor usage in HeLa cells.
MATERIALS AND METHODS
Cell lines, viruses, and antisera.
Prototype viruses HRV87 F02-3607 Corn, EV68 Fermon (lines VR-561 and VR-1076), and EV70 J670/71 were obtained from the American Type Culture Collection (ATCC; Manassas, Va.). The HRV87 prototype was also kindly provided by Janssen Pharmaceuticals, Beerse, Belgium. Rhinovirus prototypes HRV1B and HRV14 were obtained from the National Institute for Public Health and the Environment, Bilthoven, The Netherlands. HRV1B, HRV14, and HRV87 were passaged twice in the Ohio strain of HeLa cells, kindly provided by Eurico Arruda (University of Virginia, Charlottesville), before being used in subsequent experiments. EV68 line VR-561 was passaged first in the human rhabdomyosarcoma cell line (RD), which was provided by Mark A. Pallansch (Centers for Disease Control and Prevention, Atlanta, Ga.), and then once in HeLa Ohio cells. EV68 line VR-1076 was propagated twice in RD cells, and EV70 was propagated once in HeLa Ohio cells before being used as described below. Antisera to HRV87 (VR-1197AS/GP) and EV68 (VR-1076AS/HO) were purchased from ATCC.
RT-PCR and sequencing.
One hundred microliters of infected cell culture was freeze-thawed three times, clarified by centrifugation at 235 × g for 10 min, and used in RNA extraction with an RNeasy total RNA kit (Qiagen GmbH, Hilden, Germany). RNA was eluted in 30 μl of RNase-free water and stored at −70°C. For reverse transcription (RT)-PCR of the 5′ UTR, two different primer sets were used: ncr1 (1) with HRV primer 1 (3) and HRV primer 2 (3) with 9565 (25). RT-PCR of the VP4/VP2 region was carried out as described previously (25), and RT-PCR of the 5′ end of the VP1 region was carried out with the primers 187, 188, 189, and 222 (21). Part of the 3D polymerase gene was amplified with the primers 3D+ and 3D− (24). The PCR products were visualized under UV light after electrophoresis on a 2% agarose gel, purified with a QIAquick gel extraction kit (Qiagen), and used in cycle sequencing (ABI Prism BigDye terminator cycle sequencing ready reaction kit; Applied Biosystems, Espoo, Finland) in an ABI 310 automated sequencer (Applied Biosystems). Sequences were analyzed with the Sequencing Analysis, version 3.1, and Sequence Navigator, version 1, programs (Applied Biosystems). Multiple sequence alignments were done with ClustalX (version 1.64b), and distance matrices were calculated using DNAdist or PROTdist (PHYLIP, version 3.572c). The neighbor-joining option and maximum-likelihood model of nucleotide substitution in the Neighbor program (PHYLIP) were used in the drawing of the dendrograms, and NJplot was used in the visualization of the phylogenetic trees.
Antigenic characterization of the viruses.
Serotyping with monotypic neutralizing sera was carried out by use of a standard method (4). Briefly, antisera at dilutions of 1:200 and 1:400 and several dilutions of viruses were mixed. After 1 h of incubation at +37°C, the virus-antiserum mixture was inoculated into HeLa Ohio [for HRV1B, HRV14, HRV87, EV68 (VR-561), and EV70] or RD (for EV68 VR-1076) cells in microwell plates and incubated at +33°C. The results were assessed by microscopy at day 5. Neutralization was seen as the absence of cytopathic effect (CPE) in wells with the virus-antiserum mixture and the presence of CPE in wells with virus. Neutralization indices were determined for two specific antisera, a pool of human sera, and two individual sera from adult volunteers. At first, HRV87, EV68 (VR-561), and EV68 (VR-1076) were at a dilution of 1:10. Antisera to HRV87 and EV68 and the human serum pool were at dilutions of 1:20, and the two individual sera were at dilutions of 1:10. Equal volumes of virus and serum dilutions were mixed and incubated for 1 h at +37°C for neutralization, and the titers of remaining infectious viruses were determined in microwell cultures of RD cells at +33°C. The neutralization indices of the sera were calculated as the logarithm of the ratio of the titer of the virus without sera to that of the virus with the test sera.
