Abstract
In July 2000, symptomatic acute hepatitis C was diagnosed in five patients who had attended the emergency room of a municipal hospital on the same day, about 6 weeks before. Investigation of the remaining 65 patients visited at the emergency room on that day disclosed that 8 patients had a positive anti-hepatitis C virus (anti-HCV) test and 4 of them had biochemical evidence of acute anicteric hepatitis. HCV RNA was detected in 12 of the 13 anti-HCV-positive patients. Phylogenetic analysis of the nonstructural 5A (NS5A) and E2 regions showed that 10 patients, including all 9 with acute hepatitis, were infected with a closely related HCV strain, while the remaining 2 patients harbored unrelated strains. Flushing of intravenous catheters with heparin retrieved from a multidose heparin solution in saline was carried out for all the patients involved in the hepatitis outbreak but in only 1 of 23 (4%) matched controls recruited among HCV-noninfected patients attending the emergency room on the same day, and this was the only significant difference concerning risk factors for HCV infection between patients and controls. Thus, accidental contamination of a multidose heparin solution with blood from an unrecognized HCV carrier was identified as the source of this nosocomial outbreak of hepatitis C.
Hepatitis C virus (HCV) infection is mainly transmitted by parenteral route, but nearly 40% of patients with acute or chronic hepatitis C do not report risk factors for parenteral exposure (33). A history of previous hospitalization is quite common in patients with sporadic hepatitis C, suggesting that some of them may have been infected in the hospital setting (6), where HCV transmission may occur through accidental or inadvertent nonobservance of universal precaution measures (5). Nosocomial transmission of HCV infection is currently well documented by epidemiological and molecular investigations (26).
Phylogenetic analysis of HCV isolates is an important tool in epidemiological studies of HCV infection. Phylogenetic information makes it possible to know if different patients are infected with closely related or with different viral strains and is also helpful in discriminating between patients previously infected and those infected in an epidemic outbreak (17, 32). These data may also have important medico-legal relevance to solve claims from patients who ask compensation for presumed damage.
We report here an epidemic outbreak of HCV infection in the emergency ward of a municipal hospital in which classical and molecular epidemiological investigations identified a presumably contaminated multidose vial of heparin solution as the most likely source of infection.
In July 2000, five adult patients with acute hepatitis C were seen within a short period of time at the Gastroenterology Unit of the Hospital de Figueres, a municipal hospital of 155 beds. All complained from symptoms suggesting acute hepatitis, and four of them were jaundiced. Levels of alanine transaminase (ALT) in serum were elevated (323 to 3,720 IU/liter) and anti-HCV test was positive in all patients. Onset of symptoms occurred between 10 and 21 of July. The five patients had been suffering from unrelated diseases on 4 June at the emergency room of the same hospital, where placement of a heparinized intravenous line for blood sampling and fluid perfusion was the only invasive procedure carried out on these subjects.
The medical area of the emergency premises has a nursing station that serves six beds allocated into two contiguous rooms. Nursing staff takes care of all admitted patients, with no assignment of a particular nurse to a specific bed.
By request of public health authority (Direcció General de Salut Pública de la Generalitat de Catalunya), an epidemiological investigation was undertaken. Since all the infections appeared to have occurred on the same day, a common source of exposure was suspected. After informed consent, the 65 patients who were admitted to the medical area of the emergency ward on 4 June were investigated. Liver functions, including aspartate transaminase and ALT levels, were determined by routine automated procedures, and anti-HCV antibodies were tested by a third-generation enzyme-linked immunosorbent assay (Ortho Diagnostics, Raritan, N.J.). Serological examination disclosed eight additional anti-HCV positive patients. The level of ALT was normal in three patients, slightly elevated in three patients, and higher than 200 IU/liter in the remaining two patients.
