Abstract
Antananarivo, the capital city of Madagascar, has an endemic focus of tuberculosis (TB). We specifically studied patients with extrapulmonary TB (EPTB) and grouped patients according to infected body site. The strains were characterized by IS6110 fingerprinting and compared with those isolated from patients with pulmonary TB (PTB) during the same period in order to determine the possible association between the genotype and the clinical expression of TB. A total of 316 TB patients were included in this study: 151 individuals with EPTB, 10 with both PTB and EPTB, and 155 with PTB alone. Pleural TB was the major EPTB localization (77%) and was found more often in older patients, while PTB or EPTB in which the localization was other than pleural (other EPTB) was found in younger patients. The male-to-female ratio was slightly higher in pleural TB patients (3.06:1) than in patients with other EPTB (1.35:1). There was no significant difference in the BCG status among patients with PTB, pleural TB, and other EPTB. Analysis of IS6110 patterns showed that 167 patients (52.8%) were assigned to 37 clusters of 2 to 34 patients. Analysis of the IS6110 clusters and the IS6110 families did not show any association with a particular clinical expression of the disease. Patients with PTB or other EPTB were more likely to have strains with one IS6110 copy than patients with pleural TB. The clustering rate was found to be significantly higher in female patients (62%) than in male patients (48%) (P = 0.029), suggesting that Malagasy women were more likely to progress to disease after infection than men.
Although tuberculosis (TB) usually attacks the lungs, resulting in the pulmonary TB (PTB) form, other organs can be affected, leading to extrapulmonary TB (EPTB) or disseminated TB. The importance of EPTB among all forms of TB has not yet been ascertained in developing countries. This situation is due to the difficulty of the clinical diagnosis, which must be confirmed by culturing of Mycobacterium tuberculosis or hispathologic analysis, not always available. This situation can also be attributed to the lack of reliable data. In industrialized countries, prior to the human immunodeficiency virus (HIV) epidemic, about 15% of TB cases were declared extrapulmonary (6, 13). Since the emergence of HIV, EPTB has been detected more frequently (more than 30%) among HIV-infected individuals in Europe and in the United States (13, 20). All organs can be affected, but the frequencies of the different clinical sites of EPTB vary according to country (1, 6, 10, 12, 19). In Madagascar, a country in which TB is hyperendemic but in which the rate of HIV infection is low (2), the main extrapulmonary forms have been found to be pleural TB followed by lymph node TB (3, 5, 15, 17). The reason why some organs are more affected than others is not well understood. However, as was observed in the preantibiotic era, it is known that pleural TB often has a favorable outcome, in contrast to PTB (9). Thus, we can hypothesize that pleural TB may be due to less virulent M. tuberculosis strains. Alternatively, some M. tuberculosis strains may be more virulent and cause PTB or disseminate more easily than others and thus affect preferentially specific sites. A study in Thailand suggested that some strains may be more capable of causing meningitis than others (16).
In this study, the distribution of the different sites of EPTB found in patients from Antananarivo, the capital of Madagascar, is reported. Molecular fingerprinting with the IS6110 genetic marker was used to characterize the M. tuberculosis complex strains isolated from EPTB and PTB patients. The objectives of the study were to determine whether there is an association between the genotype and the clinical expression of disease and to compare the clustering rates.
MATERIALS AND METHODS
Study population.
This study was carried out in the city of Antananarivo. Clinical samples were obtained from all consecutive patients suspected of having EPTB in the six main health centers of Antananarivo between May 1994 and July 1995. These centers handled one-third of the EPTB cases diagnosed and declared in Antananarivo. A total of 689 clinical specimens (426 pleural, 55 sputum, 45 lymph node, 47 peritoneal, 33 cerebrospinal fluid, 34 urine, 14 bone, 15 genital, 11 pericardial, 3 cutaneous, and 6 miscellaneous specimens) were obtained from 465 individuals with clinical symptoms of EPTB. One hundred sixty-one cases were confirmed by bacteriologic analysis; 151 patients had EPTB and 10 had PTB associated with an extrapulmonary site. For 34 patients, two or three strains were isolated from the same patient, either from the same clinical site or from different clinical sites.
