Abstract
Minute virus of canines (MVC), also known as canine parvovirus type 1, was initially believed to be a nonpathogenic agent, since it was first isolated from canine fecal specimens in the late 1960s. However, subsequent pathological as well as epidemiological studies suggested that MVC is a pathogen of neonatal puppies and is widely distributed among domestic dogs in the United States. The virus also has been shown to cause fetal deaths. Nevertheless, the virus was not detected in dogs outside the United States until recently, presumably because of a lack of widespread availability of the only susceptible canine cell line, WRCC/3873D, used for MVC isolation. We examined 470 clinical specimens from 346 dogs by PCR and detected MVC-specific gene fragments from four diseased puppies (positive rate, 1.2%). Viruses were recovered from three PCR-positive rectal specimens by using WRCC/3873D and MDCK cells. The isolates possessed antigenic and genomic properties similar to those of the U.S. reference strain GA3 and were identified as MVC. In addition, seroepidemiological evidence that 5.0% of dogs possessed anti-MVC antibodies also indicated the presence of MVC infection among dogs in Japan. From this study and several recent European reports describing MVC field cases, it is evident that MVC is distributed among domestic dogs worldwide.
Minute virus of canines (MVC) is an autonomous parvovirus of dogs that was first discovered in the feces of normal dogs in 1967 (2). It was known as the only canine parvovirus (CPV) until a second CPV emerged about 10 years later. The latter CPV is the now-well-known CPV type 2 (CPV-2), which has become a highly virulent pathogen throughout the world. In this context, MVC has also been called CPV-1, but no antigenic relationship between CPV-1 and -2 has been shown (for reviews, see references 3 and 13).
Epidemiological studies have indicated relatively high prevalences of MVC infections in adult dogs in the United States (2, 4, 5, 10). To our knowledge, it was not until recently that reports began appearing from outside the United States. Newborn puppies with respiratory illness were reported in 1996 in Sweden (9), and in the same year an abortion case was described in Germany (16); more recently (in 1999), cases were found in Italy (14). In the last case, 35-day-old puppies died from MVC infection with pulmonary and cardiac signs, which was different from the previous natural cases, where puppies less than 3 weeks of age had enteritis as the prominent sign. Several experimental studies (4, 5, 10), as well as field case studies (6, 9, 14, 16) indicate that the infection with MVC is generally asymptomatic or milder than that caused by CPV-2. However, MVC and canine herpesvirus are now becoming recognized as important pathogens for the canine fetus and young puppies (3).
Virus characterization has not been fully described, but a recent genomic study (15) allowed us to use PCR with clinical specimens to perform molecular epidemiological investigations of MVC infection in different Japanese dog populations. The aim of the present study was to obtain virologic evidence of MVC infection status among dogs in Japan.
MATERIALS AND METHODS
Viruses and cell cultures.
The MVC strain GA3 (4) was used as the reference strain. Two different canine cell lines, the Walter Reed canine cell/3873D (WRCC) cell line (2) and the Madin-Darby canine kidney (MDCK) cell line, were used for virus isolation and propagation of MVC. WRCC cells were obtained from the James A. Baker Institute of Cornell University and used for virus isolation. They were passaged by using a combined medium of McCoy's 5A and Leibovitz's L-15 media (GIBCO BRL, Grand Island, N.Y.), consisting of an equal volume of each medium and 15% fetal bovine serum (GIBCO BRL). MDCK cells were cultured by using Eagle's minimal essential medium (Nissui, Tokyo, Japan) supplemented with 7.5% fetal bovine serum. The p85 strain of the CPV-2b antigenic type grown in MDCK cells was used as a reference for CPV-2.
Clinical specimens.
