Abstract
Ovine enzootic abortion (OEA) resulting from infection of sheep and goats with Chlamydophila abortus is of major economic importance worldwide. Over the last 50 years the serological diagnosis of infection has been based mainly on the complement fixation test (CFT), which lacks both sensitivity and specificity because of cross-reactive antibodies to other gram-negative bacteria, including another common chlamydial pathogen of sheep, Chlamydophila pecorum. In the present study, a series of overlapping recombinant antigens representing the polymorphic outer membrane protein POMP90 of C. abortus was assessed by enzyme-linked immunosorbent assay (ELISA) with a panel of 143 serum samples from sheep experimentally infected with C. abortus, from sheep clinically free of OEA, and from specific-pathogen-free lambs experimentally infected with different subtypes of C. pecorum. The results were compared to those obtained by CFT and another recently described test, an indirect ELISA (iELISA) with the recombinant OMP91B (rOMP91B) fragment (rOMP91B iELISA) (D. Longbottom, E. Psarrou, M. Livingstone, and E. Vretou, FEMS Microbiol. Lett. 195:157-161, 2001). The rOMP90-3 and rOMP90-4 ELISAs were identified as being more sensitive and specific than CFT. Assays with both fragments were evaluated further with a panel of 294 field serum samples from flocks with documented histories of abortion, from flocks with no clinical histories of abortion but which had a high proportion of samples seropositive by CFT, and from animals with no histories of abortion but from which various C. pecorum subtypes had been isolated. ELISAs with both POMP90 fragments outperformed CFT with serum samples from C. pecorum-infected animals, producing no false-positive results. However, the ELISA with the rOMP90-4 fragment appeared to be more sensitive than the one with rOMP90-3, as it identified more of the OEA-positive samples. The ELISA with the rOMP90-4 fragment was also able to identify apparently healthy animals that were infected with an enteric strain of C. abortus in flocks that were probably infected with both enteric C. abortus and C. pecorum strains. The identification of animals infected with enteric C. abortus is extremely important in controlling the spread of OEA. Overall, the new rOMP90-4 ELISA was found to be a more sensitive and specific test than CFT for differentiating animals infected with C. abortus from those infected with C. pecorum.
The family Chlamydiaceae consists of obligate, intracellular gram-negative bacteria that cause a broad range of disease in both humans and animals, including sexually transmitted diseases, trachoma, psittacosis, abortion, pneumonia, conjunctivitis, enteritis, polyarthritis, encephalomyelitis, and metritis. The family Chlamydiaceae, which previously contained the single genus Chlamydia, has recently undergone reclassification into two genera, Chlamydia and Chlamydophila, and nine species (11). The most economically important animal pathogen of small ruminants is Chlamydophila abortus (previously classified as Chlamydia psittaci serotype 1), which causes abortion in sheep (ovine enzootic abortion [OEA]) and goats (10, 30). In the United Kingdom, chlamydial abortion accounts for about 50% of all diagnosed abortions, resulting in losses estimated to be in excess of £20 million (US$28 million) each year. C. abortus can also cause abortion in cattle (15, 27) and pigs (42) and represents a significant zoonotic risk to pregnant women (6, 39).
Diagnosis of C. abortus infection can be achieved by various antigen detection techniques, including histochemical staining of smears of placental tissue or vaginal swab samples and histochemical or immunological staining of chlamydial inclusions following isolation of the organism in cell culture (see the Office International des Epizooties website [http://www.oie.int/eng/normes/mmanual/A_summry.htm]). However, all of these tests are dependent on the acquisition of good-quality, well-preserved diagnostic material or the availability of specialist culturing facilities and expertise.
The simplest methods for detecting infected animals rely on the detection of chlamydial antibodies in animal sera, such as by immunofluorescence tests, enzyme-linked immunosorbent assays (ELISAs), and the complement fixation test (CFT), which is the procedure most widely used in veterinary laboratories. In the United Kingdom, CFT, which is based on the genus-specific lipopolysaccharide (LPS), is used by the national veterinary laboratories, which operate the Premium Health Scheme for Sheep in Scotland and the Sheep and Goat Health Scheme in England and Wales and which certify that flocks are free of OEA. However, diagnosis of OEA by CFT is complicated because of the presence of cross-reactive antibodies resulting from other infections, such as inapparent enteric infections caused by another chlamydial species, Chlamydophila pecorum, and because of the presence of other serologically cross-reactive gram-negative bacteria. In addition to inapparent intestinal infections (9), C. pecorum can cause a variety of other conditions in small ruminants, such as polyarthritis, conjunctivitis, and pneumonia (3, 16, 35). Although infection with the enteric subtype of C. pecorum has been reported to be widespread in sheep (9), the prevalence of infection with the other subtypes of C. pecorum is unknown.
Several serodiagnostic tests based on chlamydial antigen preparations (1, 2, 8, 25) and purified chlamydial LPS (13, 36) have been reported; however, these tests lack specificity because the main components of these preparations, the major outer membrane protein (MOMP) and LPS, are the two most common cross-reactive chlamydial antigens. Recently, a competitive ELISA (cELISA) based on a C. abortus MOMP-specific monoclonal antibody (32) and an indirect ELISA (iELISA) based on a recombinant protein fragment of the C. abortus polymorphic outer membrane protein (POMP) POMP91B (rOMP91B iELISA) (22) have been shown to have improved specificities compared to that of CFT. The POMP proteins have been suggested to be important serodiagnostic antigen candidates because they have been shown to react strongly in immunoblotting experiments with sera from sheep infected with C. abortus (7, 12, 24, 33) but not with sera from sheep infected with C. pecorum (20, 33). They were also suggested to be important serodiagnostic antigen candidates because Southern blotting failed to detect any related C. abortus POMP gene sequences in various C. pecorum subtypes, indicating probable low degrees of homology of the sequences between species (23). The aim of this study was to develop and evaluate an improved iELISA for detecting C. abortus-specific antibodies based on recombinant protein fragments of another member of the POMP family, POMP90 (23), which has been shown to be expressed on the surface of both the elementary body (EB) and the reticulate body (21).
MATERIALS AND METHODS
Chlamydia.
C. abortus strain S26/3 was grown in McCoy cells (26). EBs were purified from infected cells by centrifugation through a continuous gradient of Urografin 340 (Schering Health Care Ltd., Burgess Hill, United Kingdom), and genomic DNA was isolated as described previously (26).