From a previous study on genetic polymorphisms of PTB strains (18), 155 patients were randomly sampled from among 559 PTB patients seen at three health centers. They corresponded to 23% and were representative of the PTB cases declared in Antananarivo between August 1994 and December 1995. The strains isolated from these PTB patients were compared with the strains isolated from the EPTB patients.
All the patients analyzed in this study were Malagasy and HIV seronegative.
Bacteriologic analysis.
All the clinical specimens were cultured on standard Lowenstein-Jensen (LJ) medium (Diagnostics Pasteur, Paris, France) and on LJ medium without glycerol but supplemented with 0.5% pyruvate. Mycobacterial isolates were identified according to growth on LJ medium, colony morphology, and the following biochemical tests: niacin production, catalase, urease, and nitrate reductase (7). The French National Reference Center for Mycobacteria (Pasteur Institute, Paris, France) was the reference laboratory for the quality control of strain identification.
All the strains isolated from each EPTB patient (197 strains) and one strain per PTB patient (155 strains) were analyzed by restriction fragment length polymorphism (RFLP) typing.
RFLP typing and analysis of IS6110 patterns.
Genomic DNA was extracted from M. tuberculosis colonies by the method described by van Soolingen et al. (24). DNA fingerprints were obtained by using the standardized RFLP technique with the IS6110 insertion sequence (IS6110 patterns) (23). Computer-assisted analysis of IS6110 patterns was performed by using Taxotron software (P. A. D. Grimont, Institut Pasteur, Paris, France). The patterns were compared by the unweighted pair-group clustering method of averages (21). The matching of patterns was further confirmed by visual examination.
A cluster of M. tuberculosis strains was defined as two or more strains with identical RFLP patterns. A cluster of patients was defined as two or more patients with identical strains. Yeh et al. (25) reported that IS6110 genotypes could change at a relatively high rate for strains with 8 to 14 bands and suggested that strains with a two-band difference could be considered related. Therefore, we classified all the RFLP profiles with more than seven bands into IS6110 families. Profiles were assigned to the same IS6110 family when they differed by only one or two hybridizing bands.
Statistical analysis was carried out by using the χ2 test or the Fisher's exact test for sample size values of ≤5. Differences were considered significant when the P value was <0.05.
RESULTS
General characteristics of the patients.
A total of 316 TB patients were included in this study: 151 individuals with EPTB, 10 individuals with both PTB and EPTB, and 155 individuals with PTB alone. The general characteristics of the patients classified according to the clinical site of TB are reported in Table 1. In summary, of the 151 EPTB patients, 117 had pleural TB (77.4%), 13 had lymph node TB (8.6%), 11 had peritoneal TB (7.2%), 3 had bone TB, 2 had meningitis, 1 had cutaneous TB, 1 had synovial TB, 1 had ear, nose, and throat TB, 1 had pericardial TB, and 1 had both pleural TB and peritoneal TB. For the 10 patients with both PTB and EPTB, the extrapulmonary site was pleural for 6 patients, lymph node for 3 patients, and peritoneal for 1 patient. The male-to-female ratio (sex ratio) was 2.1:1. The age of the patients ranged from 1 to 74 years (mean age, 33.2 years). The BCG status was known for 306 of the 316 patients (97%); 239 of 306 (78%) were vaccinated. During the course of the treatment, seven patients died: two men with pleural TB and five patients (three men and two women) with PTB.
TABLE 1.