Clinical specimens were collected in the Laboratory of Clinical Microbiology, Kyoritsu Seiyaku Corporation, Tokyo, Japan, and were examined for MVC. A total of 301 swab specimens, 44 from upper respiratory (nasal or oral) sites and 257 from rectal sites, were taken from dogs with respiratory or enteric clinical signs. The samples had been submitted by animal hospitals in various parts of Japan during the years from 1997 to 2000 for the examination of general canine viral pathogens and stored at −80°C until analyzed. In addition, a total of 169 specimens (from cerebrum, cerebellum, cerebrospinal fluid, thymus, lung, spleen, and various lymph nodes and conjunctival, oropharyngeal, nasal, and rectal swabs) were obtained from 45 dogs suspected of having canine distemper. Postmortem examination of the dogs had been performed at the Veterinary Pathology Laboratory of Azabu University, and the results of examination for canine distemper virus (CDV) were described previously (8, 11). In all, 470 clinical specimens from 346 dogs were examined for MVC.
Cell staining.
Cultured cells were stained to determine the presence of intranuclear inclusion bodies by Giemsa staining and of MVC antigens by indirect immunofluorescence antibody (IFA) staining. Both tests were performed in the usual manner. In IFA staining for original virus isolation, an anti-MVC GA3 dog serum, obtained from the James A. Baker Institute, was applied to specimens and then stained with fluorescein isothiocyanate-conjugated anti-dog immunoglobulin G rabbit serum (Sigma-Aldrich Japan, Tokyo, Japan). For characterization of the isolates, a combination of anti-MVC HM-6 rabbit immune serum and fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G sheep serum (Sigma-Aldrich Japan) was used.
Virus isolation.
Virus isolation from the PCR-positive specimens was attempted with both MDCK and WRCC cells. All samples were pretreated with immune serum against the p85 strain of CPV-2 before inoculation. An equal volume of 1:10-diluted antiserum (estimated neutralization titer of 1:1,000, based on the data in Table 2) was mixed with each sample and incubated at 37°C for 1 h and subsequently at 4°C overnight. As a control, p85 strain virus was treated with the same antiserum to confirm complete neutralization of the virus. Next, 0.2-ml amounts of each treated sample were inoculated into a 6-cm-diameter culture dish (Iwaki Glass, Tokyo, Japan) containing 4 × 105 cells suspended in 4 ml of medium. The dishes were incubated at 37°C for 7 days, and the cells were examined for inclusion bodies by Giemsa or IFA staining. If negative for both tests, the cells were trypsinized, resuspended in fresh medium, and incubated for another 7 days, and the assays were repeated for a total of three cycles.
TABLE 2.
Cross neutralization between MVC and CPV-2 strains
| Virus | Titer with rabbit immune serum against:
|
|||
|---|---|---|---|---|
| MVC strain:
|
CPV-2 strain p85 | |||
| GA3 | HM-6 | 97-114 | ||
| MVC | ||||
| GA3 | 10,240 | 10,240 | 2,560 | <10 |
| HM-6 | 10,240 | 20,480 | 10,240 | <10 |
| 97-047 | 5,120 | 10,240 | 10,240 | <10 |
| 97-114 | 1,280 | 5,120 | 5,120 | <10 |
| CPV-2 p85 | <10 | <10 | <10 | 10,240 |
Immune sera against MVC isolates HM-6 and 97-114.
Culture fluid of MDCK cells infected with the isolate HM-6 or 97-114, obtained as described above, was concentrated approximately 100 times by ultracentrifugation (100,000 × g for 3 h), and cell debris was eliminated by sucrose (40%, wt/vol) cushion centrifugation of the concentrate. A pH 8.0 Tris-EDTA buffer was used throughout the concentration and purification steps. The resultant purified viruses were mixed in an equal volume with either Freund's complete adjuvant for the initial inoculation the incomplete adjuvant for the second inoculation. Two rabbits per isolate were immunized on the following schedule. Two doses of adjuvanted virus (1 ml) were inoculated intramuscularly with a 3-week interval between inoculations. Two weeks after the second inoculation, booster inoculations of virus, without adjuvant, were given intravenously two times, 2 weeks apart. The immune serum was then obtained 1 week after the last inoculation.
Serum samples.