Prevalence of specific antibodies.
The prevalence of neutralizing antibodies to HRV87 and EV68 (VR-1076) in the pool of human sera and in the sera of seven adult volunteers was assayed in RD cells by use of a standard microneutralization method (4). The twofold dilution series of the sera was used. The results were read at day 6, after the cells were stained with crystal violet. The last dilution completely inhibiting the viral CPE was regarded as the end point titer of the serum.
Acid lability.
The acid sensitivities of the prototype strains were tested by a standard protocol (4). Briefly, an equal volume of buffer A, 0.1 M citric acid buffer, pH 4, or buffer B, 0.1 M phosphate buffer, pH 7, was added to a virus-infected cell culture supernatant. The mixtures were incubated for 1 h at +37°C and then neutralized by 0.5 M phosphate buffer, pH 7.2. The infectivities of the acid-treated and untreated viruses were assayed by titration in HeLa Ohio or RD cells at +33°C. The virus was considered acid sensitive if the titer of the acid-treated aliquot was at least 100 times lower than that of the neutral one. The acid sensitivities were also assessed according to the method of Schieble et al. (27). The 10-fold dilution series of the viruses was prepared in Eagle's minimum essential medium (MEM) without bicarbonate (final pH 3.5) and in MEM adjusted to pH 7.3. The diluted viruses were incubated for 3 h at room temperature and neutralized with 0.1 M Tris buffer, and the infectivities were assayed as described above.
Cell protection assay.
We assayed the cell protective ability of an antibody to decay-accelerating factor (DAF) SCR-3 in HRV87 and EV68 infections. The SCR-3 epitope of DAF is involved in the receptor activity for EV70 in HeLa cells (13). First, confluent monolayers of HeLa Ohio cells in 96-well plates were preincubated with 30 μl of monoclonal antibody to DAF SCR-3 (IH-4; kindly provided by D. Lublin) in basal MEM with 2% fetal calf serum. After 2 h of incubation at +33°C, the cells were challenged with 10 to 100 50% tissue culture infective doses of HRV87 or EV68. Virus adsorption onto the cells was facilitated by centrifugation at 700 × g for 2 h at +33°C. The appearance of CPE was monitored at regular intervals by light microscopy. Finally, when CPE was evident in cultures of infected controls, all wells were stained with crystal violet.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the HRV87 and EV68 sequences derived from this work are AY062273 to AY062283.
RESULTS
Sequencing and phylogenetic analysis.
Four genomic regions of HRV87 prototype strain F02-3607 Corn and two sublines of EV68 prototype Fermon (VR-561 and VR-1076) were analyzed by RT-PCR and amplicon sequencing. For comparison, the sequence of EV70 was obtained from GenBank (accession no. NC_001430). The lengths of the reliable nucleotide sequences, which were used in subsequent phylogenetic analysis, were 598 nucleotides (nt) for the 5′ UTR beginning from nt 106 (according to the numbering of EV70); 408 nt for the beginning of the polyprotein-coding region, including 207 nt for the VP4 region and 201 nt for the VP2 region; 324 nt for VP1 region (nt 2512 to 2835); and 435 nt for the 3D RNA polymerase gene (nt 6682 to 7068). The nucleotide identities of the two sublines of EV68 Fermon ranged from 99.7 to 100% for the four genomic segments analyzed. The VP4/VP2 region of EV68 (VR-1076) was 100% identical to the previously published 211-nt segment of EV68 (GenBank accession no. E68RNAPA) (24). The VP1 region of EV68 (VR-1076) had 99.7% identity to the previously published sequence with GenBank accession no. AF081348 (20). The four segments of EV68 were also 99.7 to 99.8% identical to the complete genome sequence of EV68 (M. S. Oberste [Centers for Disease Control and Prevention], personal communication).