General characteristics of patients
| Clinical site of TB (no. of patients) | Avg age, yr (range) | No. of patients
|
|||
|---|---|---|---|---|---|
| Vaccinated with BCGa
|
Male | Female | |||
| Yes | No | ||||
| Pulmonary (155) | 32 (13-72) | 116 | 30 | 98 | 57 |
| Pulmonary + pleural (6) | 35.5 (24-44) | 6 | 5 | 1 | |
| Pulmonary + lymph node (3) | 26.3 (22-34) | 3 | 2 | 1 | |
| Pulmonary + peritoneal (1) | 40 | 1 | 1 | ||
| Pleural (117) | 36.3 (10-79) | 87 | 29 | 89 | 28 |
| Pleural + peritoneal (1) | 21 | 1 | 1 | ||
| Peritoneal (11) | 31 (18-50) | 10 | 1 | 4 | 7 |
| Lymph node (13) | 33 (1-73) | 7 | 6 | 7 | 6 |
| Bone (3) | 9.6 (3-18) | 3 | 3 | ||
| Meningitis (2) | 21 | 2 | 2 | ||
| Cutaneous (1) | 14 | 1 | 1 | ||
| Synovial (1) | 37 | 1 | 1 | ||
| Ear, nose, and throat (1) | 27 | 1 | 1 | ||
| Pericardial (1) | 15 | 1 | 1 | ||
For nine patients with PTB and one patient with pleural TB, the BCG status was not known.
Pleural TB and PTB are both classified as respiratory TB. In this study, pleural TB, alone or associated with a pulmonary site, was the most frequent extrapulmonary form observed (77%). In the study group, 117 patients had only pleural TB, 1 patient had both pleural TB and peritoneal TB, and 6 patients had both PTB and pleural TB. Therefore, patients were grouped into patients with PTB, pleural TB, and EPTB in which the localization was other than pleural (other EPTB). The mean age of the patients with pleural TB (36.2 years) was higher than those of the patients with other EPTB (28.3 years; P = 0.0042) or with PTB (32 years; P = 0.008). The male-to-female ratio was also higher for the patients with pleural TB (3.06:1) than for the patients with other EPTB (1.35:1) or PTB (1.75:1) (P = 0.05). No significant differences were found among these three groups in BCG vaccination status.
IS6110 RFLP profiles of M. tuberculosis complex strains isolated from PTB and EPTB patients.
We determined whether the RFLP type could be associated with a particular clinical site by comparing the isolates from the different types of patients. The IS6110 RFLP types of the 155 PTB strains (152 M. tuberculosis and 3 M. bovis strains) were previously determined (18). One M. tuberculosis strain did not contain any IS6110 band. Of the 197 strains from the 151 patients with EPTB and the 10 patients with both PTB and EPTB, 195 were identified as M. tuberculosis and 2 were identified as M. bovis (1 from a bone biopsy and 1 from a lymph node). All of the strains were typed by the RFLP method with the IS6110 marker. Analysis of the PTB and EPTB strains revealed 191 different patterns with a number of IS6110 bands, ranging from 1 to 22 (mean, 9.3 IS6110 copies). Thirty-four patients had two TB strains, but only 1 patient with pleural TB had two strains with different RFLP patterns.
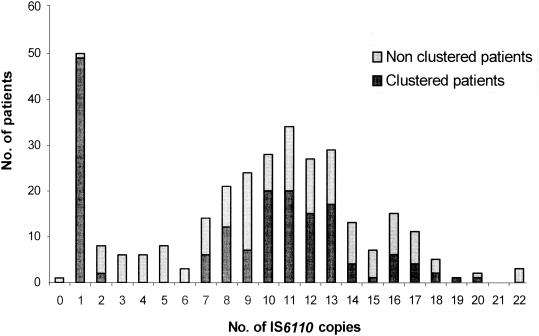
Of the 316 patients, 50 patients (15.8%) had strains with a single IS6110 hybridizing band pattern and that were assigned to four different profiles: 34 strains had a band at 1.5 kb, 10 had a band at 4.7 kb, and 5 had a band at 1.8 kb (all M. bovis), and 1 had a band at 5.1 kb. These clusters of strains with one IS6110 copy were both pulmonary and extrapulmonary. In the 118 pleural TB patients, the frequency of strains with one IS6110 copy was 11 of 118 (9.3%); in comparison, the frequencies in the patients with PTB and the patients with other EPTB were 30 of 165 (18.3%) and 9 of 33 (27.3%), respectively (P = 0.021). Of the 316 patients, 176 (55.7%) had strains with an intermediate number of IS6110 bands (8 to 14), while 45 (14.2%) had strains with a low number of bands (2 to 7) and 44 (13.9%) had strains with a high number of bands (more than 14) (Fig. 1). When we excluded the one-copy strains, we found no significant relationship between the clinical localization of the disease and the number of IS6110 copies.