During the period from January 1999 to November 2001, serum samples from 241 dogs seen at the Teaching Animal Hospitals of Hokkaido, Tokyo, and Yamaguchi Universities were used for seroepidemiology. In addition, samples were obtained from eight dogs presented for postmortem examination at the Veterinary Pathology Laboratory of Azabu University. As healthy controls, 47 samples from two different commercial specific-pathogen-free (SPF) dog colonies were used. In all, 296 sera were examined for MVC antibodies.
HA and HI assays.
Hemagglutination (HA) and HA inhibition (HI) assays for MVC were performed by using procedures described previously (2, 10). The HI assay was used for elucidating antigenic relations between MVC and CPV-2 strains and for serological studies. Briefly, HA antigens were prepared from virus-infected culture fluid, concentrated 10 to 100 times by ultracentrifugation, and then suspended in pH 8.8 Veronal buffer. The Veronal buffer was prepared as follows; a mixture of 50 ml of 0.2 M sodium barbitone and 4 ml of 0.2 M HCl was diluted to a total of 200 ml with distilled water. Chilled barbitone-acetate buffer (BBS) (pH 6.6 to 6.8) containing 0.1% bovine serum albumin, 0.15 M NaCl, 1.5 mM Ca2+, and 0.75 mM Mg2+ was used as the diluent for both antigen and sera in a 96-well V-bottom plate (Iwaki Glass). Fresh rhesus monkey erythrocytes were used at a 0.5% cell concentration in BBS. Both antigen and erythrocyte suspensions in BBS were prepared just prior to use. Reactions were performed at 4°C. HA titers were read as the reciprocal of the highest antigen dilution showing complete HA, and HI titers were defined as the reciprocal of the highest serum dilution completely inhibiting HA. HA and HI assays for CPV-2 were performed as described previously (12).
Neutralization assay.
By using polyclonal immune sera against MVC (GA3, HM-6, and 97-114 strains) and CPV-2 (p85 strain), neutralizing antigenic relationships between the isolates and the reference parvovirus strains were examined. Reaction mixtures contained 100 μl of each serum dilution and 100 μl of virus fluid containing approximately 100 50% tissue culture infective doses in a 96-well flat-bottom plate (Iwaki Glass). The reaction mixtures were incubated at 37°C for 1 h, and 50 μl of each mixture was inoculated into a Lab-Tek II Chamber Slide well (Nalge Nunc International, Naperville, Ill.) containing 4 × 104 MDCK cells in 0.4 ml of Eagle's minimal essential medium with 7.5% fetal bovine serum. Two chambers were used for each serum dilution. The slides were incubated at 37°C for 7 days and then stained by IFA. The titer was defined as the reciprocal of the highest serum dilution at which virus growth was completely inhibited.
PCR amplification.
An MVC-specific DNA fragment was amplified by using primers designed according to the MVC genomic sequence (15): primer 227, 5′-GCGAATTCGTGGTATGCACCTATATACAACGGAC-3′ (identical to nucleotides 3506 to 3539) and primer 226, 5′-CGGGATCCGGATGCGACATAGGCAGAGTTCCATC-3′ (complementary to nucleotides 4772 to 4739). The clinical specimens were routinely tested undiluted and at 1:10 dilutions made with sterile distilled water. Each sample (0.5 μl) was then subjected to PCR. Reactions were performed in a final volume of 50 μl, which contained the following: 5 μl of 10× PCR buffer (Takara, Tokyo, Japan), 3 μl of 25 mM MgCl2, 4 μl of 2.5 mM deoxynucleoside triphosphate mixture, 0.5 μl of each primer (50 μM), 0.25 μl (2.5U) of a recombinant Taq DNA polymerase (Takara), and 37.25 μl of distilled water. After the mixtures had been incubated at 94°C for 5 min, amplification was performed for 30 cycles in a Trio-ThermoBlock (Biometra Inc., Tampa, Fla.). One amplification cycle consisted of denaturation at 94°C, primer annealing at 55°C, and extension at 72°C, and each step was done for 30 s. The final extension reaction was performed at 72°C for 5 min. Ten-microliter amounts of the reaction products were run on a 2% (wt/vol) agarose gel with Tris-acetate-EDTA buffer. A fragment of 1,266 bp was expected from the MVC sequence.
