Abstract
An increased human parvovirus B19 infection rate has been observed in immunocompromised hosts. In this study, we sought to determine the prevalence of parvovirus B19 infection in adult cancer patients receiving multiple courses of systemic chemotherapy. From March 1999 through April 2000, 59 men and 68 women, with a median age of 49 (18 to 79) years, were enrolled in this study. They had received an average of 7.1 (4 to 32) courses of systemic chemotherapy. The median duration from the date of starting chemotherapy to the date of blood sampling was 11 (4 to 88) months. Serum B19 immunoglobulin G (IgG) and IgM levels were examined by an enzyme-linked immunosorbent assay, and B19 DNA was examined by a nested PCR. A group of 400 healthy blood donors served as the control group. The overall prevalences of anti-B19 IgG in adult cancer patients and healthy blood donors were 61.4 and 25.0%, respectively (P < 0.01). Anti-B19 IgM and B19 DNA were not detectable in these anti-B19 IgG-seropositive individuals. A further age-stratified comparison revealed that only patients younger than 40 years had a significantly higher anti-B19 IgG seropositivity rate than the controls (19 of 39 versus 53 of 310; P < 0.001). The increased prevalence of B19 infection in these 39 adult patients younger than 40 years might be clinically significant, since unexplained anemia, a common sequela of B19 infection, was detected in 3 of 20 seronegative patients (15.0%) and in 12 of 19 seropositive patients (63.2%) (P < 0.005). The results of this study suggest that adult patients younger than 40 years and receiving multiple courses of systemic chemotherapy may have a significantly increased risk of B19 infection. Prospective studies to define the time course and clinical consequence of B19 infection in this group of patients are needed.
Human parvovirus B19, the only parvovirus known to be pathogenic for humans, is a small DNA virus with a single-stranded linear genome which encodes one nonstructural protein, NS-1, and two viral capsid proteins, VP1 (83 kDa) and VP2 (58 kDa) (23). The virus exhibits a remarkable tropism for erythroid progenitor cells (6) and is frequently associated with anemia. Parvovirus B19 infection has also been implicated in a wide range of clinical manifestations, the outcome of which depends heavily on the physiologic status of the individual and the immune response against the virus (29).
In immunologically healthy hosts, B19 may cause a number of acute, generally self-limiting diseases, notably, fifth disease or erythema infectiosum in children, acute polyarthritis in adults, and aplastic crisis in patients with chronic hemolytic anemia such as sickle cell anemia or hereditary spherocytosis (3, 15, 24, 26). In pregnant women, B19 infection may result in the lysis of nucleated fetal red cells, hydrops fetalis, and subsequent spontaneous abortion and fetal death (16). B19 also has been found to be associated with glomerulonephritis, vasculitis, peripheral neuropathies, myocarditis, and fulminant hepatic failure (31). In immunocompromised hosts, B19 infection may persist and lead to chronic anemia, red cell aplasia and, less frequently, thrombocytopenia, neutropenia, and pancytopenia (1, 9, 19). In this study, we sought to clarify whether B19 infection poses a significant problem in cancer patients receiving multiple courses of chemotherapy.
MATERIALS AND METHODS
Patients.
Serum samples were collected from cancer patients who had previously received more than four courses of systemic chemotherapy at National Taiwan University Hospital. Serum samples from 400 healthy blood donors, randomly selected from a national blood bank, served as controls. The medical records of these patients were carefully reviewed. Pertinent clinicopathologic features were correlated with the serologic markers of B19 infection.
Serologic examinations.
All the serum samples had been stored at −20°C until tested for parvovirus B19 immunoglobulin G (IgG) antibody, IgM antibody, and DNA. For testing the prevalence of B19 infection in cancer patients, a parvovirus B19 IgG and IgM enzyme-linked immunosorbent assay (Biotrin International, Dublin, Ireland) was used according to the manufacturer's instructions. This enzyme-linked immunosorbent assay is a μ-capture sandwich enzyme immunoassay, and the antigen used is the purified parvovirus B19 recombinant VP2 protein. Following a wash step, peroxidase-labeled rabbit anti-human IgG or IgM is added and binds to the human parvovirus B19 IgG or IgM present. The whole complex is then detected by the addition of a substrate (tetramethylbenzidene), which turns blue in the presence of peroxidase. The results were expressed as the absorbance at 450 nm and interpreted as positive, equivocal, or negative by using the manufacturer's recommended cutoff values.