Serum HCV RNA extraction and reverse transcription-PCR amplification of the 5′ noncoding (5′NC) region of HCV genome was carried out with samples from the 13 patients positive for anti-HCV, as previously described (23). HCV RNA was quantified using a commercial kit (Amplicor HCV Monitor test, version 2.0; Roche Diagnostic Systems Inc., Branchburg, N.J.). HCV genotype was determined by restriction fragment length polymorphism (14), and specific partial amplification of the E2 region, including hypervariable region 1, and the NS5A region were carried out as described elsewhere (15, 24). Amplified fragments were purified, and a bidirectional sequence analysis was performed using a commercial kit (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom). HCV RNA-positive serum samples from nine well-characterized HCV-infected patients filed at the hospital clinic were retrieved for control purposes. Measures to prevent PCR contamination were strictly observed (13). Two independent RNA extractions from two serum samples taken from each patient several weeks apart were amplified in separate reactions by reverse transcription-PCR. Analyses were performed in October using the first available serum sample from each patient that was stored at −20°C. Sequence alignment and phylogenetic analyses were carried out using PHYLIP (phylogeny inference package, version 3.5c; J. Felsenstein) as previously described (24, 30).
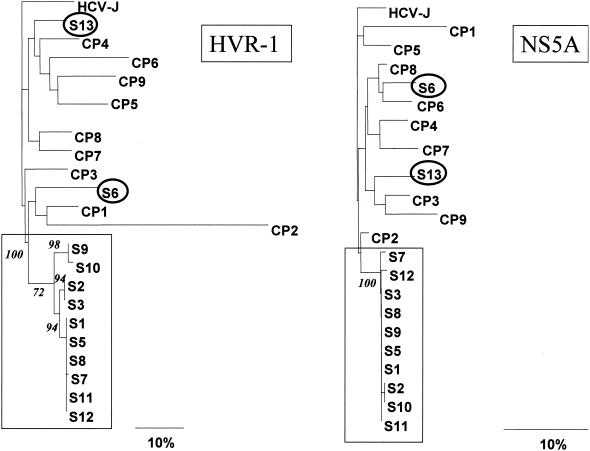
HCV RNA amplification was successful in specimens from 12 of the 13 anti-HCV positive patients. Up to four attempts to amplify HCV RNA from three different samples from the remaining patient gave negative results. Genotype 1b was identified in all of them, but this finding does not support a common source of infection, since genotype 1b is highly prevalent in our area (14). Nucleotide alignment of the 12 HCV RNA-positive samples disclosed that 10 of the 12 patients were infected by a genetically very closely related viral strain. Nucleotide genetic distances within these 10 isolates were 0 to 4.8% in hypervariable region 1 and 0 to 1.5% in the NS5A region, while the values in the remaining two samples were 13.5 to 22.8% and 7.3 to 9.3%, respectively. The latter are similar to those found between nonrelated HCV genotype 1b isolates from our geographical area (15.5 to 52.3% and 4.5 to 14.5%, respectively). The genetic distance between these 10 isolates was similar to that previously documented among viral isolates in other instances of HCV transmission through different mechanisms, such as colonoscopy (4), mother to infant (20, 31), and others (19, 28, 29). In contrast, the genetic distance between virus from the remaining two cases was much greater and similar to that previously described among epidemiologically unrelated strains from the same geographical area (9, 24, 25, 27), suggesting that these individuals were not involved in the epidemic outbreak under consideration. Close genetic relatedness of these 10 isolates was confirmed by phylogenetic analysis, since all of them clustered together with high bootstrap values and were clearly separated from the remaining samples analyzed (Fig. 1).
FIG. 1.
Phylogenetic tree of the partial E2 and NS5A sequences from patients investigated in the outbreak (S) and control samples (PC). Branch lengths are drawn to scale. Prototype HCV-J (10) sequence is included as the outgroup sequence. Numbers in italics represent bootstrap proportions in support of the adjacent node based on 100 resampling iterations. Only bootstrap values higher than 70% are shown. The squares and circles include HCV strains with and without a common genetic origin, respectively, obtained from patients who attended the same emergency ward on the same day.
Acute hepatitis was diagnosed by clinical and/or biochemical criteria for 9 of the 10 patients harboring genetically closely related HCV sequences. The remaining patient (S2) had normal transaminase levels. Individual clinical and virological features of patients positive for anti-HCV are shown in Table 1.
TABLE 1.