Sequencing.
Nucleotide sequencing of the PCR product was performed by a method described previously (11) Briefly, the product was purified with the Wizard PCR Preps DNA purification system (Promega, Madison, Wis.). TA cloning was then performed with the Regular pT7Blue(R)T-vector kit (Novagen, Madison, Wis.), and ligation mixtures were transformed into Epicurian Coli XL2-Blue MRF′ competent cells (Stratagene, La Jolla, Calif.). For each sample, 10 plasmids containing the PCR product were purified with the Wizard Minipreps DNA purification system (Promega), sequenced with Cy5-labeled M13 primers, and further sequenced with Cy5-labeled primers designed from sequences that were obtained by using the AutoRead sequencing kit (Amersham Pharmacia Biotech, Tokyo, Japan). Nucleic acid sequences were translated into amino acid sequences, which were analyzed by using Genetyx-Mac, version 8.5 (Software Development Co., Tokyo, Japan).
Nucleotide sequence accession numbers.
The nucleotide sequence data produced in this paper have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence database under accession no. AB079376, AB079377, AB079378, and AB079379 for strains HM-6, 97-047, 97-114, and 12D, respectively.
RESULTS
PCR detection of MVC in clinical specimens.
Four specimens, designated 97-047, 97-114, 12D, and HM-6, were shown to be positive by PCR (Fig. 1). The first three specimens were from Japanese dogs, and HM-6 was from a dog imported from the Republic of Korea (8). In total, 4 out of 346 dogs were found to be MVC positive, and the positive rate was 1.2%. As shown in Table 1, specimens 97-047, 97-114, and HM-6 were rectal swabs of diarrheic puppies; specimen 12D was from the lung of a puppy that died from canine distemper. The age of puppy 97-114 was unknown; the others were between 2.5 and 4 months old.
FIG. 1.
PCR detection of MVC in clinical specimens. A fragment of 1,266 bp was expected for MVC-positive samples. Lanes: 1, 100-bp ladder size markers; 2 and 10, reference MVC strain GA3; 3 to 6, swab extracts of representative positive clinical samples 12D, 97-047, 97-114, and HM-6, respectively; 7 and 8, swab extracts of representative negative clinical samples 97-023 and 97-060, respectively; 9, reference CPV-2b strain p85.
TABLE 1.
MVC-positive clinical specimens
| Specimen (site)a | Yr and place of collection | Age and clinical signs of animal | Virus isolation in:
|
Other agent(s) detectedb | |
|---|---|---|---|---|---|
| MDCK cells | WRCC cells | ||||
| 97-047 (rectum) | 1997; Okayama, Japan | 3 mo; diarrhea, anorexia | + | − | CCV |
| 97-114 (rectum) | 1997; Aomori, Japan | Puppy; diarrhea | + | + | CPV-2 |
| 12D (lung) | 1999; Tokyo, Japan | 2.5 mo; respiratory and nervous signs, diarrhea | − | − | CDV, CPV-2 |
| HM-6 (rectum) | 1999; importedc | 4 mo; respiratory signs, eye discharge, diarrhea | + | + | CDV |
Only rectal swabs were examined for specimens 97-047 and 97-114. In the case of 12D, lung was positive but conjunctival and tracheal swabs, thymus, cerebrum, and cerebellum were negative. In the case of HM-6, only rectal but not nasal and conjunctival swabs were positive.
Both CDV and CCV were examined by reverse transcription-PCR (1,11), and CPV-2 was examined by PCR (12).
A dog imported from the Republic of Korea. Swab samples were taken while the dog was quarantined upon arrival in Japan (8).
Isolation and identification of viruses from PCR-positive specimens.