Plasmid construction and antigen production.
Twelve 200- to 300-bp overlapping fragments representing the entire pomp90 gene (excluding the predicted signal sequence; GenBank accession number U65943) were amplified by PCR with the Expand Long Template PCR system (Roche Molecular Biochemicals, Lewes, United Kingdom) and primers with engineered BamHI and EcoRI restriction enzyme sites to allow cloning in-frame into expression vector pGEX-4T-1 (Amersham Biosciences UK Limited, Little Chalfont, United Kingdom). Details about the primer pairs used to generate each fragment are shown in Table 1. Ligated products were used to transform Epicurian Coli XL-1 Blue competent cells (Stratagene, Amsterdam, The Netherlands), according to the instructions of the manufacturer. Recombinant plasmid DNA was purified with a QIAprep Spin Plasmid Miniprep kit (Qiagen Ltd., Crawley, United Kingdom), and the constructs were checked by sequencing on an ABI 377 automated DNA sequencer. Clones were expressed as soluble glutathione S-transferase (GST) fusion proteins by induction with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and incubation for 3 h at 30°C. The GST fusion proteins were affinity purified using glutathione sepharose beads as detailed in the Amersham Biosciences GST Gene Fusion System Handbook (Amersham Biosciences UK Limited). Recombinant proteins were analyzed by immunoblotting with pooled postabortion sheep sera and affinity-purified antibodies to the recombinant amino- or carboxy-terminal halves of POMP90 (21), as described previously (24). GST was also expressed from the empty vector (i.e., no insert) by the procedures described above for use as a GST background control in the ELISAs. Protein concentrations were determined with the BCA protein assay reagent (Perbio Science UK Ltd., Tattenhall, United Kingdom) with bovine serum albumin as the standard.
TABLE 1.
Primers used to generate pomp90 gene fragment constructs
| pomp90 gene fragment | Forward primera | Reverse primera | POMP90 fragmentsb |
|---|---|---|---|
| pomp90-1 | 5′-GCCTAGGATCCTCGAATTCTTTGAGCTT-3′ | 5′-CCCGAATTCTAGGAAGGTAAGGTTATC-3′ | 1Ser-71Leu |
| pomp90-2 | 5′-AGGGAAAGGATCCGGTCTAAAGAAAAGTTG-3′ | 5′-TGTATGAATTCGTAACCAGTTGTGCCTG-3′ | 56Gly-121Tyr |
| pomp90-3 | 5′-TTTTCAGGATCCTATTGTCCTCCAGGCA-3′ | 5′-GTGAATTCATCAGCATAAATAGCCCCG-3′ | 113Tyr-187Asp |
| pomp90-4 | 5′-TCTCAGAAGGATCCTCCACTTCAAAAGG-3′ | 5′-GATGAATTCGTTCCCATCGAAGGTA-3′ | 177Ser-238Asn |
| pomp90-5 | 5′-GAATGTAGCGGATCCGCTGATCTCG-3′ | 5′-ACCTGAGAATTCGATCTTTCCTGTATAA-3′ | 228Ala-301Ile |
| pomp90-6 | 5′-ACTGGGGGAGGATCCGATGAAC-3′ | 5′-TGGTCCCGAATTCCATGACAACGG-3′ | 285Asp-357Met |
| pomp90-7 | 5′-ATCTGGATCCGCAAAACAAGTAACGC-3′ | 5′-CATAAGCATTGAATTCAGCATCGA-3′ | 344Ala-415Ala |
| pomp90-8 | 5′-GCTAAACTCGGATCCAATACAGCAAGTC-3′ | 5′-TTCGGGAATTCAGGTCCTTGACGTTCTG-3′ | 399Asn-496Pro |
| pomp90-9 | 5′-AACAAACTGGATCCTCCCCTAACCC-3′ | 5′-GTTGGCAGAATTCCGCACTGAA-3′ | 488Ser-572Ala |
| pomp90-10 | 5′-GGATACGCTTTAGGATCCTACGCA-3′ | 5′-CACCCTTGAATTCTGCATACGTTG-3′ | 561Tyr-654Ala |
| pomp90-11 | 5′-CCAACATGACGACTGGATCCGCTCCTCG-3′ | 5′-AGAATAAGCAGCAGTGAATTCATAAGAAGC-3′ | 647Ala-740Tyr |
| pomp90-12 | 5′-TCTCTGCCTATCGGATCCAAGTTTGA-3′ | 5′-GGAACGGGGTAGGAATTCTGGATGAG-3′ | 728Lys-823Phe |
Boldface sequences indicate BamHI (forward primers) and EcoRI (reverse primers) restriction enzyme sites for cloning.
Fragment sequences are based on the published sequence of the predicted mature POMP90B protein (GenBank accession number U65943).
iELISA.
The iELISA procedure was performed essentially as described previously (22), with a few modifications. Briefly, 96-well microtiter plates (Immulon; Dynex Technologies, Ashford, United Kingdom) were coated overnight at 4°C with antigen at a concentration of 0.1 μg of protein/well in 0.1 M sodium carbonate/bicarbonate buffer (pH 9.6); odd-numbered rows were coated with GST, and even-numbered rows were coated with the GST-rOMP90 fragments or the GST-rOMP91B fragments. Following three washes with 0.05% Tween 20-phosphate-buffered saline (PBST; pH 7.4), the plates were blocked with 10% nonfat dried milk (NFDM) in bicarbonate coating buffer for 60 min at 37°C. The plates were washed as described above, and then sheep sera (diluted 1:100 in 5% NFDM-PBST) were added to the appropriate wells and the plates were incubated for 60 min at 37°C. After further washing, horseradish peroxidase-conjugated donkey anti-sheep immunoglobulin G (diluted 1:1,000 in 5% NFDM-PBST) was added, and the mixture was incubated for 60 min at 37°C. Bound antibody was detected with an o-phenylenediamine dihydrochloride peroxidase substrate set (Fast o-phenylenediamine dihydrochloride tablet set; Sigma Chemical Co., Poole, United Kingdom), according to the instructions of the manufacturer. The reaction was stopped after 10 min by the addition of 3 M sulfuric acid, and the absorbance at 492 nm was measured on a Labsystems iEMS MF microplate reader (Thermo Life Sciences, Hampshire, United Kingdom). Net optical density (OD) values were obtained by subtracting the absorbance values for the wells with GST from those for the wells with GST-rOMP90 or GST-rOMP91B fragments. The cutoff for the rOMP91B iELISA was previously determined to be 0.3 (22).
cELISA.