Demographic, clinical, biochemical, and virological features of the patients in this study
| Patient no. | Sexa | Age (yr) | Peak ALT (IU/liter) | Viral load (103 IU/ml) | Clinical diagnosis | Outcome | Phylogenetic adscription to the outbreak |
|---|---|---|---|---|---|---|---|
| S1 | M | 59 | 323 | 76.0 | Acute anicteric hepatitis | Chronic HCV infection | Yes |
| S2 | M | 29 | 39 | 10.5 | HCV infection without evidence of hepatic disease | Unchanged | Yes |
| S3 | M | 62 | 1,936 | 857.4 | Acute icteric hepatitis | Resolved with treatment | Yes |
| S4 | M | 38 | 55 | Undetectable | Past HCV infection | Unchanged | No |
| S5 | M | 47 | 1,648 | 703.4 | Acute icteric hepatitis | Resolved with treatment | Yes |
| S6 | M | 90 | 33 | 76.9 | Chronic HCV infection | Unchanged | No |
| S7 | M | 81 | 62 | 7.4 | Acute anicteric hepatitis | Spontaneous resolution | Yes |
| S8 | M | 82 | 552 | 361.5 | Acute anicteric hepatitis | Chronic HCV infection | Yes |
| S9 | M | 69 | 114 | 8.0 | Acute anicteric hepatitis | Spontaneous resolution | Yes |
| S10 | F | 68 | 2,320 | 5.0 | Acute icteric hepatitis | Spontaneous resolution | Yes |
| S11 | F | 66 | 1,029 | 90.9 | Acute anicteric hepatitis | Chronic HCV infection | Yes |
| S12 | M | 46 | 2,570 | 508.8 | Acute icteric hepatitis | Resolved with treatment | Yes |
| S13 | F | 82 | 15 | 19.4 | Chronic HCV infection | Unchanged | No |
Abbreviations: M, male; F, female.
Among the 10 patients with HCV infection likely related to a common exposure, the first patient attending the emergency ward on 4 June entered the hospital at 5 h 24 min. He was a 59-year-old male, without previous history of hepatic disease. Eleven weeks later, he remained asymptomatic but had elevated liver enzymes (aspartate transaminase, 162 IU/liter; ALT, 323 IU/liter). The second HCV RNA-positive subject was admitted to the hospital at 5 h 28 min. He was a 29-year-old male without previously known hepatic disease. He did not have symptoms suggesting hepatitis, and his liver function tests were normal at his first visit but showed elevated serum transaminase levels in subsequent visits (less than 80 IU/liter). This individual was considered to be an HCV carrier with chronic hepatic disease. The remaining eight patients involved in the outbreak arrived at the hospital 4 to 12 h later, and all of them developed acute hepatitis.
In three patients with acute hepatitis, disease resolved spontaneously. Three patients were treated with interferon (3 MU, three times week) plus ribavirin (1 g/day), and all had a favorable sustained response with normalization of ALT and clearance of HCV RNA. HCV viremia persisted in the remaining three patients who did not receive treatment due to old age or to contraindications for antiviral therapy (Table 1).
The two patients infected with a viral strain genetically unrelated to that causing the outbreak had normal ALT levels and were 90 and 82 years old, respectively. A case-control study aimed at identifying the factors implicated in HCV transmission in the hospital was undertaken. Patients whose HCV RNAs were highly homologous, suggesting a common source of infection, were considered as subjects (n = 10). Adult patients visited at the emergency ward during 4 June without evidence of HCV infection, matched by age and gender to the subjects, served as controls (n = 23). The following risk factors for HCV infection during their visit to the hospital were considered: blood or blood product transfusion, minor or major surgery, and intravenous line placement, as well as other risk factors, such as intravenous illegal drug use, sexual promiscuity, and attendance at a hospital in the 3 months prior to 4 June. Differences of the prevalence in risk factors between subjects and controls were analyzed by the chi-square test or Fisher's exact test.
Risk factors of exposure to infectious agents other than those associated with their visit to the hospital were not identified for any of the anti-HCV positive patients. The case-control study disclosed that all subjects, but only three (12%) control individuals, were attended in one of the two areas of the emergency ward, which was usually dedicated to more severe medical disorders (P < 0.01). A heparin-flushed intravenous line was placed in 100% of the subjects and in only one of the controls (4%) (P < 0.01). Intravenous lines were routinely placed by nurses and heparinized by flushing with a dose retrieved from a heparin solution which was prepared by diluting 5 ml of 1% heparin from a multidose vial into a 100-ml flask of isotonic saline. The heparin solution was prepared daily and discarded within 24 h.
Inadvertent contamination of the diluted heparin solution with HCV-infected blood was most likely the cause of HCV spread throughout intravenous catheters to other patients, although this could not be definitely proven, because the heparin solution was not available for virological studies.