Purified DNA equivalent to 2 μl of serum was used for nested PCR as described by Fridell et al. (12). Serum samples with and without parvovirus B19 were prepared in the same way as the test serum samples and used as positive and negative controls. In addition, dilution buffer was used as a reagent control. The nested PCR product was a fragment of DNA sequence coding for VP1 of parvovirus B19. The first-round PCR was performed with nucleotides (nt) 2955 to 2974 and nt 3364 to 3349, yielding a product of 410 bp. The second-round PCR was performed with nt 3002 to 3020 and nt 3291 to 3272 as primers, giving a product of 289 bp. The final composition of the reaction mixture was 10 mM Tris HCl (pH 9.6), 2 mM MgCl2, 50 mM NaCl, 0.2 mM each deoxynucleoside triphosphate, 0.1 μM each primer, 1 U of Taq DNA polymerase (Perkin-Elmer Cetus, Foster City, Calif.), purified DNA (or diluted first-round PCR product), and water in a volume of 25 μl. Twenty-five cycles of both first- and second-round amplifications were performed under the following conditions: 95°C for 20 s, 55oC for 30 s, and 72oC for 30 s (Perkin-Elmer Cetus DNA thermal cycler 9600).
Unexplained anemia.
Most B19 infection-related symptoms and signs are usually nonspecific and hence left unrecognized; the presence of anemia, on the other hand, can be reliably determined from the medical records of patients receiving chemotherapy and was therefore chosen as the surrogate clinical marker of B19 infection. In this study, unexplained anemia was defined as a sudden drop of more than 2.5 g of hemoglobin/dl without a readily attributable etiology, such as acute and chronic blood loss, accelerated red blood cell destruction, iron deficiency, vitamin B12 deficiency, renal insufficiency, drug-induced marrow suppression, and tumor involvement of the marrow.
Statistical analysis.
The relationship between parvovirus B19 infection and clinicopathologic variables, including sex, age, history of blood transfusion, duration, regimen, and course of chemotherapy, and cancer type, was evaluated by the χ2 test, Fisher's exact test, or the t test. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed with the use of SAS software (SAS Institute, Cary, N.C.).
RESULTS
Clinicopathologic features of the patients.
From March 1999 through April 2000, a total of 127 cancer patients were enrolled in this study. Pertinent clinicopathologic features of the patients are tabulated in Table 1. There were 59 men and 68 women, with a median age of 49 (18 to 79) years. They received an average of 7.1 (4 to 32) courses of systemic chemotherapy. The median duration from the date of starting chemotherapy to the date of blood sampling was 11 (4 to 88) months. The seropositive patients were significantly older than the seronegative patients; no other significant difference in clinicopathologic features was detected.
TABLE 1.
Pertinent clinicopathologic features of all patients
| Variable | B19 IgGa
|
Pd | |
|---|---|---|---|
| Positive | Negative | ||
| Patient sample | |||
| Total | 78 (61.4) | 49 (38.6) | |
| Male | 41 (69.5) | 18 (30.5) | 0.08 |
| Female | 37 (54.4) | 31 (45.6) | |
| Age (yr)e | 52.5 ± 14.9 | 43.8 ± 14.7 | 0.002 |
| Cancer category | 0.21 | ||
| Gastrointestinal | 26 (66.7) | 13 (33.3) | 0.42 |
| Breast | 12 (48.0) | 13 (52.0) | 0.12 |
| Lung | 18 (75.0) | 6 (25.0) | 0.13 |
| Other malignanciesb | 22 (56.4) | 17 (33.6) | 0.77 |
| History of blood transfusion | 53 (63.9) | 30 (36.1) | 0.44 |
| No. of courses of chemotherapye | 7.4 ± 4.4 | 6.6 ± 2.7 | 0.23 |
| Mo before samplingc,e | 15.4 ± 12.6 | 13.2 ± 13.5 | 0.15 |
Reported as number (percentage) of patients, unless otherwise indicated.
Other malignancies: Non-Hodgkin's lymphoma, nasopharyngeal cancer, hepatocellular carcinoma, germ cell tumor, and sarcoma.
Time from date of starting chemotherapy to date of blood sampling.
The relationships between parvovirus B19 infection and the given clinicopathologic variables were evaluated by statistical analysis.
Values are means ± SD.
Prevalence of B19 infection.