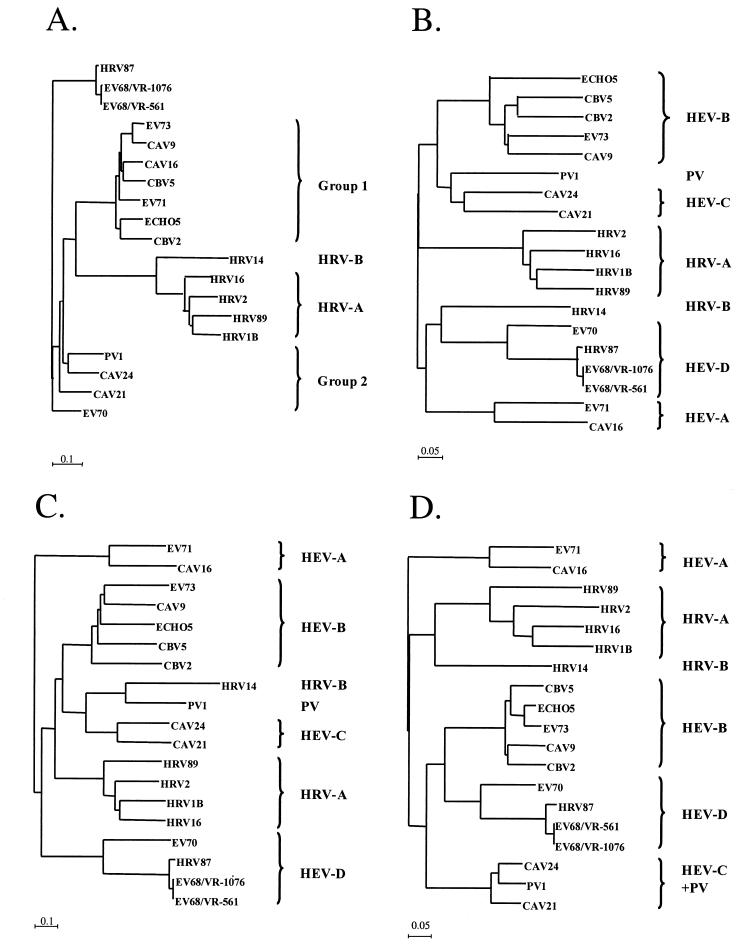
The nucleotide sequence identities between HRV87 and EV68 were 97% for the 5′ UTR, 97.8% for the VP4/VP2 region, 95.2% for the VP1 region, and 95.9% for the 3D RNA polymerase gene. The identities at the deduced amino acid level were 100% for VP4/VP2, 98.1% for VP1, and 97.9% for 3D RNA polymerase. The amino acid alignments of VP1 and 3D RNA polymerase are shown in Fig. 1. The similarities between HRV87 and EV70 were 74.3% for the 5′ UTR, 71.6% for the VP4/VP2 region, 41.8% for the VP1 region, and 69.6% for the 3D RNA polymerase gene at the nucleotide level and 91.4% for VP4/VP2, 54.4% for VP1, and 85.6% for 3D RNA polymerase at the amino acid level. The nucleotide sequence of the 5′ UTR of HRV87 was 100% identical to the sequence found in GenBank under accession no. AF108187. The data presented above are derived from an HRV87 strain obtained from ATCC. We initially sequenced the VP4/VP2 region of a strain from another source; that sequence was identical to the sequence of the strain discussed here (data not shown). Representative sequences of all known HEV and HRV genetic groups were chosen from GenBank and used for phylogenetic analysis of the four genomic fragments (Fig. 2A to D). HRV87 definitely clustered into the HEV-D group with EV68 and EV70 with regard to the two capsid regions and the 3D region, whereas for the 5′ UTR, HRV87 and the two sublines of EV68 showed a cluster of their own.
FIG. 1.
ClustalX (version 1.64b) multiple sequence alignment of a 108-amino-acid region of VP1 (A) and a 145-amino-acid region of 3D RNA polymerase (B) from prototype strains of HRV87, EV68 (lines VR-561 and VR-1076), and EV70. Amino acids identical in all four sequences are marked by asterisks, and amino acid differences between HRV87 and EV68 are indicated by shading.