The cELISA procedure was performed as described previously (32) with anti-MOMP monoclonal antibody 188 (32, 40) as the competitor antibody and periodate-treated EB as the antigen. An inhibition value greater than 55% specifically identified OEA-infected animals (32).
CFT.
CFT was performed as described previously (1, 34). Samples were tested at twofold dilutions from 1/32 to 1/512. CFT titers were expressed as the highest serum dilution giving 50% or less hemolysis: 50% hemolysis was graded 2+, and 0% hemolysis was graded 4+. A titer of 4+ at a dilution of 1/32 or greater was assumed to be positive, whereas a titer of 2+ at a dilution of 1/32 was assumed to be equivocal. Equivocal results were counted as positive results for calculation purposes.
Animal sera.
The experimental and field sera used in this study were grouped as follows. Group 1 consisted of sera from 70 ewes that were experimentally infected at 70 to 75 days gestation with C. abortus isolate S26/3, as described by Anderson et al. (2). This group could be subdivided further into sera from 58 ewes that subsequently yielded heavily infected placentas or aborted (group 1A) and sera from 12 ewes that lambed normally with either lightly infected placentas or no placental lesions but that were shown to be positive by cell culture (group 1B). Group 2 consisted of sera from 64 sheep which were known through their participation in the Premium Health Scheme for Sheep to be clinically free of OEA and which were used as negative controls in experimental trials. None of these animals aborted or showed evidence of placental lesions, and no organisms could be recovered from any of their placentas by cell culture. Group 3 consisted of samples from nine specific-pathogen-free (SPF) lambs, three of which had been immunized with C. pecorum isolate P787 (an ovine arthritogenic isolate), two of which had been immunized with C. pecorum isolate 84-796 (an ovine conjunctival isolate), and four of which had been immunized with C. pecorum isolate 84-604 or W73 (ovine enteric isolates). The animals were immunized as described by Jones et al. (19). Group 4 comprised 38 field samples from a flock of ewes from Scotland in which there was clinical evidence of abortion. Group 5 comprised 32 field serum samples from a flock of ewes from Scotland with no previous clinical history of chlamydial abortion but in which individual animals had reacted positively by CFT. Group 6 consisted of 11 serum samples from a flock of ewes from the Republic of Ireland from which an enteric subtype of C. pecorum (subtype W73) (25) had been isolated and 30 samples from a herd of goats from New Zealand from which an arthritogenic subtype of C. pecorum had been isolated (J. O'Keefe, G. F. Mackereth, R. Kittleberger, W. Stanislawek, and D. Longbottom, unpublished data). Neither the flock nor the herd had a clinical history of abortion. Group 7 comprised 99 field samples from flocks of ewes from Greece with documented histories of OEA. Group 8 consisted of 84 serum samples from Greek flocks with no documented clinical histories of OEA but with a large number of samples with seropositive results by CFT and with the isolation of C. abortus from the feces of one positive reactor. All serum samples were randomly assigned a unique reference number to ensure that the tests were performed in a blinded manner.
RESULTS
In order to maximize the yield of soluble protein, the solubility of each recombinant GST-POMP90 fusion protein was assessed following induction at 30 or 37°C with 0.1 or 1 mM IPTG. Fragments POMP90-1 to POMP90-7 were the most soluble, with yields of 1 to 5 mg of protein per 800 ml of starting culture. POMP90-8 and POMP90-11 were considerably less soluble, although sufficient amounts of soluble protein (yields of 0.1 to 0.5 mg of protein per 800 ml of starting culture) could be purified for evaluation by ELISA. POMP90-9 and POMP90-10 were completely insoluble, and little expression could be achieved for POMP90-12 under any conditions. Three new 200-bp constructs representing the POMP90-9 and POMP90-10 gene fragments were produced in an attempt to improve solubility. Unfortunately, this failed to improve the yield of soluble protein, and consequently, these fragments were investigated no further. The purity and immunoreactivity of each of the remaining nine recombinant GST-POMP90 proteins were confirmed by staining of the proteins with Coomassie brilliant blue and immunoblot analysis with either pooled postabortion sera (22) or monospecific antisera to the expressed recombinant amino- and carboxy-terminal halves of POMP90 (21) (data not shown).