Viruses were isolated from specimens 97-047, 97-114, and HM-6, but not from 12D in MDCK or WRCC cells (Table 1). No virus was recovered from specimen 97-047 in WRCC cells, but virus isolation succeeded when MDCK cells were used, and the isolate could also be passaged in WRCC cells thereafter. Typical inclusion bodies appeared in the second MDCK cultures of specimens 97-047 and HM-6 and in the third of specimen 97-114, and MVC-specific antigens were detected in the nuclei (Fig. 2). Inclusion bodies also were observed in WRCC cells infected with the isolates. Those culture fluids were also found to be PCR positive for MVC but negative for canine coronavirus (CCV), CDV, and CPV-2 by reverse transcription-PCR or PCR assays (references 1, 11, and 12 and data not shown).
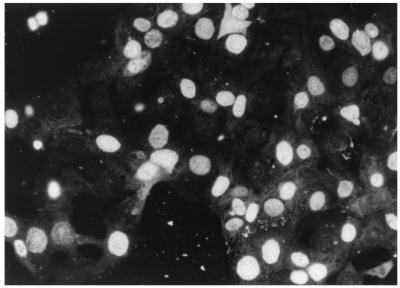
FIG. 2.
Indirect immunofluorescence staining of MDCK cells infected with MVC isolate HM-6 by using anti-MVC GA3 strain immune serum. Specific antigens were detected in the nuclei.
Antigenic relationships between MVC isolates.
Antigenic relationships determined by neutralization are shown in Table 2. No antigenic relationship was detected between MVC and CPV-2 strains. Among MVC strains, including GA3, no obvious antigenic difference was observed.
Table 3 shows antigenic relationships examined by HI assay. HA assay of MVC was found to be very difficult to perform, especially for the GA3 and 97-114 strains, so HI could not be achieved for those strains in the present study. The data shown in Table 3 indicate similar antigenic properties of the HM-6 and 97-047 strains but no relationship between those MVC strains and the CPV-2 p85 strain.
TABLE 3.
Cross HI between MVC and CPV-2 strains
| Virus | Titer with rabbit immune serum against:
|
|||
|---|---|---|---|---|
| MVC strain:
|
CPV-2 strain p85 | |||
| GA3 | HM-6 | 97-114 | ||
| MVC | ||||
| GA3a | ||||
| HM-6 | 640 | 2,560 | 2,560 | <10 |
| 97-047 | 640 | 2,560 | 2,560 | <10 |
| 97-114a | ||||
| CPV-2 p85 | <10 | <10 | <10 | 640 |
No HA could be observed for strains GA3 and 97-114 under the conditions suitable for strains HM-6 and 97-047.
Nucleotide and amino acid sequences of the MVC-specific PCR products.
The sizes of PCR products from the four clinical specimens and the reference strain GA3 were identical, namely, 1,266 bp. In the cases of 97-047, 97-114, and HM-6, the same PCR products could again be amplified from each virus grown in MDCK cells. Figure 3 shows the predicted amino acid sequence alignment; only a portion (251 residues) could be presented for strains 97-114 and 12D because of a stop codon at nucleotide 790 of the their PCR products. Both nucleotide and amino acid sequence similarities among MVC strains are presented in Table 4; they were calculated from the nucleotide sequences of the PCR products, excluding the primer positions, and the amino acid sequences shown in Fig. 3, respectively. Including the reference GA3 strain, they showed high degrees of identity for nucleotide (97.8 to 99.3%) as well as amino acid (97.2 to 99.6%) sequences.
FIG. 3.
Deduced amino acid sequence alignment of the PCR products. Four hundred residues are shown for strains GA3, 97-047, and HM-6, but only 251 residues were available for strains 97-114 and 12D (see Table 4, footnote a). Dots indicate positions of sequence identity with the MVC GA3 strain.
TABLE 4.