FIG. 1.
Numbers of IS6110 copies in strains from TB patients.
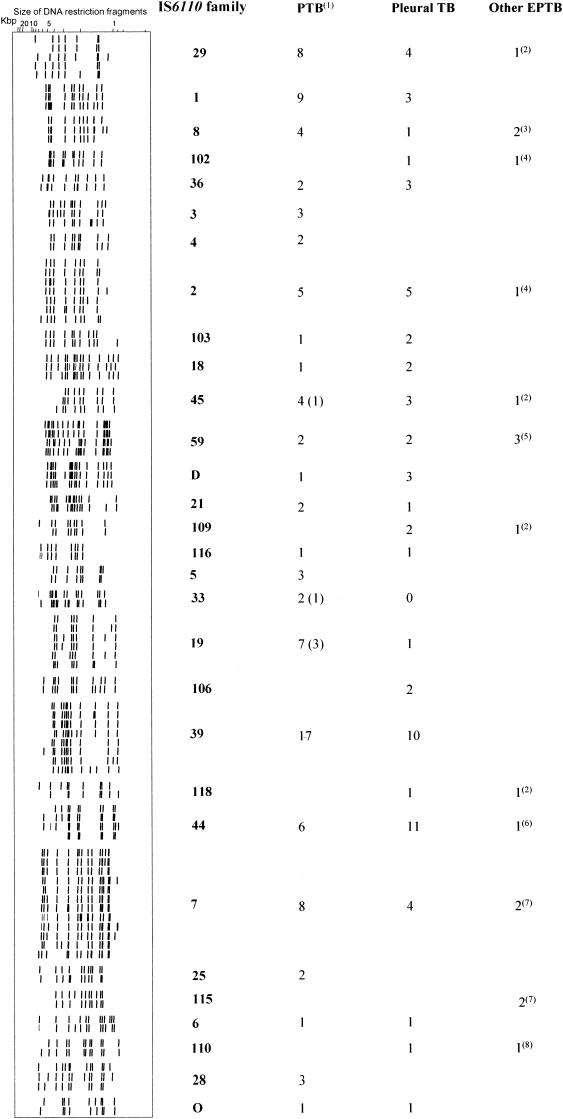
An analysis of the IS6110 profiles showed that 149 patients had strains with a unique IS6110 genotype and that 167 patients (52.8%) were assigned to 37 clusters of 2 to 37 patients. Of the 37 clusters, there were 24 clusters with fewer than 4 patients (20 clusters with 2 patients and 4 clusters with 3 patients). When we examined the clusters with more than four patients, no RFLP profile could be obviously associated with a clinical form of TB or a particular site of disease. As the majority (69.6%) of the patients had strains with more than seven bands (Fig. 1), we classified the RFLP profiles into IS6110 families. Thus, according to Yeh et al. (25), we identified 30 IS6110 families (Fig. 2), of which 6 contained at least 10 patients (families 1, 2, 7, 29, 39, and 44). The other families were too small and so were excluded from the analysis. IS6110 families 1 and 39 contained only PTB and pleural TB patients. The other four families contained patients with all forms of TB, so there was no evidence of any association with the clinical expression of the disease.
FIG. 2.
Distributions of patients with PTB, pleural TB, and other EPTB in IS6110 families. The IS6110 family numbers are arbitrary numbers used in our laboratory. Key to superscript numbers: 1, this group contains patients with PTB and patients with both PTB and EPTB; 2, lymph node TB; 3, one meningitis and one cutaneous TB; 4, peritoneal TB; 5, two peritoneal TB and one lymph node TB; 6, meningitis; 7, one peritoneal TB and one bone TB; 8, ear, nose, and throat TB.