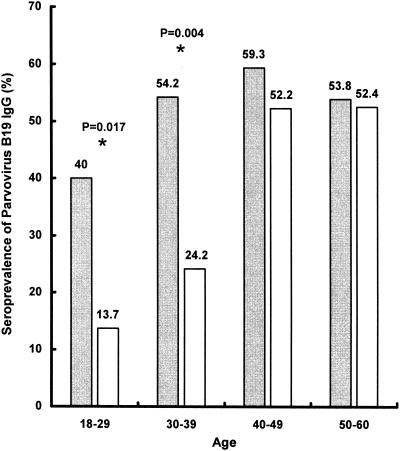
Parvovirus B19 IgG antibodies were detected in 78 (61.4%) of the 127 cancer patients and in 100 (25%) of the 400 healthy blood donors. B19 IgM antibodies and B19 DNA were not detected in any of the patients or donors. A further age-stratified comparison of B19 infection between cancer patients and healthy blood donors is shown in Fig. 1. The higher seropositivity rate for B19 IgG was significantly increased only in cancer patients younger than 40 years.
FIG. 1.
Age-stratified comparison of B19 IgG seroprevalence rates between cancer patients (gray bars) and healthy blood donors (white bars). An asterisk indicates a statistically significant difference. P values were calculated by the χ2 test or Fisher's exact test.
Association of B19 with unexplained anemia.
A further analysis of the clinicopathologic variables in young cancer patients revealed a significantly higher incidence of unexplained anemia in seropositive patients (Table 2). Unexplained anemia was detected in 3 of 20 seronegative and 12 of 19 seropositive patients (P < 0.005). Eight patients presented with unexplained anemia 3 to 4 months following chemotherapy. The mean drop in the hemoglobin level in the 12 seropositive patients was 3.7 ± 1.2 mg/dl (value is the mean ± the standard deviation). The mean duration between the date of diagnosis of unexplained anemia and the date of blood sampling in this study was 6.5 ± 2.7 months. It is important that the seropositive young cancer patients had a significantly longer hospital stay than the seronegative patients. Other clinicopathologic features, including the frequency of blood transfusion and the number of courses of chemotherapy, were not significantly different between the two groups (Table 2). In patients older than 40 years, the history of blood transfusion also was not significantly different between the two groups (P = 0.83).
TABLE 2.
Pertinent clinicopathologic features of patients younger than 40 years
| Variable | B19 IgGa
|
P | |
|---|---|---|---|
| Positive | Negative | ||
| Patient sample | |||
| Total | 19 (48.7) | 20 (51.3) | |
| Female | 8 (47.1) | 9 (52.9) | 0.86 |
| Male | 11 (50.0) | 11 (50.0) | |
| Age (yr)e | 32.4 ± 6.2 | 29.3 ± 7.4 | 0.16 |
| Cancer category | 0.32 | ||
| Gastrointestinal | 8 (66.7) | 4 (33.3) | 0.43 |
| Breast | 1 (20.0) | 4 (80.0) | 0.17 |
| Lung | 1 (33.6) | 2 (66.7) | 0.58 |
| Other malignanciesb | 9 (47.4) | 10 (52.6) | 0.88 |
| History of blood transfusion | 11 (52.4) | 10 (47.6) | 0.62 |
| No. of courses of chemotherapye | 6.7 ± 3.5 | 6.5 ± 2.9 | 0.78 |
| Mo before samplingc,e | 13.3 ± 8.0 | 9.5 ± 4.7 | 0.07 |
| Days in hospitald,e | 74.3 ± 74.4 | 32.1 ± 23.4 | 0.02 |
| Unexplained anemia | 12 (63.2) | 3 (15.0) | 0.002 |
Reported as number (percentage) of patients, unless otherwise indicated.
Other malignancies: Non-Hodgkin's lymphoma, nasopharyngeal cancer, hepatocellular carcinoma, germ cell tumor, and sarcoma.
Time from date of starting chemotherapy to date of blood sampling.
Total days of hospitalization from date of starting chemotherapy to date of blood sampling.
Values are means ± SD.