Nucleotide and predicted amino acid identities between the PCR products of MVC strains
| Strain | % Identitya with strain:
|
||||
|---|---|---|---|---|---|
| GA3 | 97-047 | HM-6 | 97-114 | 12D | |
| GA3 | 97.8 | 98.1 | 98.2 | 98.2 | |
| 97-047 | 98.3 | 98.8 | 98.5 | 98.7 | |
| HM-6 | 98.0 | 99.5 | 98.7 | 98.9 | |
| 97-114 | 97.6 | 98.4 | 98.8 | 99.3 | |
| 12D | 97.2 | 98.8 | 99.2 | 99.6 | |
Nucleotide identities are based on a 1,198-bp sequence, excluding the primer positions, and are shown in the upper half of the table. The sequence alignments of strains 97-114 and 12D used for calculation contained a stop codon at nucelotide position 790. Amino acid identities are based on the sequence presented in Fig. 3 and are shown in the lower half. For caluculation, 400 residues were used for strains GA3, 97-047, and HM-6, and 251 residues were used for strains 97-114 and 12D.
Seroprevalence in dog populations.
HA of the HM-6 strain was used as an antigen for the survey. Since HI titers of 1:10 to 1:20 were obtained in about 15% of the SPF dogs (Table 5), comparable to results in previous surveys conducted in the United States and Japan (5, 7), only titers of 1:40 or more were regarded as significant. As a result, a prevalence of 5% (12 of 238) was obtained for the field dog populations.
TABLE 5.
Serologic survey of MVC antibodies in dog populations
| Antibody titera | No. of sera collected at University of:
|
No. of sera from SPF dog colonies | |||
|---|---|---|---|---|---|
| Hokkaido | Tokyo | Azabu | Yamaguchi | ||
| Unknownb | 6 | 3 | 0 | 2 | 6 |
| <10 | 62 | 74 | 4 | 64 | 34 |
| 10 | 4 | 6 | 2 | 0 | 6 |
| 20 | 1 | 7 | 1 | 1 | 1 |
| 40 | 2 | 4 | 1 | 0 | 0 |
| 80 | 1 | 2 | 0 | 1 | 0 |
| 160 | 1 | 0 | 0 | 0 | 0 |
| >160 | 0 | 0 | 0 | 0 | 0 |
| Total no. of samples | 77 | 96 | 8 | 68 | 47 |
| Mean antibody titer | 1:25.2 | 1:21.5 | 1:16.8 | 1:40 | 1:11 |
| Antibody positive rate (%)c | 5.6 (4/71) | 6.5 (6/93) | 12.5 (1/8) | 1.5 (1/66) | 0 (0/41) |
Antibody titers were determined by an HI assay using strain HM-6.
No titer was obtained by nonspecific agglutination of the serum sample.
Titers of 1:40 or more were considered significant.
DISCUSSION
Until recently, MVC has been regarded as a novel and less virulent parvovirus of dogs, with a limited geographical distribution (i.e., inside the United States). The most probable reason for finding such an epidemiological peculiarity may have been the fastidious nature of MVC in vitro (2, 3). It was believed that MVC can grow only in WRCC cells, established from a subdermoid cyst of an irradiated male dog (2), and this cell line has not been distributed extensively. Thus far, studies demonstrating MVC have been reported only from European laboratories where specific antibodies and WRCC cells are available (9, 14, 16).
About a decade ago, a seroepidemiological survey was conducted for 266 dogs from the Tokai area of Japan, in which it was found that 41 dogs (15.4%) had HI antibody against MVC with a titer range of 1:40 to 1:1,280 (7). It was serologically confirmed by the present study that MVC has circulated among dogs for at least a decade now, but direct evidence for MVC has not been presented. This is the first report demonstrating MVC from Asian domestic dogs. The present report describes a PCR assay based on the genomic information for MVC. Although it is uncertain if the assay could detect all cases of MVC infections efficiently, at least four puppies (1.2%) were found to be PCR positive, and MVC was recovered from three of them, confirming that MVC is prevalent in domestic dogs in Japan.
In addition to this epidemiological finding, another biologically significant point found in the present study was that all MVC strains, including the reference U.S. strain GA3, could grow also in MDCK cells, albeit to various degrees. Susceptibility of MDCK cells to MVC has not been systematically studied, although in one report it was described that primary dog kidney did not support MVC growth in vitro (2). One isolate (HM-6) indeed grew more efficiently in MDCK cells than in WRCC cells, and this unexpected finding may promote the study of MVC elsewhere.