FIG. 2.
Neighbor-joining tree showing phylogenetic clustering of representative serotypes of HRVs and enteroviruses. (A) 5′ UTR; (B) VP4/VP2 region; (C) VP1 region; (D) 3D RNA polymerase region. The accession numbers of the previously published sequences are as follows: NC_001435, HRV1B; X02316, HRV2; NC_001490, HRV14; NC_001752, HRV16; A10937, HRV89; NC_002347, coxsackie A virus 9 (CAV9); NC_001612, CAV16; NC_001428, CAV21; D90457, CAV24; NC_00081, coxsackie B virus 2 (CBV2); NC_001342, CBV5; NC_002601, echovirus 5 (ECHO5); NC_001430, EV70; NC_001769, EV71; NC_002684, EV73; and V01150, poliovirus 1/Sabin (PV1).
Biological comparisons between proposed HEV-D strains.
Typing of the viruses by specific antisera was carried out by use of a standard method. HRV87 was neutralized by validated antisera raised against HRV87 and EV68, while EV68 was neutralized only by the antiserum to EV68. No effect on HRV1B-, HRV14-, or EV70-induced CPE was seen with any of the antisera. The observed one-way cross-reactivity was confirmed by determination of the neutralization indices for the antisera. The antiserum to HRV87 reduced the titers of the two EV68 strains only marginally, while the EV68 antiserum very effectively neutralized all three tested viruses. Interestingly, human sera used in the assay revealed antigenic differences between the two EV68 strains as well (Table 1).
TABLE 1.
Neutralizing indices of selected sera to indicated viruses tested in RD cells
| Serum | Neutralizing index of sera to:
|
||
|---|---|---|---|
| HRV87 | EV68 (VR-561) | EV68 (VR-1076) | |
| HRV87 antiserum | 3.0 | 2.0 | 0.75 |
| EV68 antiserum | 3.0 | 3.75 | 4.5 |
| Human serum pool | 2.75 | 2.75 | 1.0 |
| Serum 1 | 1.5 | 1.0 | 0 |
| Serum 2 | 3.0 | 3.25 | 1.0 |
Infections caused by HRV87 and/or EV68 appeared to be relatively common, as an unselected pool of human sera had a relatively high titer of neutralizing antibodies. Furthermore, the seven individual sera tested contained neutralizing antibodies to HRV87 and the end point titers were very similar to those of antibodies against EV68 (Table 2).
TABLE 2.
Microneutralization assay for HRV87 and EV68 (VR-1076)
| Serum | End point titer of neutralizing antibodies to:
|
|
|---|---|---|
| HRV87 | EV68 (VR-1076) | |
| Virus antisera | ||
| HRV87 antiserum | 128 | 64 |
| EV68 antiserum | 512 | 4,096 |
| Human sera | ||
| Serum pool | 128 | 128 |
| Serum 1 | 8 | 16 |
| Serum 2 | 64 | 64 |
| Serum 3 | >512 | >512 |
| Serum 4 | 4 | <4 |
| Serum 5 | 32 | 8 |
| Serum 6 | 256 | 256 |
| Serum 7 | 64 | 32 |
HRV87 and the two sublines of EV68 Fermon were sensitive to a 1-h acid treatment at +37°C. The acid-treated and untreated aliquots of the viruses were assayed in both HeLa Ohio and RD cells. After acid treatment, the titers of the 50% cell culture infectious doses of HRV87, EV68 (VR-561), and EV68 (VR-1076) were 102.5, 102, and 104 times lower in HeLa Ohio and 104.5, 103.5, and 104 times lower in RD cells, respectively. The acid sensitivity test was repeated for selected viruses by the protocol described by Schieble et al. (27). After a 3-h acid treatment at room temperature, the titers of HRV 87 and EV68 (VR-1076) were 102.5 and 102 times lower, respectively, in HeLa Ohio cells and 102 and 102.5 times lower, respectively, in RD cells. EV70 was even resistant to a 4-h acid treatment.