Patient S2 was presumably the source of the infection, because he was the sole patient involved in the outbreak not developing acute hepatitis and came to the emergency room early in the morning. It is likely that HCV was transferred into the heparin solution by inadvertent reuse of a needle or a syringe contaminated with blood from this patient. However, nurses in charge did not ratify that inadequate manipulations might have occurred.
The HCV outbreak in the emergency ward described here appears to be a rare event. So far, only two nosocomial outbreaks traced to a single source of exposure have been reported, including five patients inadvertently infected by a cardiac surgeon (8) and five patients infected by an anesthesiology assistant (22). Most nosocomial epidemic outbreaks of HCV infection, either in hemodialysis units (1, 7, 16, 18) or in other hospital settings (26), have involved multiple separate instances of patient-to-patient transmission and were likely related to repeated violations of universal precaution measures (5).
Contaminated multidose vials of saline, heparin, or anesthetics have been reported as the vehicle of transmission of HBV, HCV, human immunodeficiency virus, and malaria in the hospital setting (2, 3, 11, 12, 21). In all these instances, contamination has been attributed to the accidental reinsertion of a contaminated needle in a vial or the reuse of a syringe. Therefore, extremely careful manipulation is essential to minimize contamination risks when multidose vials are used. Use of single-dose vials would prevent the reuse of contaminated devices, or the reinsertion of a contaminated needle in a common container, thus reducing the risk of nosocomial transmission of blood-borne pathogens. Beside these measures, it is also important to remember that strict adherence to the standard precautions for infection control is essential to prevent transmission of infectious agents.
Finally, the present study also highlights the value of molecular epidemiology as a tool for solving medico-legal conflicts that may rise in the context of nosocomial transmission of HCV.
Nucleotide sequence accession numbers.
The HCV sequences in this study have been submitted to GenBank under accession numbers AF4806030 through AF486071.
Acknowledgments
This work was supported in part by SAF (grant 01-1799). S.F. was supported by a grant from the Fundació Clinic (FC), and M.G.-B. was supported by the Instituto de Salud Carlos III (ICIII).
REFERENCES
- 1.Allander, T., C. Medin, S. H. Jacobson, L. Grillner, and M. A. Persson. 1994. Hepatitis C transmission in a hemodialysis unit: molecular evidence for spread of virus among patients not sharing equipment. J. Med. Virol. 43:415-419. [DOI] [PubMed] [Google Scholar]
- 2.Al-Saigul, A. M., R. E. Fontaine, and Q. Haddad. 2000. Nosocomial malaria from contamination of a multidose heparin container with blood. Infect. Control Hosp. Epidemiol. 21:329-330. [DOI] [PubMed] [Google Scholar]
- 3.Alter, M. J., J. Ahtone, and J. E. Maynard. 1983. Hepatitis B virus transmission associated with a multiple-dose vial in a hemodialysis unit. Ann. Intern. Med. 99:330-333. [DOI] [PubMed] [Google Scholar]
- 4.Bronowicki, J. P., V. Venard, C. Botté, N. Monhoven, I. Gastin, I. Chone, H. Hudziak, B. Rihn, C. Delanoe, A. LeFaou, M. A. Bigard, and P. Gaucher. 1997. Patient to patient transmission of hepatitis C virus during colonoscopy. N. Engl. J. Med. 337:237-240. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. 1988. Universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health care settings. JAMA 260:462-465. [PubMed] [Google Scholar]