DISCUSSION
This study suggests that adult patients who are younger than 40 years and are receiving multiple courses of chemotherapy are at increased risk of parvovirus B19 infection. B19 infection in this group of patients may cause important sequelae, as exemplified in this series by the significantly higher incidence of unexplained anemia, which is a common complication of B19 infection. To date, parvovirus B19 has been recognized as an important cause of severe anemia in immunocompromised patients, including organ transplants recipients (1), patients with congenital and acquired immunodeficiencies (9, 19), and leukemia patients receiving maintenance or consolidation chemotherapy (18). This is the first report describing an increased prevalence of B19 infection among young cancer patients who have received multiple courses of chemotherapy. Since B19 infection usually presents with nonspecific symptoms and signs and is easily overlooked, a high degree of suspicion and a careful search for clinical evidence of infection are needed.
In general, an acute B19 infection is diagnosed by detecting B19 IgM antibodies or DNA, and past infection is diagnosed by detecting B19 IgG antibodies to VP1 and VP2 (2, 8). The mean duration of the IgM response is 4.8 months but can be shorter than 2 months (22). In contrast, the IgG response persists for years and, perhaps, for life. The persistence of serum B19 DNA after infection, as detected by PCR, may last for 2 to 4 months (22). Therefore, the laboratory evidence of B19 infection may go unnoticed if the infection is not clinically suspected. We could not detect B19 IgM and B19 DNA in our B19 IgG-seropositive patients, indicating that their B19 infection likely occurred at least 2 months before blood sampling. Unexplained anemia frequently developed 3 to 4 months after the start of chemotherapy in our patients, and the timing appeared to correlate approximately with the development of B19 infection in immunocompromised hosts (4, 7, 17, 25, 28, 30). Since the mean duration between the onset of unexplained anemia and the date of blood sampling was 6.5 ± 2.7 months in this study, IgM tests and nested PCR may both yield negative results for sera from patients with B19 infection that began 2 to 6 months earlier. Prospective studies to verify this hypothesis are needed.
Serologic studies of the prevalence of antibodies to the B19 virus showed that 60% or more of adults 16 to 40 years old in Western populations are seropositive (3, 10), whereas the antibody prevalence rates in young adults (15 to 39 years old) in Taiwan range from 16 to 36% (21). Lower prevalence rates in young adults have also been observed in Japan and Hong Kong (20, 32). In our study, the prevalence of anti-B19 IgG in adult cancer patients younger than 40 years was significantly higher than that in healthy blood donors of a similar age. It is well known that immune suppression predisposes individuals to unusual manifestations of B19 infection, such as persistent infection and chronic anemia (1, 9, 19). However, the reasons why only young adults were susceptible to B19 infection after multiple courses of chemotherapy remain to be defined. We suspect that nosocomial infection may have played a role in this scenario. In Taiwan, adult cancer patients younger than 40 years are more often hospitalized to receive chemotherapy and hence may have an increased risk of nosocomial infection with B19 by airborne transmission and person-to-person contact (2, 5, 27). Our data also indicate that the seropositive group of adult cancer patients younger than 40 years had a significantly longer hospital stay than the seronegative group. Nosocomial spread of B19 infection can occur in the late incubation period or early in the acute stage of disease and can even occur during the chronic phase of infection (11). However, it remains difficult to explain why there was no significant difference in the seropositivity rate between older cancer patients and older healthy blood donors. The already much higher rate of B19 seropositivity in the older age group may be one of the reasons (2, 10, 21, 32). Another possibility is that older individuals who remain uninfected by B19 may represent a population subgroup that is inherently more resistant to this virus.
Although B19 infection may be transmitted parenterally from contaminated blood products, the risk for acquiring B19 from single-unit and single-donor blood transfusions is low (13, 14). In this study, we have demonstrated that blood transfusion is not a risk factor for B19 infection in cancer patients undergoing systemic chemotherapy. This finding is in accordance with the report by Lim and colleagues, in which the prevalence of anti-B19 IgG among patients with Cooley's anemia after multiple transfusions was only 16.7%, similar to that for the same age group in the general population (20).
In conclusion, the seroprevalence of human parvovirus B19 infection is increased in adult patients who are younger than 40 years and receiving multiple courses of systemic chemotherapy. The infection may be associated with significant anemia. A high degree of clinical suspicion is the only way to make an early diagnosis of this easily overlooked infection. Prospective studies are needed to delineate the course of serologic and clinical responses of patients.
Acknowledgments
This work was supported by the National Health Research Institute, Taipei, Taiwan, and by research grants NTUH 87S2002 from National Taiwan University Hospital, Taipei, Taiwan, and NSC 89-2320-B-002-085 from the National Science Council, Taipei, Taiwan.