We studied whether the DAF-specific monoclonal antibody is capable of blocking infection caused by HRV87. The HRV87-induced CPE was clearly inhibited in HeLa Ohio cells pretreated with monoclonal antibody to the SCR-3 epitope of DAF. Practically similar results were obtained with EV68 and EV70 (data not shown).
DISCUSSION
In this work, we have demonstrated the close genetic similarity in four genomic regions between HRV87 and EV68, which is classified in the HEV-D species together with EV70 (15). It has been shown recently that the nucleotide sequence encoding VP1 correlates well with enterovirus serotype (20). Our VP1 gene sequences did not cover the entire VP1 region, but compared with the intraserotypic divergence limits of 25% for the nucleotide sequence and 12% for the amino acid sequence, the observed identities of 95.2 and 98.1%, respectively, are clearly indicative of the same serotype. EV70 is of a different serotype but is a member of the same genetic cluster. Antigenic characterization of the strains confirmed this conclusion. The one-way antigenic cross-reactivity between HRV87 and EV68 is typical of the prototype strain-prime strain relationship previously described for both enteroviruses (17) and rhinoviruses (5). Analysis of individual human sera suggested antigenic differences between strains now proposed to belong to a single serotype. However, this kind of variability of response is not new and has been described, for instance, for poliovirus type 3 (10).
The clustering of HRV87 and EV68 with EV70 was evident for the coding regions as well for the 5′ UTR. The apparent divergence between the first two viruses and EV70 in the 5′ UTR was definitely greater than the corresponding interserotypic differences in the HEV-B species. The divergence of HRV87 from other HRVs with regard to the 5′ UTR has been described previously (1).
The initial characterization of the EV68 Fermon strain showed that it was resistant to low pH, so it was classified as an enterovirus (27). However, according to the data presented here, the titer of the acid-treated virus aliquot was somewhat lower than that of the untreated virus. We performed the acid sensitivity test by use of a standard method (4) and also repeated the test by the protocol used by Schieble et al. (27). The acid sensitivities of HRV87 and EV68 were obvious in both tests, but the differences between the titers of the acid-treated and untreated viruses were greater when the current standard method was used.
Originally, EV68 strain Fermon was recovered from a throat swab of a 10-month-old female with pneumonia. Despite a careful search for rhinovirus and enterovirus isolation data, no references were found in the literature to the isolation of either EV68 or HRV87 from clinical specimens after their first appearance and characterization. Serotyping of rhinovirus isolates is laborious and has been carried out in only a few studies (6, 14, 16, 18). However, the typing of enteroviruses has been widespread and has resulted in lengthy reports of clinical enterovirus findings. Even so, no isolation of EV68 has previously been reported (7, 19, 28). In this study, neutralizing antibodies to these virus strains were found in all tested sera. Although the number of sera was small, these results indicate that infections caused by these viruses are not uncommon. The absence of clinical isolates of HRV87 or EV68 in the literature may be due to the acid-sensitive nature of these viruses and the infrequent serotyping of acid-sensitive viruses in routine virus diagnostics.
Approximately 90% of rhinovirus serotypes bind to intercellular adhesion molecule 1, while all but one of the remaining serotypes use the low-density lipoprotein receptor (9, 29). The attachment of HRV87 to the cell surface is known to require sialic acid (29), which has also been reported for EV70 (30). Our present results suggest that the receptor might be the DAF and might thus be shared by EV70 and several echovirus serotypes (13).
In conclusion, we have shown in this study that HRV87 and EV68 are genetically and antigenically highly similar and that they also share homology with EV70. Therefore, we propose that this virus pair, HRV87 and EV68, should be reclassified as a single HEV-D serotype. Our results also demonstrate a conflict between the classical and modern phylogenetic classification criteria. HRV87/EV68 and EV70 definitely belong to the same genetic cluster and appear to share receptor specificity in HeLa cells. However, according to acid sensitivity assays, HRV87 and EV68 should be classified as rhinoviruses whereas EV70 is a typical enterovirus. The dividing line between these two genera is even more blurred than previously thought.