- 6.Chiaramonte, M., T. Stroffolini, U. Lorenzoni, F. Minniti, S. Conti, A. Flo, E. Ntakirutimana, A. Vian, T. Ngatchu, and R. Naccarato. 1996. Risk factors in community acquired chronic hepatitis C virus infection: a case-control study in Italy. J. Hepatol. 24:129-134. [DOI] [PubMed] [Google Scholar]
- 7.De Lamballerie, X., M. Olmer, D. Bouchouareb, C. Zandotti, and P. De Micco. 1996. Nosocomial transmission of hepatitis C virus in haemodialysis patients. J. Med. Virol. 49:296-302. [DOI] [PubMed] [Google Scholar]
- 8.Esteban, J. I., J. Gómez, M. Martell, B. Cabot, J. Quer, J. Camps, A. González, T. Otero, A. Moya, R. Esteban, and J. Guardia. 1996. Transmission of hepatitis C virus by a cardiac surgeon. N. Engl. J. Med. 334:555-560. [DOI] [PubMed] [Google Scholar]
- 9.Giménez-Barcons, M., S. Franco, Y. Suárez, X. Forns, S. Ampurdanés, F. Puig-Basagoiti, A. Sánchez-Fueyo, J. M. Barrera, J. M. Llovet, J. Bruix, J. M. Sánchez-Tapias, J. Rodés, and J. C. Sáiz. 2001. High amino acid variability within the NS5A of hepatitis C virus (HCV) is associated with hepatocellular carcinoma in patients with HCV-1b related cirrhosis. Hepatology 34:158-167. [DOI] [PubMed] [Google Scholar]
- 10.Kato, N., M. Hijikata, Y. Ootsuyama, M. Nakagawa, S. Ohkoshi, T. Sugimura, and K. Shimotohno. 1990. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc. Natl. Acad. Sci. USA 87:9524-9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzenstein, T. L., L. B. Jorgensen, H. Permin, J. Hansen, C. Nielsen, R. Machuca, and J. Gerstoft. 1999. Nosocomial HIV-transmission in an outpatient clinic detected by epidemiological and phylogenetic analyses. AIDS 13:1737-1744. [DOI] [PubMed] [Google Scholar]
- 12.Krause, G., S. Whisenhunt, M. Trepka, D. Katz, O. Nainan, S. Wiersma, and R. S. Hopkins. 2000. Patient-to-patient transmission of hepatitis C virus associated with use of multidose saline vials in a hospital. Infect. Control Hosp. Epidemiol. 21:261. [DOI] [PubMed]
- 13.Kwok, S., and R. Higuchi. 1989. Avoiding false positive with PCR. Nature 18:237-238. [DOI] [PubMed] [Google Scholar]
- 14.López-Labrador, F. X., S. Ampurdanés, X. Forns, A. Castells, J. C. Sáiz, J. Costa, J. Bruix, J. M. Sánchez-Tapias, M. T. Jiménez de Anta, and J. Rodés. 1997. Hepatitis C virus (HCV) genotypes in spanish patients with HCV infection: relationship between HCV genotype 1b, cirrhosis and hepatocellular carcinoma. J. Hepatol. 27:959-965. [DOI] [PubMed] [Google Scholar]
- 15.López-Labrador, F. X., S. Ampurdanés, M. Giménez-Barcons, M. Guilera, J. Costa, M. T. Jiménez de Anta, J. M. Sánchez-Tapias, J. Rodés, and J. C. Sáiz. 1999. Relationship of the genomic complexity of hepatitis C virus with liver disease severity and response to interferon in patients with chronic HCV genotype 1b infection. Hepatology 29:897-903. [DOI] [PubMed] [Google Scholar]
- 16.Mc Laughlin, K. J., S. O. Cameron, T. Good, E. Mc Cruden, J. C. Ferguson, F. Davidson, P. Simmonds, R. A. Mactier, and M. A. McMillan. 1997. Nosocomial transmission of hepatitis C virus within a British dialysis centre. Nephrol. Dial. Transplant. 12:304-309. [DOI] [PubMed] [Google Scholar]
- 17.Mizokami, M., and J. Y.-N. Lau. 1998. Molecular evolutionary analysis: its aplication in the study of hepatitis C virus, 147-158. In J. Y.-N. Lau (ed.), Hepatitis C protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 18.Mizuno, M., T. Higuchi, K. Kanmatsuse, and M. Esumi. 1998. Genetic and serological evidence for multiple instances of unrecognized transmission of hepatitis C virus in hemodialysis units. J. Clin. Microbiol. 36:2926-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norder, H., A. Bergstrom, I. Uhnoo, J. Alden, L. Weiss, J. Czajkowski, and L. Magnius. 1998. Confirmation of nosocomial transmission of hepatitis C virus by phylogenetic analysis of the NS5-B region. J. Clin. Microbiol. 36:3066-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohto, H., S. Terazawa, N. Sasaki, K. Hino, C. Ishiwata, M. Kako, N. Ujiie, C. Endo, A. Matsui, H. Okamoto, and S. Mishino. 1994. Transmission of hepatitis C virus from mothers to infant. N. Engl. J. Med. 330:744-750. [DOI] [PubMed] [Google Scholar]