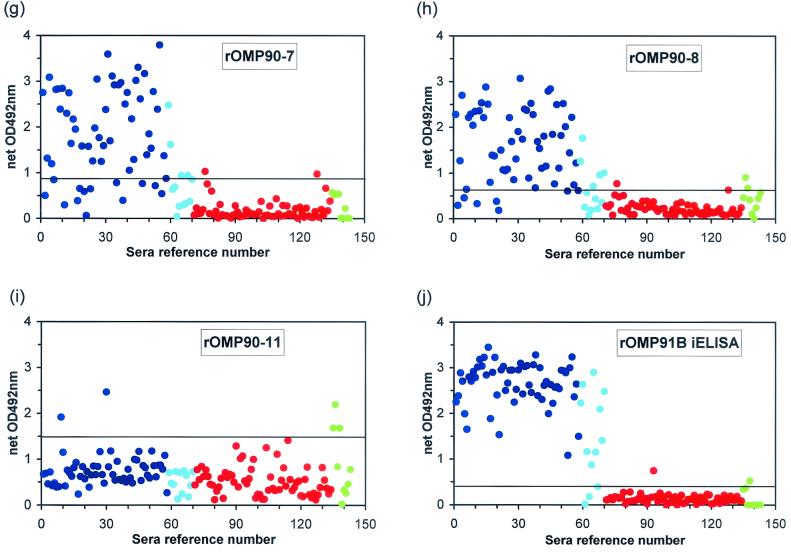
As a preliminary screen, each of the nine rOMP90 fragments was assessed by using a panel of 143 serum samples from sheep experimentally infected with C. abortus (group 1), from OEA-free sheep (group 2), and from SPF lambs experimentally infected with different subtypes of C. pecorum (group 3). The reactivities of each individual serum sample with each of the recombinant proteins are shown in Fig. 1. For comparison the results obtained by the rOMP91B iELISA (22) are also shown. The cutoff for each of the fragments (as shown in Fig. 1) was calculated as the mean net OD value for the group 2 sera plus 3 times the standard deviation. For rOMP90-4, the cutoff was calculated from the group 5 and group 6 OEA-free field sera (see later) because all of the group 2 serum samples were completely negative by ELISA with this fragment. The performance of the ELISA with each rOMP90 fragment compared with those of the rOMP91B iELISA and CFT is summarized in Table 2. Overall, the sensitivities (the minimum number of false-negative results in group 1) of the ELISAs with rOMP90 fragments 3, 4, 5, and 6 was either comparable to (rOMP90-5 and rOMP90-6) or exceeded (rOMP90-3 and rOMP90-4) the sensitivity of CFT. The rOMP90-3 ELISA performed the best and was as sensitive as the rOMP91B iELISA. In terms of the specificities (the minimum number of false-positive results in group 2) of the ELISAs with these four rOMP90 fragments, the assays with rOMP90-3 and rOMP90-4 were 100% specific and performed better than either CFT or the rOMP91B iELISA. The rOMP90-5 ELISA was as specific as both CFT and the rOMP91B iELISA, whereas the rOMP90-6 ELISA did not perform as well. Furthermore, the graphs in Fig. 1 show that of the assays with these four rOMP90 fragments, only the assays with rOMP90-3 (Fig. 1c) and rOMP90-4 (Fig. 1d) clearly differentiated the group 1 positive sera from the group 2 negative sera, comparable to the results obtained by the rOMP91B ELISA (Fig. 1j). Fragment rOMP90-5 also differentiated the two groups quite well (Fig. 1e), but in contrast to rOMP90-3 and rOMP90-4, the cutoff was very large and some of the group 3 C. pecorum-positive sera (Table 2 and Fig. 1e) were extremely positive with this fragment. On the other hand, the rOMP90-6 ELISA had a lower cutoff, but the positive OD values were considerably lower (Fig. 1f) than those of the rOMP90-3 and rOMP90-4 ELISAs, and again, some of the group 3 sera were also positive (Table 2 and Fig. 1f). Interestingly, all of the false-positive results obtained with the group 3 C. pecorum-positive sera were for samples with the arthritogenic and conjunctival subtypes, apart from one serum sample with the enteric subtype in the assay with rOMP90-5 (Table 2). The CFT performed the worst with the group 3 sera, with four of the five samples with arthritogenic or conjunctival subtypes being positive (Table 2). Overall, the rOMP90-3 and rOMP90-4 ELISAs performed better than CFT and the rOMP91B iELISA. The few false-negative results obtained by assays with rOMP90-3, rOMP90-4, and rOMP91B with the group 1 sera were all for animals that lambed normally with no placental lesions but that were positive by cell culture (group 1B): two of the samples were negative by all three tests, one was negative by both the rOMP90-3 and the rOMP90-4 ELISAs, and one was negative only by the rOMP90-4 ELISA. In addition to four false-negative results and one borderline positive result obtained with the group 1B sera by CFT, a further two results were negative and a further six results were equivocal or borderline with the group 1A sera.
FIG. 1.
Results of recombinant POMP90 ELISAs and rOMP91B iELISA with experimental sera. Recombinant antigens were screened with sera from sheep which were experimentally infected with C. abortus and which yielded heavily infected placentas or aborted (group 1A, serum samples 1 to 58 [dark blue circles]) or which lambed normally with lightly infected placentas or with placentas that were positive by cell culture (group 1B, serum samples 59 to 70 [light blue circles]); with sera from sheep clinically free of OEA (group 2, serum samples 71 to 134 [red circles]); and with sera from SPF lambs immunized with different subtypes of C. pecorum (group 3, sheep 135 to 143 [green circles]). For experimental details, see Materials and Methods. The cutoff for each fragment is depicted on each graph by a horizontal line.
TABLE 2.
Evaluation of assays with recombinant POMP90 fragments with experimental seraa
| Test | Sensitivity (%) with group 1 sera | Specificity (%) with group 2 sera | No. of positive samples in group 3
|
|
|---|---|---|---|---|
| Arth/conj | Enteric | |||
| rOMP90-1 ELISA | 47.1 | 96.9 | 1 | 0 |
| rOMP90-2 ELISA | 32.9 | 100 | 2 | 0 |
| rOMP90-3 ELISA | 95.7 | 100 | 0 | 0 |
| rOMP90-4 ELISA | 94.3 | 100 | 0 | 0 |
| rOMP90-5 ELISA | 91.4 | 98.4 | 3 | 1 |
| rOMP90-6 ELISA | 91.4 | 96.9 | 2 | 0 |
| rOMP90-7 ELISA | 72.9 | 96.9 | 0 | 0 |
| rOMP90-8 ELISA | 78.6 | 98.4 | 2 | 0 |
| rOMP90-11 ELISA | 2.9 | 100 | 3 | 0 |
| rOMP91B iELISA | 95.7 | 98.4 | 1 | 0 |
| CFT | 91.4 | 98.4 | 4 | 0 |
Group 1, sera from experimentally infected ewes (n = 70); group 2, sera from OEA-free free flocks (n = 64); group 3, sera from SPF lambs vaccinated with arthritogenic or conjunctival (Arth/conj; n = 5) or enteric (n = 4) subtypes of C. pecorum. Sensitivity = [number of true positives/(number of true positives + number of false negatives)] × 100; specificity = [number of true negatives/(number of true negatives + number of false positives)] × 100.
Following the assessment of all the rOMP90 fragments, rOMP90-3 and rOMP90-4 were chosen for further analysis with a panel of 294 field serum samples (Table 3). All serum samples were also tested by CFT and either by the rOMP91B iELISA (groups 4 to 6) or by the cELISA (groups 7 and 8). rOMP90-4 identified 23 of the 38 serum samples from group 4 (which had evidence of OEA) as being positive, with the results agreeing well with those of both the rOMP91B iELISA and CFT, but the results are in contrast to those obtained by the rOMP90-3 ELISA, by which only 11 samples were positive.
TABLE 3.