Acknowledgments
This work was partly supported by Wyeth-Lederle Vaccines and by grants from the Academy of Finland and the Päivikki and Sakari Sohlberg Foundation, Helsinki, Finland.
We thank Douglas Lublin (Washington University School of Medicine, St. Louis, Mo.) for the DAF SCR-3-specific murine monoclonal antibody 1H4 used in the study. The kind advice of Mirja Stenvik and expert technical assistance by Kristiina Aitkoski, Mervi Eskelinen, and Päivi Hirttiö are gratefully acknowledged.
REFERENCES
- 1.Andeweg, A. C., T. M. Bestebroer, M. Huybreghs, T. G. Kimman, and J. C. de Jong. 1999. Improved detection of rhinoviruses in clinical samples by using a newly developed nested reverse transcription-PCR assay. J. Clin. Microbiol. 37:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andries, K., B. Dewint, J. Snoekes, H. Wouters, H. Moereels, P. J. Lewi, and P. A. J. Janssen. 1990. Two groups of rhinoviruses revealed by a panel of antiviral compounds present sequence divergence and differential pathogenicity. J. Virol. 64:1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomqvist, S., A. Skyttä, M. Roivainen, and T. Hovi. 1999. Rapid detection of human rhinoviruses in nasopharyngeal aspirates by a microwell reverse transcription-PCR-hybridization assay. J. Clin. Microbiol. 37:2813-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couch, R. B. 1992. Rhinoviruses, p. 709-729. In E. H. Lennette (ed.), Laboratory diagnosis of viral infections. Marcel Dekker, Inc., New York, N.Y.
- 5.Couch, R. B. 1996. Rhinoviruses, p. 713-734. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Channock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Strauss (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 6.Fox, J. P., M. K. Cooney, C. E. Hall, and H. M. Foy. 1985. Rhinoviruses in Seattle families, 1975-1979. Am. J. Epidemiol. 101:122-143. [DOI] [PubMed] [Google Scholar]
- 7.Grist, N. R., E. J. Bell, and F. Assaad. 1978. Enteroviruses in human disease. Prog. Med. Virol. 24:114-157. [PubMed] [Google Scholar]
- 8.Hamparian, V. V., R. J. Colonno, M. K. Cooney, E. C. Dick, J. M. Gwaltney, Jr., J. H. Hughes, W. S. Jordan, Jr., A. Z. Kapikian, W. J. Mogabgab, A. Monto, C. A. Phillips, R. R. Rueckert, J. H. Schieble, E. J. Stott, and D. A. J. Tyrrell. 1987. A collaborative report: rhinoviruses—extension of the numbering system from 89 to 100. Virology 159:191-192. [DOI] [PubMed] [Google Scholar]
- 9.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blaas. 1994. Members of the low-density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hovi, T., K. Cantell, A. Huovilainen, E. Kinnunen, T. Kuronen, K. Lapinleimu, T. Pöyry, M. Roivainen, N. Salama, M. Stenvik, A. Silander, C.-J. Thoden, S. Salminen, and P. Weckström. 1986. Outbreak of paralytic poliomyelitis in Finland: widespread circulation of antigenically altered poliovirus type 3 in a vaccinated population. Lancet ii:1427-1432. [DOI] [PubMed] [Google Scholar]
- 11.Kapikian, A. Z., R. M. Conant, V. V. Hamparian, R. M. Chanock, P. J. Chapple, E. C. Dick, J. D. Fenters, J. M. Gwaltney, Jr., D. Hamre, J. C. Holper, W. S. Jordan, Jr., E. H. Lennette, J. L. Melnick, W. J. Mogabgab, M. A. Mufson, C. A. Phillips, J. H. Schieble, and D. A. J. Tyrrell. 1967. Rhinoviruses: a numbering system. Nature (London) 213:761-763. [Google Scholar]
- 12.Kapikian, A. Z., R. M. Conant, V. V. Hamparian, R. M. Chanock, E. C. Dick, J. M. Gwaltney, Jr., D. Hamre, W. S. Jordan, Jr., G. E. Kenny, E. H. Lennette, J. L. Melnick, W. J. Mogabgab, C. A. Phillips, J. H. Schieble, E. J. Stott, and D. A. J. Tyrrell. 1971. A collaborative report: rhinoviruses—extension of the numbering system. Virology 43:524-526.5543842 [Google Scholar]