Rate of clustering.
Based on the RFLP patterns, clustering rates were compared for M. tuberculosis strains and clinical infection sites (Table 2). The mean number of IS6110 bands was 9.3 per strain. When we excluded the 50 patients having strains with one copy, the percentages of clustered patients were 53.9% for those having strains with an intermediate number of bands and 17.7% (P = 0.0003) and 34.1% (P = 0.028) for patients having strains with low and high numbers of bands, respectively (Fig. 1). Thus, strains with an intermediate number of IS6110 copies were more likely to be clustered.
TABLE 2.
Rate of clustering and sex of patients
| Clinical site of TB (no. of patients) | Avg no. of IS6110 bands (range) | No. of patients
|
Pa | |||
|---|---|---|---|---|---|---|
| Clustered
|
Nonclustered
|
|||||
| Male | Female | Male | Female | |||
| Pulmonary (155) | 9.3 (0-22) | 45 | 38 | 53 | 19 | 0.019 |
| Both pulmonary and extrapulmonary (10) | 8.9 (1-14) | 4 | 3 | 3 | 1 | 0.53 |
| Pulmonary + pleural (6) | 10 (7-14) | 3 | 1 | 2 | ||
| Pulmonary + lymph node (3) | 9.3 (8-12) | 1 | 1 | 1 | ||
| Pulmonary + peritoneal (1) | 1 | 1 | ||||
| Pleural (118) | 9.5 (1-22) | 44 | 15 | 45 | 14 | 1 |
| Other extrapulmonary (33) | 8 (1-19) | 10 | 9 | 9 | 5 | 0.75 |
| Peritoneal (11) | 10.4 (2-19) | 1 | 3 | 3 | 4 | |
| Lymph node (13) | 5.9 (1-18) | 4 | 6 | 3 | ||
| Bone (3) | 8.6 (1-14) | 2 | 1 | |||
| Meningitis (2) | 10.5 (10-11) | 2 | ||||
| Cutaneous (1) | 9 | 1 | ||||
| Synovial (1) | 4 | 1 | ||||
| Ear, nose, and throat (1) | 12 | 1 | ||||
| Pericardial (1) | 1 | 1 | ||||
| Pleural + peritoneal (1) | 8 | 1 | ||||
Comparison of rates of clustering of male and female groups.
We did not observe any differences in the rates of clustering among patients with PTB, pleural TB, and other EPTB (P = 0.68). With regard to the groups of clustered and nonclustered patients, no significant difference was found for age or BCG vaccination status (data not shown). However, the male-to-female ratio was lower for clustered patients (1.6:1) than for nonclustered patients (2.8:1). In other words, the rate of clustering was significantly higher for the female patients (62%) than for the male patients (48%) (P = 0.029). This difference was marked for the PTB patients (P = 0.019). Moreover, while for the male patients the rates of clustering were the same for all age ranges, these rates for the female patients varied with age (Table 3): they were 35.7% for patients less than 21 years old, 57.1% for 21- to 30-year-old patients, 75% for 31- to 40-year-old patients, 55.5% for 41- to 50-year-old patients, and 90% for patients more than 50 years old (P = 0.039). This difference was even more marked when only the PTB form was considered: the rates of clustering for these age ranges were, respectively, 42.8, 50, 89.4, 100, and 80% (P = 0.022).
TABLE 3.