Evaluation of fragments rOMP90-3 and rOMP90-4 with field sera
| Test | No. (%) of samples positivea
|
|||||
|---|---|---|---|---|---|---|
| Group 4 | Group 5 | Group 6
|
Group 7 | Group 8 | ||
| Arthritogenic | Enteric | |||||
| rOMP90-3 ELISA | 11 (28.9) | 0 (0) | 0 (0) | 0 | 32 (32.3) | 6 (7.1) |
| rOMP90-4 ELISA | 23 (60.5) | 0 (0) | 0 (0) | 0 | 49 (49.5) | 17 (20.2) |
| rOMP91B iELISA | 21 (55.3) | 1 (3.1) | 1 (3.3) | 0 | ND | ND |
| cELISA | NDb | ND | ND | ND | 68 (68.7) | 18 (21.4) |
| CFT | 25 (2c) (65.8) | 14 (4c) (43.8) | 14 (4c) (46.7) | 0 | 86 (18c) (86.9) | 44 (25c) (52.4) |
Group 4, sera from a flock of ewes with a documented history of OEA (n = 38); group 5, sera from a flock of ewes with no clinical history of OEA but with a large number of samples seropositive by CFT (n = 32); group 6, sera from a flock of ewes infected with an enteric subtype of C. pecorum (n = 11) and from a herd of goats infected with an arthritogenic subtype of C. pecorum (n = 30); group 7, sera from flocks of ewes with a documented history of OEA (n = 99); group 8, sera from flocks of ewes with no clinical history of OEA but with a large number of samples seropositive by CFT and with the isolation of C. abortus from the feces of one positive reactor (n = 84).
ND, not determined.
The number of samples with equivocal results included among the total number of samples with positive results (see Materials and Methods).
The results obtained with the group 5 and 6 sera are particularly interesting. The flock from which the group 5 sera were obtained was known through its history to be clinically free of OEA, whereas the flocks from which the group 6 sera were obtained were known to be infected with an arthritogenic or enteric subtype of C. pecorum. Both groups contained a large proportion of individual serum samples with low or borderline CFT-positive titers; in contrast, among these samples, only one sample in group 5 and one sample in group 6 were positive by the rOMP91B iELISA, and all samples were found to be negative by the rOMP90-3 and rOMP90-4 ELISAs. All of the serum samples in group 6 positive by CFT and the rOMP91B iELISA were from goats known to be infected with an arthritogenic subtype of C. pecorum.
The CFT results with the group 7 field sera from flocks of ewes with a documented history of OEA identified 86 of the 99 samples as positive. This is in contrast to the results of the cELISA, which identified 68 of the samples as positive, and is in particular contrast to the results of the ELISAs with the rOMP90-4 and rOMP90-3 fragments, which identified only 49 and 32 of the samples as positive, respectively. A similar pattern of results was also obtained with the group 8 sera from flocks of ewes with no clinical history of abortion but with a large number of samples with CFT-positive titers and with the isolation of C. abortus from the feces of one positive reactor. In this case, 31% more of the samples were found to be positive by CFT than by cELISA and the ELISA with the rOMP90-4 fragment, and 45% more of the samples were positive by CFT than by the ELISA with the rOMP90-3 fragment.
The concordances between the tests based on the results obtained with the individual experimental serum samples and the field serum samples are shown in Table 4. The concordances between the ELISAs with the rOMP90-3 and rOMP90-4 fragments and the rOMP91B iELISA (90.6 to 96.9%) were significantly better than those between the ELISA with the rOMP90-3 or rOMP90-4 fragment and the cELISA or CFT (66.6 to 72.3%). This result is largely accounted for by the proportionally large number of negative-positive correlations between rOMP90-3-rOMP90-4 ELISAs and CFT-cELISA. The best correlation was between the rOMP90-4 fragment and the rOMP91B iELISA.
TABLE 4.
Concordance of the results for the individual serum samples with the five serological tests
| Test and result | No. of samples with the indicated result by the following test:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| rOMP90-4
|
rOMP91B iELISA
|
cELISA
|
CFT
|
|||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| rOMP90-3 ELISA | ||||||||
| Positive | 115 | 1 | 78 | 0 | 32 | 6 | 111 | 5 |
| Negative | 40 | 281 | 15 | 161 | 54 | 91 | 141 | 180 |
| % Concordancea | 90.6 | 94.1 | 67.2 | 66.6 | ||||
| rOMP90-4 ELISA | ||||||||
| Positive | 87 | 2 | 47 | 19 | 143 | 12 | ||
| Negative | 6 | 159 | 39 | 78 | 109 | 173 | ||
| % Concordance | 96.9 | 68.3 | 72.3 | |||||
| rOMP91B iELISA | ||||||||
| Positive | 86 | 7 | ||||||
| Negative | 36 | 125 | ||||||
| % Concordance | 83.1 | |||||||
| cELISA | ||||||||
| Positive | 86 | 0 | ||||||
| Negative | 44 | 53 | ||||||
| % Concordance | 76.0 | |||||||
Concordance is the sum of positive-positive values and negative-negative values expressed as a percentage of the total number of serum samples.
DISCUSSION
In this study we have screened a series of overlapping recombinant protein fragments of POMP90 with a panel of sera obtained from ewes and SPF lambs experimentally infected with C. abortus and various C. pecorum subtypes, respectively, to identify a fragment that may be suitable for use in a serological test to specifically identify animals with OEA. The results obtained in assays with each recombinant antigen and the experimental sera were compared to those obtained by CFT, the most widely used serological test for the diagnosis of OEA, and the rOMP91B iELISA (22). Two fragments in particular, rOMP90-3 and rOMP90-4, were identified as being the most sensitive and specific, showing no cross-reactivity with sera from SPF lambs infected with different subtypes of C. pecorum. These two fragments represent contiguous regions within the amino half of POMP90, which has previously been shown in electron microscopy surface localization studies with monoclonal antibodies and affinity-purified polyclonal antibodies to be exposed on the surface of C. abortus EBs (21). The monoclonal antibodies used in that study have been shown to specifically bind to fragments 4 and 5 (E. Vretou, P. Giannikopoulou, D. Longbottom, and E. Psarrou, unpublished data).