REFERENCES
- 1.Ahsan, N., M. J. Holman, C. D. Gocke, J. A. Groff, and H. C.Yang. 1997. Pure red cell aplasia due to parvovirus B19 infection in solid organ transplantation. Clin. Transpl. 11:265-270.M. J.L. J. [PubMed] [Google Scholar]
- 2.Anderson, L. J., C. Tsou, R. A. Parker, T. L. Chorba, H. Wulff, P. Tattersall, and P. P. Mortimer. 1986. Detection of antibodies and antigens of human parvovirus B19 by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:522-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, M. J., P. G. Higgins, L. R. Davis, J. S. Willman, S. E. Jones, I. M. Kidd, J. R. Pattison, and D. A. Tyrrell. 1985. Experimental parvoviral infection in humans. J. Infect. Dis. 152:257-265. [DOI] [PubMed] [Google Scholar]
- 4.Azzi, A., P. A. Macchia, C. Favre, M. Nardi, K. Zakrzewska, and O. B. Corsi. 1989. Aplastic crisis caused by B19 virus in a child during induction therapy for ALL. Haematologica 74:191-194. [PubMed] [Google Scholar]
- 5.Bell, L. M., S. J. Naides, P. Stoffman, R. L. Hodinka, and S. A. Plotkin. 1989. Human parvovirus B19 infection among hospital staff members after contact with infected patients. N. Engl. J. Med. 321:485-491. [DOI] [PubMed] [Google Scholar]
- 6.Brown, K. E., S. M. Anderson, and N. S. Young. 1993. Erythrocyte P antigen: cellular receptor of B19 parvovirus. Science 262:114-117. [DOI] [PubMed] [Google Scholar]
- 7.Carstensen, H., K. Ornvold, and B. I. Cohen. 1989. Human parvovirus B19 infection associated with prolonged erythroblastopenia in a leukemic child. Pediatr. Infect. Dis. J. 8:56. [DOI] [PubMed] [Google Scholar]
- 8.Cassinotti, P., M. Weitz, and G. Siegl. 1993. Human parvovirus B19 infections: routine diagnosis by a new nested polymerase chain reaction assay. J. Med. Virol. 40:228-234. [DOI] [PubMed] [Google Scholar]
- 9.Chernak, E., G. Dubin, D. Henry, S. J. Naides, R. L. Hodinka, R. R. MacGregor, and H. M. Friedman. 1995. Infection due to parvovirus B19 in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 20:170-173. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, B. J., and M. M. Buckley. 1988. The prevalence of antibody to human parvovirus B19 in England and Wales. J. Med. Microbiol. 25:151-153. [DOI] [PubMed] [Google Scholar]
- 11.Dowell, S. F., T. J. Torok, J. A. Thorp, J. Hedrick, D. D. Erdman, S. R. Zaki, C. J. Hinkle, W. L. Bayer, and L. J. Anderson. 1995. Parvovirus B19 infection in hospital workers: community or hospital acquisition? J. Infect. Dis. 172:1076-1079. [DOI] [PubMed] [Google Scholar]
- 12.Fridell, E., A. N. Bekassy, B. Larsson, and B. M. Eriksson. 1992. Polymerase chain reaction with double primer pairs for detection of human parvovirus B19 induced aplastic crises in family outbreaks. Scand. J. Infect. Dis. 24:275-282. [DOI] [PubMed] [Google Scholar]
- 13.Hino, M., O. Ishiko, K. L. Honda, T. Yamane, K. Ohta, T. Takubo, and N. Tatsumi. 2000. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br. J. Haematol. 108:194-195. [DOI] [PubMed] [Google Scholar]
- 14.Jordan, J., B. Tiangco, J. Kiss, and W. Koch. 1998. Human parvovirus B19: prevalence of viral DNA in volunteer blood donors and clinical outcomes of transfusion recipients. Vox Sang. 75:97-102. [PubMed] [Google Scholar]
- 15.Kelleher, J. F., N. L. Luban, P. P. Mortimer, and T. Kamimura. 1983. Human serum “parvovirus”: a specific cause of aplastic crisis in children with hereditary spherocytosis. J. Pediatr. 102:720-722. [DOI] [PubMed] [Google Scholar]