The pathogenic potential of MVC is not fully clear. In experimental studies, only fetuses and newborn puppies can be infected transplacentally or horizontally (4, 5, 10). A limited number of spontaneous field cases also suggested that only young puppies (less than 1 month old) are affected by MVC infection (6, 9, 14, 16). The clinical conditions of the four MVC-positive puppies described in the present study (Table 1) suggest that MVC may have a tropism to both the enteric and respiratory tracts of puppies, which is consistent with previous reports (5, 6, 9, 10, 14). However, more-pathogenic agents, such as CPV-2, CDV, and CCV, were also involved in the present MVC-positive field cases, obscuring the results. Therefore, no conclusion can be made about the pathogenic participation of MVC in the present cases. However, the case of sample 12D may be notable in a pathological respect; that is, only the lung, and not other parts (including upper respiratory tract) was positive, showing a possible preference of MVC for lung as seen in controlled experimental cases (5) or spontaneous cases (14). It will obviously be valuable to study the pathological profiles of different MVC strains experimentally.
Antigenic properties of MVC strains revealed in the present study were also of interest. In agreement with findings of an earlier study (10), we confirmed here that MVC strains are antigenically distinguishable from CPV-2 by both neutralization and HI assays (Tables 2 and 3). No obvious antigenic difference was observed among MVC strains by the assays using polyclonal immune sera, although analyses by HI assay were incomplete because no HA was available for some strains under the conditions of the present study. The reason for the lack of HA is presently unknown.
The complete genome of MVC is now being sequenced (C. Parrish, unpublished data), and MVC has a closer genetic relationship to bovine parvovirus than to other mammalian parvoviruses studied (3, 15). What is known so far is that the MVC genome sequence contains three large open reading frames (left, middle, and right), and the right-hand open reading frame encodes a 570-residue protein, starting with a methionine at nucleotide 3329, that is predicted to be the VP2 homologue (15). The primer set 227-226 was designed to amplify a portion of VP2 homologous protein gene. We demonstrated that it could detect MVC from clinical specimens. The PCR product from the lung of animal 12D was also considered to be MVC specific, and as a whole, the MVC strains examined here showed high sequence similarities to each other (Table 4). The reason why a stop codon appeared at nucleotide 790 of the PCR products of strains 97-114 and 12D is unknown, and further examinations are in progress.
When referring to the computerized genetic databases for homology to the limited sequences of our MVC strains obtained with the FASTA program, the best-matching sequences obtained were found to be of bovine parvovirus origin, as described by Schwartz et al. (15). Nucleotide (791 bp) and predicted amino acid (263 amino acids) identities of CPV-2 (strain CPV-N, accession no. M19296) to the corresponding parts of the MVC strains were lower (25.7 to 26.4% and 12.6 to 12.9%, respectively). In all cases, our results, together with previous reports (3, 10, 15), suggest that MVC is a distinct parvovirus species of canine origin with worldwide distribution.
In conclusion, MVC strains antigenically and genetically related to the previously known MVC strain GA3 were recovered from diseased puppies in Japan. Further clinical and pathological investigations will be needed to study MVC infections of dogs, since we must consider that MVC is more widely distributed than previously recognized.
Acknowledgments
Dog serum samples were kindly provided by Nobuo Sasaki and Yukinobu Tohya (Tokyo University) and Toshifumi Ohnishi and Ken Maeda (Yamaguchi University).
REFERENCES
- 1.Bandai, C., S. Ishiguro, N. Masuya, T. Hohdatsu, and M. Mochizuki. 1999. Canine coronavirus infections in Japan: virological and epidemiological aspects. J. Vet. Med. Sci. 61:731-736. [DOI] [PubMed] [Google Scholar]
- 2.Binn, L. N., E. C. Lazar, G. A. Eddy, and M. Kajima. 1970. Recovery and characterization of a minute virus of canines. Infect. Immun. 1:503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmichael, L. E. 23November1999, posting date. Neonatal pup diseases. Current status of canine herpesvirus (CHV) and minute virus of canines (MVC, canine parvovirus-type 1, CPV-1). In L. E. Carmichael (ed.), Document no. A0102.1199. Recent advances in canine infectious diseases. [Online.] International Veterinary Information Service, Ithaca, N.Y. http://www.ivis.org.