Assays with fragments rOMP90-3 and rOMP90-4 performed better than CFT in terms of sensitivity with the sera from the experimentally infected ewes (group 1), identifying with certainty the results deemed to be equivocal by CFT. In terms of specificity, CFT performed almost equally as well as assays with fragments 3 and 4 with the sera from ewes with no clinical history of abortion (group 2); in contrast, false-positive results were obtained with the sera from SPF lambs experimentally infected with various C. pecorum subtypes (group 3). The false-positive results obtained by CFT and assays with some of the rOMP90 fragments with the group 3 C. pecorum-infected sera were almost uniquely obtained with sera infected with the conjunctival or arthritogenic subtypes rather than the enteric subtype. Clinically inapparent intestinal infections caused by the enteric subtypes of C. pecorum have been reported to be prevalent in both OEA-affected and unaffected flocks in the United Kingdom (17). Although it has been reported that the CFT antibody titers are low in these apparently healthy sheep (31, 37), in this study both the experimentally infected (group 3) and naturally infected (group 6) animals were found to be negative. This suggests that the lack of specificity of CFT in the field may be due to the arthritogenic and conjunctival subtypes rather than the more common enteric subtype, as suggested previously (19, 22). CFT also performed better than has been reported by other groups, in which the sensitivity has been reported to be lower with either naturally or experimentally infected sheep (5, 19, 25), although six borderline positive samples and two negative samples were obtained from ewes that either aborted or lambed with heavily infected placentas (group 1A). The specificity of CFT and the variability of CFT antibody titers, in which very low or borderline titers are produced in some animals following abortion, reinforce the arguments that the test should be used only for flocks and not for the diagnosis of infection in individual animals (30).
Following the identification of rOMP90-3 and rOMP90-4 as the most promising antigens in terms of sensitivity and specificity, they were then assessed further in assays with a panel of field sera from flocks with known clinical histories. The results with the field sera from the Scottish (group 4) and Greek (group 7) OEA-affected flocks indicate that the rOMP90-3 fragment is perhaps less sensitive than the rOMP90-4 fragment, as assays with the fragment detected considerably fewer positive samples. On the other hand, the results of the assay with rOMP90-4 agreed closely with those of the rOMP91B iELISA and CFT with the group 4 sera, although this was not the case with the group 7 sera, in which both the cELISA and CFT in particular identified many more animals as being positive for infection. In view of the poor specificity of CFT with the sera from animals experimentally infected (group 3) and naturally infected (group 6) with C. pecorum compared to the results of the rOMP90-4 iELISA, the results obtained with the group 7 sera indicate that the flocks are likely to be infected with both C. pecorum and C. abortus. Such mixed infections have been suggested to be a common occurrence in flocks (5). The CFT results with the group 3 and 6 sera also suggest that the ewes in group 5, which had no clinical history of abortion but which had large numbers of samples seropositive by CFT, are infected with C. pecorum and that this infection is more likely due to an arthritogenic or conjunctival subtype rather than an enteric subtype. This explanation is tempting when we consider that these subtypes appear to be less prevalent than the enteric subtype, which would agree with the low level of false-seropositive results observed in the field when testing for OEA. Another possibility is that the flock is infected with a nonpathogenic or commensal strain of C. abortus. Evidence of the existence of such strains in the feces of sheep from flocks with no clinical history of abortion is emerging (14, 28, 32), although the prevalence of these enteric strains and their ability to induce detectable levels of serum antibody are unknown at this time. Indeed such “avirulent” C. abortus strains, which have been isolated from the feces of clinically normal sheep with no history of abortion, have been reported in flocks in Greece (32). Serum samples from such sheep are represented in group 8, in which over 50% of the samples were positive by CFT, whereas 20% were positive by the cELISA and the assay with the rOMP90-4 fragment. Interestingly, 25 of the 44 samples with CFT-positive results were classified as having equivocal or borderline positive results, and the results for all 25 of these samples were determined to be within the 30 to 55% grey zone of the cELISA that has previously been attributed to either C. abortus or C. pecorum infection (32). Furthermore, 18 of the 25 samples with borderline CFT-positive results were determined to be negative by the assay with the rOMP90-4 fragment, suggesting that these samples were from C. pecorum-infected animals. The results for an additional two of the samples with borderline CFT-positive results were equivocal, just above the rOMP90-4 ELISA cutoff of 0.2, while the remaining five were positive. Of these five rOMP90-4 ELISA-positive samples, four (ODs, between 0.62 and 1.41) were also positive by the rOMP90-3 ELISA, while the fifth one (OD, 0.44) was negative by the rOMP90-3 ELISA. Overall, these results suggest that the flocks represented by the group 8 sera are infected with both enteric C. abortus and C. pecorum. The rOMP90-3 fragment again appears to be less sensitive than the rOMP90-4 fragment. As stated by Salti-Montesanto et al. (32), the identification of apparently healthy flocks infected with enteric C. abortus remains a major challenge to controlling OEA. Both the cELISA and the rOMP90-4 test appear to be sufficiently sensitive and specific for achieving this aim. It is interesting that in a recent study, when one of the enteric isolates was administered by oronasal inoculation to pregnant ewes, it resulted in abortion (38). Surprisingly, under the experimental conditions used, the enteric isolate was even more virulent than the control abortifacient isolate. It is not clear why infection with an enteric C. abortus isolate that has been carried silently in untreated sheep for years would be so invasive in pregnant ewes and result in abortion, although it should be noted that similar results have been observed following intravenous inoculation of pregnant ewes with enteric C. pecorum, which is considered nonpathogenic in nature (29, 31).
Recently, a commercial ELISA based on one of the C. abortus 90-kDa POMP antigens was evaluated with field sera (5). In the absence of defined sera from experimentally infected animals with complete clinical histories, like those used in this study, a comparative microimmunofluorescence test for differentiation of animals infected with C. abortus from those infected with C. pecorum was used. In contrast to the results presented here, however, it was found that a substantial proportion of the sera from animals infected with C. pecorum were positive, presumably because the antigen used in the commercial test is based on a fragment of one of the POMPs containing some of the cross-reactive epitopes. However, in view of the absence of the identity and the size of the recombinant antigen used in the test, it is impossible to be certain about this.