- 13.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 70:5143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellner, G., T. Popow-Kraupp, C. Binder, I. Goedl, M. Kundi, and C. Kunz. 1991. Respiratory tract infections due to different rhinovirus serotypes and the influence of maternal antibodies on the clinical expression of the disease in infants. J. Med. Virol. 35:267-272. [DOI] [PubMed] [Google Scholar]
- 15.King, A. M. Q., F. Brown, P. Christian, T. Hovi, T. Hyypiä, N. J. Knowles, S. M. Lemon, P. D. Minor, A. C. Palmenberg, T. Skern, and G. Stanway. 2000. Family Picornaviridae, p. 657-683. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, D. J. McGeoch, J. Maniloff, M. A. Mayo, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 16.Krilov, L., L. Pierik, E. Keller, K. Maham, D. Watson, M. Hirsch, V. Hamparian, and K. McIntosh. 1986. The association of rhinoviruses with lower respiratory tract disease in hospitalized patients. J. Med. Virol. 19:345-352. [DOI] [PubMed] [Google Scholar]
- 17.Melnick, J. L. 1996. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 655-712. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Channock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Strauss (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 18.Monto, A. S., E. R. Bryan, and S. Ohmit. 1987. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J. Infect. Dis. 156:43-49. [DOI] [PubMed] [Google Scholar]
- 19.Nairn, C., and G. B. Clements. 1999. A study of enterovirus isolations in Glasgow from 1977 to 1997. J. Med. Virol. 58:304-312. [PubMed] [Google Scholar]
- 20.Oberste, M. S., K. Maher, D. R. Kilpatrick, and M. A. Pallansch. 1999. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73:1941-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberste, M. S., K. Maher, M. R. Flemister, G. Marchetti, D. R. Kilpatrick, and M. A. Pallansch. 2000. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J. Clin. Microbiol. 38:1170-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberste, M. S., D. Schnurr, K. Maher, S. al-Busaidy, and M. A. Pallansch. 2001. Molecular identification of new picornaviruses and characterization of a proposed enterovirus 73 serotype. J. Gen. Virol. 82:409-416. [DOI] [PubMed] [Google Scholar]
- 23.Pöyry, T., L. Kinnunen, T. Hyypiä, B. Brown, C. Horsnell, T. Hovi, and G. Stanway. 1996. Genetic and phylogenetic clustering of enteroviruses. J. Gen. Virol. 77:1699-1717. [DOI] [PubMed] [Google Scholar]
- 24.Pulli, T., P. Koskimies, and T. Hyypiä. 1995. Molecular comparison of coxsackie A virus serotypes. Virology 212:30-38. [DOI] [PubMed] [Google Scholar]
- 25.Savolainen, C., M. N. Mulders, and T. Hovi. 2002. Phylogenetic analysis of rhinovirus isolates collected during successive epidemic seasons. Virus Res. 85:41-46. [DOI] [PubMed] [Google Scholar]
- 26.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of prototype strains of all 102 human rhinovirus serotypes: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83:333-340. [DOI] [PubMed] [Google Scholar]
- 27.Schieble, J. H., V. L. Fox, and E. H. Lennette. 1967. A probable new human picornavirus associated with respiratory disease. Am. J. Epidemiol. 85:297-310. [DOI] [PubMed] [Google Scholar]
- 28.Strikas, R. A., L. J. Anderson, and R. A. Parker. 1986. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970-1983. J. Infect. Dis. 153:346-351. [DOI] [PubMed] [Google Scholar]
- 29.Uncapher, C. R., C. M. DeWitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 30.Utagawa, E. T., K. Miyamura, A. Mukoyama, and R. Kono. 1982. Neuraminidase-sensitive erythrocyte receptor for enterovirus type 70. J. Gen. Virol. 63:141-148. [DOI] [PubMed] [Google Scholar]