- 4.Carmichael, L. E., D. H. Schlafer, and A. Hashimoto. 1991. Pathogenicity of minute virus of canines (MVC) for the canine fetus. Cornell Vet. 81:151-171. [PubMed] [Google Scholar]
- 5.Carmichael, L. E., D. H. Schlafer, and A. Hashimoto. 1994. Minute virus of canines (MVC, canine parvovirus type-1): pathogenicity for pups and seroprevalence estimate. J. Vet. Diagn. Investig. 6:165-174. [DOI] [PubMed] [Google Scholar]
- 6.Harrison, L. R., E. L. Styer, A. R. Pursell, L. E. Carmichael, and J. C. Nietfeld. 1992. Fatal disease in nursing puppies associated with minute virus of canines. J. Vet. Diagn. Investig. 4:19-22. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto, A., M. Takiguchi, K. Hirai, H. Kida, and L. E. Carmichael. 2001. A serological survey of minute virus of canines (MVC; canine parvovirus type-1) in dogs in the Tokai area of Japan. Jpn. J. Vet. Res. 49:249-253. [PubMed] [Google Scholar]
- 8.Hashimoto, M., Y. Une, and M. Mochizuki. 2001. Hemagglutinin genotype profiles of canine distemper virus from domestic dogs in Japan. Arch. Virol. 146:149-155. [DOI] [PubMed] [Google Scholar]
- 9.Järplid, B., H. Johansson, and L. E. Carmichael. 1996. A fatal case of pup infection with minute virus of canines (MVC). J. Vet. Diagn. Investig. 8:484-487. [DOI] [PubMed] [Google Scholar]
- 10.Macartney, L., C. R. Parrish, L. N. Binn, and L. E. Carmichael. 1988. Characterization of minute virus of canines (MVC) and its pathogenicity for pups. Cornell Vet. 78:131-145. [PubMed] [Google Scholar]
- 11.Mochizuki, M., M. Hashimoto, S. Hagiwara, Y. Yoshida, and S. Ishiguro. 1999. Genotypes of canine distemper virus determined by analysis of the hemagglutinin genes of recent isolates from dogs in Japan. J. Clin. Microbiol. 37:2936-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mochizuki, M., S. Konishi, M. Ajiki, and T. Akaboshi. 1989. Comparison of feline parvovirus subspecific strains using monoclonal antibodies against a feline panleukopenia virus. Jpn. J. Vet. Sci. 51:264-272. [DOI] [PubMed] [Google Scholar]
- 13.Parrish, C. R. 1994. Parvoviruses: cats, dogs and mink, p. 1061-1067. In R. G. Webster and A. Granoff (ed.), Encyclopedia of virology. Academic Press Inc., San Diego, Calif.
- 14.Pratelli, A., D. Buonavoglia, M. Tempesta, F. Guarda, L. Carmichael, and C. Buonavoglia. 1999. Fatal canine parvovirus type-1 infection in pups from Italy. J. Vet. Diagn. Investig. 11:365-367. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz, D., B. L. Green, and C. R. Parrish. 14August1999, posting date. Molecular analysis and characterization of canine parvovirus type-1. In Proceedings for the Canine Infectious Diseases Workshop: from clinics to molecular pathogenesis. Document no. P0126.0899. [Online.] International Veterinary Information Service, Ithaca, N.Y. http://www.ivis.org.
- 16.Truyen, U., G. Wolf, and L. E. Carmichael. 1996. Das “andere” parvovirus: Erstbeschreibung des minute virus of canines (canines parvovirus typ 1) in Deutschland. Tierarztl. Prax. 24:511-513. [PubMed] [Google Scholar]