In summary, the results of this study show that an ELISA based on the rOMP90-4 fragment of the outer membrane protein POMP90 is suitable for the diagnosis of OEA. This rOMP90 iELISA is highly sensitive and specific, showing no cross-reactivity with animals infected with C. pecorum, which gives this test an important advantage over others based on cross-reactive antigens and epitopes, such as CFT and EB-based tests. The new test is rapid and simple to perform, cheap to produce, and free from subjective interpretation. Importantly, the test should also be suitable for use in the diagnosis of human infection resulting from exposure to infected animals. Such infections have repeatedly been reported and are of major concern to pregnant women, in whom they can cause abortion or stillbirth and severe or even fatal illness for the woman (4, 6, 18, 39, 41).
Acknowledgments
We thank B. Markey, J. O'Keefe, and the Scottish Agricultural College Veterinary Services for the field sera used in this study.
This work was funded by the Scottish Executive Environment and Rural Affairs Department and the Hellenic General Secretariat for Science and Technology.
REFERENCES
- 1.Anderson, I. E., A. J. Herring, G. E. Jones, J. C. Low, and A. Greig. 1995. Development and evaluation of an indirect ELISA to detect antibodies to abortion strains of Chlamydia psittaci in sheep sera. Vet. Microbiol. 43:1-12. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, I. E., T. W. Tan, G. E. Jones, and A. J. Herring. 1990. Efficacy against ovine enzootic abortion of an experimental vaccine containing purified elementary bodies of Chlamydia psittaci. Vet. Microbiol. 24:21-27. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, A. H., P. C. Goddard, A. J. Wilsmore, and G. J. Dagnell. 1987. A chlamydial keratoconjunctivitis in a British sheep flock. Vet. Rec. 120:238-239. [DOI] [PubMed] [Google Scholar]
- 4.Bonneau, D., M. Berthier, N. Malo, G. Magnin, and C. Bonneau. 1991. Infection materno-foetale humaine par Chlamydia psittaci transmise par la chevre: une nouvelle zoonose? Bull. Acad. Vet. Fr. 64:301-307. [PubMed] [Google Scholar]
- 5.Buendia, A. J., F. Cuello, L. Del Rio, M. C. Gallego, M. R. Caro, and J. Salinas. 2001. Field evaluation of a new commercially available ELISA based on a recombinant antigen for diagnosing Chlamydophila abortus (Chlamydia psittaci serotype 1) infection. Vet. Microbiol. 78:229-239. [DOI] [PubMed] [Google Scholar]
- 6.Buxton, D. 1986. Potential danger to pregnant women of Chlamydia psittaci from sheep. Vet. Rec. 118:510-511. [DOI] [PubMed] [Google Scholar]
- 7.Cevenini, R., M. Donati, E. Brocchi, F. De Simone, and M. La Placa. 1991. Partial characterization of an 89-kDa highly immunoreactive protein from Chlamydia psittaci A/22 causing ovine abortion. FEMS Microbiol. Lett. 65:111-115. [DOI] [PubMed] [Google Scholar]
- 8.Cevenini, R., A. Moroni, V. Sambri, S. Perini, and M. La Placa. 1989. Serological response to chlamydial infection in sheep, studied by enzyme-linked immunosorbent assay and immunoblotting. FEMS Microbiol. Immunol. 1:459-464. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson, M. J., and H. L. Philips. 1997. Isolation of faecal Chlamydia from sheep in Britain and their characterization by cultural properties. Vet. J. 153:307-310. [DOI] [PubMed] [Google Scholar]
- 10.Entrican, G., D. Buxton, and D. Longbottom. 2001. Chlamydial infection in sheep: immune control versus fetal pathology. J. R. Soc. Med. 94:273-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, K. D. E., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths, P. C., H. L. Philips, M. Dawson, and M. J. Clarkson. 1992. Antigenic and morphological differentiation of placental and intestinal isolates of Chlamydia psittaci of ovine origin. Vet. Microbiol. 30:165-177. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths, P. C., J. M. Plater, M. W. Horigan, M. P. M. Rose, C. Venables, and M. Dawson. 1996. Serological diagnosis of ovine enzootic abortion by comparative inclusion immunofluorescence assay, recombinant lipopolysaccharide enzyme-linked immunosorbent assay, and complement fixation test. J. Clin. Microbiol. 34:1512-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gut-Zangger, P., E. Vretou, E. Psarrou, A. Pospischil, and R. Thoma. 1999. Chlamydial abortion in sheep: possibilities of serological diagnosis with a competitive ELISA and insight into the epidemiologic situation in Switzerland. Schweiz. Arch. Tierh. 141:361-366. [PubMed] [Google Scholar]
- 15.Holliman, A., R. G. Daniel, J. G. Parr, P. C. Griffiths, B. J. Bevan, T. C. Martin, R. G. Hewinson, M. Dawson, and R. Munro. 1994. Chlamydiosis and abortion in a dairy herd. Vet. Rec. 134:500-502. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins, J. B., E. H. Stephenson, J. Storz, and R. E. Pierson. 1973. Conjunctivitis associated with chlamydial polyarthritis in lambs. J. Am. Vet. Med. Assoc. 163:1157-1160. [PubMed] [Google Scholar]
- 17.Johnson, F. W. A. 1984. Abortion-continuing flock problem: enteric infections in sheep associated with enzootic abortion (Chlamydia psittaci). Ir. Vet. News 1984. (December 10-11): 13-15. [Google Scholar]
- 18.Johnson, F. W. A., B. A. Matheson, H. Williams, A. G. Laing, V. Jandial, R. Davidson-Lamb, G. J. Halliday, D. Hobson, S. Y. Wong, K. M. Hadley, M. A. J. Moffat, and R. Postlethwaite. 1985. Abortion due to infection with Chlamydia psittaci in a sheep farmer's wife. Br. Med. J. 290:592-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, G. E., J. C. Low, J. Machell, and K. Armstrong. 1997. Comparison of five tests for the detection of antibodies against chlamydial (enzootic) abortion of ewes. Vet. Rec. 141:164-168. [DOI] [PubMed] [Google Scholar]