Age of patients and rate of clusteringa
| Age (yr) | No. of clustered patients/total no. of patients (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| All
|
With the following type of TB:
|
|||||||
| Pulmonary
|
Pleural
|
Other extrapulmonary
|
||||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| <21 | 15/31 (48.3) | 5/14 (35.7) | 6/12 (50) | 3/7 (42.8) | 3/10 (30) | 1/5 (20) | 6/9 (66.6) | 1/2 (50) |
| 21-30 | 31/66 (46.9) | 24/42 (57) | 19/41 (46.3) | 13/26 (50) | 10/22 (45.4) | 8/11 (72.7) | 2/3 (66.6) | 3/5 (60) |
| 31-40 | 33/60 (55) | 21/28 (75) | 16/30 (53.3) | 17/19 (89.4) | 16/27 (59.2) | 1/4 (25) | 1/3 (33.3) | 3/5 (60) |
| 41-50 | 13/28 (46.4) | 5/9 (55.5) | 6/12 (50) | 3/3 (100) | 6/13 (46.1) | 2/6 (33.3) | 1/3 (33.3) | 0 |
| >50 | 11/28 (39.3) | 9/10 (90) | 2/10 (20) | 4/5 (80) | 9/17 (52.9) | 3/3 (100) | 0/1 (0) | 2/2 (100) |
P values for male and female patients, respectively, were as follows: all, 0.72 and 0.039; with PTB, 0.47 and 0.022; with pleural TB, 0.58 and 0.068; and with other EPTB, 0.56 and 0.71 (comparison of rates of clustering in different age groups).
DISCUSSION
In this study, we used molecular typing methods to determine whether certain M. tuberculosis strains had an affinity for particular organs. It should be noted that at present, this is not known. A preliminary report from Thailand on the typing of M. tuberculosis strains isolated from 15 sputum and 6 cerebrospinal fluid samples suggested the ability of such strains to cause meningitis (16). To our knowledge, no other report confirmed these results. In this study, two of our strains were responsible for meningitis. One belonged to IS6110 family 8, and the other belonged to IS6110 family 44 (Fig. 2), which also contained strains from both PTB and pleural TB patients. The most frequent IS6110 families, 2, 7, 29, and 44 (Fig. 2), contained strains from patients with PTB, pleural TB, and other EPTB. It is possible that these strains disseminate more easily than others. This notion needs to be confirmed. We cannot draw conclusions about the specificity of one family for one clinical site, since the proportion of patients with other EPTB was low.
Pleural TB usually develops via lymphatic spread of PTB but has a good prognosis and often results in “self-cure” (9, 13). One explanation could be that, following a primary infection, less virulent strains lead to pleural TB, while the most virulent strains are more likely responsible for the development of PTB or are more easily disseminated, leading to EPTB. Strains with one IS6110 copy were significantly more frequent in PTB and EPTB patients than in pleural TB patients, suggesting that these strains are more virulent than multiple-band strains. However, of the seven deceased patients, only two PTB patients had strains with single IS6110 copies and five patients had strains with other patterns; this difference was not significant (P = 0.16). Thus, the virulence of such strains cannot be the only explanation for our results. Besides, it has been suggested that strains with a single IS6110 copy are ancestral strains (4). We can thus assume that they have been circulating in the population for a longer time than other strains, explaining their high frequency in PTB patients.
Sex difference in TB notification rates have been reported often, with a higher incidence in males. This difference is generally explained by either underdiagnosis or underreporting of TB in females. A study of the sex ratio among TB patients in San Francisco (14), on the basis of clustering data, suggested that the sex difference may be due to a difference in transmission dynamics rather than underdiagnosis. It may also be due to a difference in infection and/or progression to disease (8). In this study, the male-to-female ratio in the global TB population (2.1:1) was slightly higher than the ratio of 1.5:1 reported in previous studies (3, 17). However, the global rate of clustering in female patients was higher than that in male patients. Besides, while this rate was relatively low (42.8%) in female PTB patients less than 21 years old, it increased significantly in patients more than 21 years old and reached 89% in patients between 31 and 40 years old (Table 3). A high rate of clustering is associated with recent infection and rapid progression to disease (20). Thus, our results suggested that women more than 21 years old are more likely to be infected and to develop PTB after infection than men. Considering that pleural TB can be due to reactivation, the low rate of clustering (25%) in 31- to 40-year-old women with pleural TB, compared to that of men in this age range (59%), is in agreement with these results. There may be several explanations for the higher rate of clustering in middle-aged women. It is likely that women are more concerned by immunodeficiency. Since the HIV prevalence is low in Madagascar (2, 26), it is more likely that socioeconomic and cultural factors (poverty and crowded condition of life, nutritional status, close pregnancies, prolonged breast-feeding, and so forth) influence the rates of TB infection. Although this study did not provide data proving such a hypothesis, our results are in agreement with those of other studies which stated that in their reproductive years, women may progress more rapidly to disease (8, 11). As women's health has an impact on both their families and the economy, the incorporation of sex difference in TB control programs could yield more beneficial outcomes, as recommended by Uplekar et al. (22).