- 21.Oren, I., R. C. Hershow, E. Ben-Porath, N. Krivoy, N. Goldstein, S. Rishpon, D. Shouval, S. C. Hadler, M. J. Alter, and J. E. Maynard. 1989. A common-source outbreak of fulminant hepatitis B in a hospital. Ann. Intern. Med. 110:691-698. [DOI] [PubMed] [Google Scholar]
- 22.Ross, R. S., S. Viazov, T. Gross, F. Hofmann, H. M. Seipp, and M. Roggendorf. 2000. Transmission of hepatitis C virus from a patient to an anesthesiology assistant to five patients. N. Engl. J. Med 343:1851-1854. [DOI] [PubMed] [Google Scholar]
- 23.Sáiz, J. C., S. Ampurdanés, E. Olmedo, F. X. López-Labrador, X. Forns, M. Guilera, D. Tassies, J. Costa, J. M. Sánchez-Tapias, M. T. Jiménez de Anta, and J. Rodés. 1997. Hepatitis G virus infection in chronic hepatitis C: frequency, features and response to interferon. J. Hepatol. 26:787-793. [DOI] [PubMed] [Google Scholar]
- 24.Sáiz, J. C., F. X. López-Labrador, S. Ampurdanés, J. Dopazo, X. Forns, J. M. Sánchez-Tapias, and J. Rodés. 1998. The prognostic relevance of the nonstructural 5A gene interferon sensitivity determining region is different in infections with genotype 1b and 3a isolates of hepatitis C virus. J. Infect. Dis 177:839-847. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Fueyo, A., M. Giménez-Barcons, F. Puig-Basagoiti, A. Rimola, J. M. Sánchez-Tapias, J. C. Sáiz, and J. Rodés. 2001. Influence of the dynamics of the hypervariable region 1 of hepatitis C virus (HCV) on the histological severity of HCV recurrence after liver transplantation. J. Med. Virol. 65:266-275. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Tapias, J. M. 1999. Nosocomial transmission of hepatitis C virus. J. Hepatol. 31(Suppl. 1):107-112. [DOI] [PubMed] [Google Scholar]
- 27.Soguero, C., E. Campo, T. Ribalta, J. M. Sánchez-Tapias, J. C. Sáiz, and M. Bruguera. 2000. Assessment of genotype and molecular evolution of hepatitis C virus in formalin-fixed paraffin-embedded liver tissue from patients with chronic hepatitis C virus infection. Lab. Investig. 80:851-856. [DOI] [PubMed] [Google Scholar]
- 28.Stuyver, L., H. Claeys, A. Wyseur, W. van Arnhem, H. de Beenhouwer, S. Uytendaele, J. Beckers, D. Matthijs, G. Leroux-Roels, G. Maertens, and M. Paepe. 1996. Hepatitis C virus in hemodialysis unit: molecular evidence of nosocomial transmission. Kidney Int. 49:889-895. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki, K., M. Mizokami, J. Y. Lau, N. Mizoguchi, K. Kato, Y. Mizuno, T. Sodeyama, K. Kiyosawa, and T. Gojobori. 1994. Confirmation of hepatitis C virus transmission through needlestick accidents by molecular evolutionary analysis. J. Infect. Dis. 170:1575-1578. [DOI] [PubMed] [Google Scholar]
- 30.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner, A. J., M. M. Thaler, K. Crawford, K. Ching, J. Kansopon, D. Y. Chien, J. E. Hall. F. Hu, and M. Houghton. 1993. A unique, predominant hepatitis C virus variant found in an infant born to a mother with multiple variants. J. Virol. 67:4365-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widell, A., B. Christenson, T. Wiebe, C. Schalen, H. B. Hansson, T. Allanander, and M. A. Persson. 1999. Epidemiological and molecular investigations of outbreaks of hepatitis C virus infection on a pediatric oncology service. Ann. Intern. Med 130:130-134. [DOI] [PubMed] [Google Scholar]
- 33.Zeuzem, S., G. Tuber, J. H. Lee, B. Ruster, and W. K. Roth. 1996. Risk factors for the transmission of hepatitis C. J. Hepatol. 24(Suppl. 2):3-10. [PubMed] [Google Scholar]