- 20.Layachi, K., A. Rodolakis, and D. Buzoni-Gatel. 1993. Identification by Western blots of virulence specific antigens of Chlamydia psittaci isolated from ewes. Vet. Res. 24:55-65. [PubMed] [Google Scholar]
- 21.Longbottom, D., J. Findlay, E. Vretou, and S. M. Dunbar. 1998. Immunoelectron microscopic localisation of the OMP90 family on the outer membrane surface of Chlamydia psittaci. FEMS Microbiol. Lett. 164:111-117. [DOI] [PubMed] [Google Scholar]
- 22.Longbottom, D., E. Psarrou, M. Livingstone, and E. Vretou. 2001. Diagnosis of ovine enzootic abortion using an indirect ELISA (rOMP91B iELISA) based on a recombinant protein fragment of the polymorphic outer membrane protein POMP91B of Chlamydophila abortus. FEMS Microbiol. Lett. 195:157-161. [DOI] [PubMed] [Google Scholar]
- 23.Longbottom, D., M. Russell, S. M. Dunbar, G. E. Jones, and A. J. Herring. 1998. Molecular cloning and characterization of the genes coding for the highly immunogenic cluster of 90-kilodalton envelope proteins from the Chlamydia psittaci subtype that causes abortion in sheep. Infect. Immun. 66:1317-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longbottom, D., M. Russell, G. E. Jones, F. A. Lainson, and A. J. Herring. 1996. Identification of a multigene family coding for the 90 kDa proteins of the ovine abortion subtype of Chlamydia psittaci. FEMS Microbiol. Lett. 142:277-281. [DOI] [PubMed] [Google Scholar]
- 25.Markey, B. K., M. S. McNulty, and D. Todd. 1993. Comparison of serological tests for the diagnosis of Chlamydia psittaci infection of sheep. Vet. Microbiol. 36:233-252. [DOI] [PubMed] [Google Scholar]
- 26.McClenaghan, M., A. J. Herring, and I. D. Aitken. 1984. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect. Immun. 45:384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabeya, M., K. Kaneko, H. Ogino, D. Nakabayashi, T. Watanabe, J. Murayama, K. Hayashi, H. Fukushi, T. Yamaguchi, K. Hirai, Y. Inaba, and M. Matumoto. 1991. Abortion in Japanese cows caused by Chlamydia psittaci. Vet. Microbiol. 29:261-265. [DOI] [PubMed] [Google Scholar]
- 28.Philips, H. L. 1993. Enteric and abortion chlamydia of sheep. Ph.D. thesis. University of Liverpool, Liverpool, United Kingdom.
- 29.Philips, H. L., and M. J. Clarkson. 1998. Experimental infection of pregnant ewes with Chlamydia pecorum. Infect. Immun. 66:2818-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodolakis, A., J. Salinas, and J. Papp. 1998. Recent advances on ovine chlamydial abortion. Vet. Res. 29:275-288. [PubMed] [Google Scholar]
- 31.Rodolakis, A., and A. Souriau. 1989. Variations in the virulence of strains of Chlamydia psittaci for pregnant ewes. Vet. Rec. 125:87-90. [DOI] [PubMed] [Google Scholar]
- 32.Salti-Montesanto, V., E. Tsoli, P. Papavassiliou, E. Psarrou, B. K. Markey, G. E. Jones, and E. Vretou. 1997. Diagnosis of ovine enzootic abortion, using a competitive ELISA based on monoclonal antibodies against variable segments 1 and 2 of the major outer membrane protein of Chlamydia psittaci serotype 1. Am. J. Vet. Res. 58:228-235. [PubMed] [Google Scholar]
- 33.Souriau, A., J. Salinas, C. De Sa, K. Layachi, and A. Rodolakis. 1994. Identification of subspecies- and serotype 1-specific epitopes on the 80- to 90-kilodalton protein region of Chlamydia psittaci that may be useful for diagnosis of chlamydial induced abortion. Am. J. Vet. Res. 55:510-514. [PubMed] [Google Scholar]
- 34.Stamp, J. T., J. A. A. Watt, and R. B. Cockburn. 1952. Enzootic abortion in ewes: complement fixation test. J. Comp. Pathol. 62:93-101. [DOI] [PubMed] [Google Scholar]
- 35.Stephenson, E. H., J. Storz, and J. B. Hopkins. 1974. Properties and frequency of isolation of chlamydiae from eyes of lambs with conjunctivitis and polyarthritis. Am. J. Vet. Res. 35:177-180. [PubMed] [Google Scholar]
- 36.Sting, R., and H. M. Hafez. 1992. Purification of Chlamydia psittaci antigen by affinity chromatography on polymyxin B agarose for use in the enzyme-linked immunosorbent assay (ELISA). Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 277:436-445. [DOI] [PubMed] [Google Scholar]
- 37.Storz, J. 1963. Superinfection of pregnant ewes latently infected with a psittacosis-lymphogranuloma agent. Cornell Vet. 53:469-480. [PubMed] [Google Scholar]
- 38.Tsakos, P., V. Siarkou, F. Guscetti, H. Chowdhury, N. Papaioannou, E. Vretou, and O. Papadopoulos. 2001. Experimental infection of pregnant ewes with enteric and abortion-source Chlamydophila abortus. Vet. Microbiol. 82:285-291. [DOI] [PubMed] [Google Scholar]
- 39.Villemonteix, P., G. Agius, B. Ducroz, J. Rouffineau, V. Plocoste, M. Castets, and G. Magnin. 1990. Pregnancy complicated by severe Chlamydia psittaci infection acquired from a goat flock: case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 37:91-94. [DOI] [PubMed] [Google Scholar]
- 40.Vretou, E., H. Loutrari, L. Mariani, K. Costelidou, P. Eliades, G. Conidou, S. Karamanou, O. Mangana, V. Siarkou, and O. Papadopoulos. 1996. Diversity among abortion strains of Chlamydia psittaci demonstrated by inclusion morphology, polypeptide profiles and monoclonal antibodies. Vet. Microbiol. 51:275-289. [DOI] [PubMed] [Google Scholar]
- 41.Wong, S. Y., E. S. Gray, D. Buxton, J. Finlayson, and F. W. A. Johnson. 1985. Acute placentitis and spontaneous abortion caused by Chlamydia psittaci of sheep origin: a histological and ultrastructural study. J. Clin. Pathol. 38:707-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woollen, N., E. K. Daniels, T. Yeary, H. W. Leipold, and R. M. Phillips. 1990. Chlamydial infection and perinatal mortality in a swine herd. J. Am. Vet. Med. Assoc. 197:600-601. [PubMed] [Google Scholar]