Acknowledgments
We are grateful to Elie J. Vololonirina, T. Rasolonavalona, and P. Ravololonandriana for technical assistance. We also thank the physicians from the health centers of Antananarivo Centre Hospitalier de Soavinandriana, Antananarivo Correctional Facility, Dispensaire Antituberculeux, Service des Maladies Respiratoires de l'Hopital Général de Befeletanana, Centre de Biologie Clinique de l'Institut Pasteur de Madagascar, and Hôpital Joseph Ravoahangy Andrianavalona for their collaboration in this study.
This study was supported by the French Cooperation (grant 93008) and the Raoul Follereau Foundation.
REFERENCES
- 1.Aurégan, G. 1993. Originalités de la tuberculose dans les pays en voie de développement. Ann. Inst. Pasteur 4:208-215. [Google Scholar]
- 2.Aurégan, G., J. Morvan, H. Zeller, and A. Rasamindrakotroka. 1995. SIDA et tuberculose: la situation à Madagascar. Arch. Inst. Pasteur Madag. 62:24-25. [PubMed] [Google Scholar]
- 3.Brygoo, E. R., and P. Le Noc. 1963. Note sur la tuberculose histologiques observée à Madagascar—a propos de 351 observations. Arch. Inst. Pasteur Madag. 31:183-203. [Google Scholar]
- 4.Cave, M. D., K. D. Eisenach, P. F. McDermott, J. H. Bates, and J. T. Crawford. 1991. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol. Cell. Probes 5:73-80. [DOI] [PubMed] [Google Scholar]
- 5.Coulanges, P., A. Mayoux, and E. R. Brygoo. 1970. La tuberculose histologique à Madagascar 1954-1969. A propos de 911 cas. Arch. Inst. Pasteur Madag. 39:173-209. [Google Scholar]
- 6.Farer, L. S., L. M. Lowell, and M. P. Meador. 1979. Extrapulmonary tuberculosis in the United States. Am. J. Epidemiol. 109:205-217. [DOI] [PubMed] [Google Scholar]
- 7.Helali, N. E., and P. Vergez. 1993. Identification des mycobactéries. Feuill. Biol. XXXIV(190):5-18. [Google Scholar]
- 8.Holmes, C. B., H. Haulser, and P. Nunn. 1998. A review of sex differences in the epidemiology of tuberculosis. Int. J. Tuberc. Lung Dis. 2:96-104. [PubMed] [Google Scholar]
- 9.Hopewell, P. C. 1994. Overview of clinical tuberculosis, p. 25-46. In B. B. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 10.Hubert, B., and A. Pelletier. 1991. Les cas déclarés de tuberculose en France en 1990. Wkly. Epidemiol. Rec. 48:207-208. [Google Scholar]
- 11.Hudelson, P. 1996. Gender differentials in tuberculosis: the role of socio-economic and cultural factors. Tuberc. Lung Dis. 77:391-400. [DOI] [PubMed] [Google Scholar]
- 12.Kashongwe, M., L. Kumboneki, M. Mizerero, and Y. Oorenge. 1990. Epanchements liquidiens de la plèvre chez l'adulte en milieu tropical: expérience des cliniques universitaires de Kinshasa. Pub. Med. Afriq. 106:20-26. [Google Scholar]
- 13.Lacut, J. Y., M. Dupon, and M. C. Paty. 1995. Tuberculoses extra-pulmonaires: revue et possibilités de diminution des délais d'intervention thérapeutique. Med. Mal. Infect. 25:304-320. [Google Scholar]