- 16.Kinney, J. S., L. J. Anderson, J. Farrar, R. A. Strikas, M. L. Kumar, R. M. Kliegman, J. L. Sever, E. S. Hurwitz, and R. K. Sikes. 1988. Risk of adverse outcomes of pregnancy after human parvovirus B infection. J. Infect. Dis. 157:663-667. [DOI] [PubMed] [Google Scholar]
- 17.Koch, W. C., G. Massey, C. E. Russell, and S. P. Adler. 1990. Manifestations and treatment of human parvovirus B19 infection in immunocompromised patients. J. Pediatr. 116:355-359. [DOI] [PubMed] [Google Scholar]
- 18.Kurtzman, G. J., B. Cohen, P. Meyers, A. Amunullah, and N. S. Young. 1988. Persistent B19 parvovirus infection as a cause of severe chronic anemia in children with acute lymphocytic leukemia. Lancet 2:1159-1162. [DOI] [PubMed] [Google Scholar]
- 19.Kurtzman, G. J., K. Ozawa, B. Cohen, G. Hanson, R. Oseas, and N. S. Young. 1987. Chronic bone marrow failure due to persistent B19 parvovirus infection. N. Engl. J. Med. 317:287-294. [DOI] [PubMed] [Google Scholar]
- 20.Lim, W. L., K. F. Wong, and C. S. Lau. 1997. Parvovirus B19 infection in Hong Kong. J. Infect. 35:247-249. [DOI] [PubMed] [Google Scholar]
- 21.Lin, K. H., S. L. You, C. J. Chen, C. F. Wang, C. S. Yang, and S. Yamazaki. 1999. Seroepidemiology of human parvovirus B19 in Taiwan. J. Med. Virol. 57:169-173. [PubMed] [Google Scholar]
- 22.Musiani, M., M. Zerbini, G. Gentilomi, M. Plazzi, G. Gallinella, and S. Venturoli. 1995. Parvovirus B19 clearance from peripheral blood after acute infection. J. Infect. Dis. 172:1360-1363. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa, K., and N. S. Young. 1987. Characterization of capsid and noncapsid proteins of B19 parvovirus propagated in human erythroid bone marrow culture. J. Virol. 61:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattison, J. R., S. E. Jones, J. Hodgson, L. R. Davis, J. M. White, C. E. Stroud, and L. Murtaza. 1981. Parvovirus infections and hypoplastic crisis in sickle-cell anemia. Lancet i:664-665. [DOI] [PubMed] [Google Scholar]
- 25.Rao, S. P., S. T. Miller, and B. J. Cohen. 1994. B19 parvovirus infection in children with malignant solid tumors receiving chemotherapy. Med. Pediatr. Oncol. 22:255-257. [DOI] [PubMed] [Google Scholar]
- 26.Reid, D. M., T. M. Reid, T. Brown, J. A. Rennie, and C. J. Eastmond. 1985. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet i:422-425. [DOI] [PubMed] [Google Scholar]
- 27.Seng, C., P. Watkins, D. Morse, S. P. Barrett, M. Zambon, N. Andrews, M. Atkins, S. Hall, Y. K. Lau, and B. J. Cohen. 1994. Parvovirus B19 outbreak on an adult ward. Epidemiol. Infect. 113:345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw, P. J., T. Eden, and B. J. Cohen. 1993. Parvovirus B19 as a cause of chronic anemia in rhabdomyosarcoma. Cancer 72:945-949. [DOI] [PubMed] [Google Scholar]
- 29.Shneerson, J. M., P. P. Mortimer, and E. M. Vandervelde. 1980. Febrile illness due to parvovirus. Br. Med. J. 280:1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, M. A., N. R. Shah, J. S. Lobel, P. J. Cera, G. W. Gary, and L. J. Anderson. 1988. Severe anemia caused by human parvovirus in a leukemia patient on maintenance chemotherapy. Clin. Paediatr. 27:383-386. [DOI] [PubMed] [Google Scholar]
- 31.Torok, T. J. 1997. Unusual clinical manifestations reported in patients with parvovirus B19 infection. Monogr. Virol. 20:61-92. [Google Scholar]
- 32.Yamashita, K., Y. Matsunaga, J. Taylor-Wiedeman, and S. Yamazaki. 1992. A significant age shift of the human parvovirus B19 antibody prevalence among young adults in Japan observed in a decade. Jpn. J. Med. Sci. Biol. 45:49-58. [DOI] [PubMed] [Google Scholar]