- 14.Martinez, A. N., J. T. Rhee, P. M. Small, and M. A. Behr. 2000. Sex differences in the epidemiology of tuberculosis in San Francisco. Int. J. Tuberc. Lung Dis. 4:26-31. [PubMed] [Google Scholar]
- 15.Ménard, D., J. L. Pécarrère, F. Ramaroson, J. L. Lesbordes, R. Andrianirinarisoa, I. Razafitsiarovana, M. Andriamiandrisoa, V. Raholimina Rahary, J. Rakotonizao, J. Richard, M. Peghini, P. Guyon, and S. Chanteau. 1995. Les tuberculoses extra-pulmonaires à Antananarivo: principales localisations et intérêt du diagnostic biologique. Arch. Inst. Pasteur Madag. 62:77-82. [PubMed] [Google Scholar]
- 16.Palittapongarnpim, P., S. Rienthong, and W. Panbangred. 1993. Comparison of restriction fragment length polymorphism of Mycobacterium tuberculosis isolated from cerebrospinal fluid and sputum: a preliminary report. Tuberc. Lung Dis. 74:204-207. [DOI] [PubMed] [Google Scholar]
- 17.Pécarrère, J. L., C. Raharisolo Vololonantenaina, J.-A. Dromigny, D. Ménard, G. Aurégan, J. L. Lesbordes, J. Richard, and P. De Rotalier. 1995. A propos de 660 cas de tuberculoses extra-pulmonaires étudiés à l'Institut Pasteur de Madagascar. Arch. Inst. Pasteur Madag. 61:83-89. [PubMed] [Google Scholar]
- 18.Rasolofo Razanamparany, V., H. Ramarokoto, G. Aurégan, B. Gicquel, and S. Chanteau. 2001. A combination of two genetic markers is sufficient for restriction fragment length polymorphism typing of Mycobacterium tuberculosis in areas with a high incidence of tuberculosis. J. Clin. Microbiol. 39:1530-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieder, H. L., D. E. Snider, and J. R. Cauthen. 1990. Extrapulmonary tuberculosis in the United States. Am. Rev. Respir. Dis. 141:347-351. [DOI] [PubMed] [Google Scholar]
- 20.Small, P. M., P. C. Hopewell, S. P. Singh, A. Paz, J. Parsonnet, D. C. Ruston, G. F. Schecter, C. L. Daley, and G. K. Schoolnik. 1994. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N. Engl. J. Med. 330:1703-1709. [DOI] [PubMed] [Google Scholar]
- 21.Sneath, P. H. A., and R. R. Solal. 1973. Numerical taxonomy. W. H. Freeman & Co., San Francisco, Calif.
- 22.Uplekar, M. W., S. Rangan, M. G. Weiss, J. Ogden, M. W. Borgdorff, and P. Hudelson. 2001. Attention to gender issues in tuberculosis control. Int. J. Tuberc. Lung Dis. 5:220-224. [PubMed] [Google Scholar]
- 23.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. W. M. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Soolingen, D., P. W. M. Hermans, P. E. W. de Haas, D. R. Soll, and J. D. A. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh, R. W., A. Ponce de Leon, C. B. Agasino, J. A. Hahn, C. L. Daley, P. C. Hopewell, and P. M. Small. 1998. Stability of Mycobacterium tuberculosis DNA genotypes. J. Infect. Dis. 177:107-111. [DOI] [PubMed] [Google Scholar]
- 26.Zeller, H. G., A. Ramamonjisoa, P. Boisier, B. Ravelojaona, L. Brutus, R. Randriamanga, L. Rabarijaona, M. Rakoto-Andrianarivelo, G. Auregan, F. Behets, J. F. Roux, and A. J. Rasamindrakotroka. 1997. HIV infection in Madagascar in 1995. AIDS 11:401-402. [PubMed] [Google Scholar]