Abstract
False-positive Mycobacterium tuberculosis cultures are a benchmark for the quality of laboratory processes and patient care. We studied the incidence of false-positive cultures, risk factors, and consequences for patients during the period from 1993 to 2000 in 44 peripheral laboratories in The Netherlands. The national reference laboratory tested 8,889 M. tuberculosis isolates submitted by these laboratories. By definition, a culture was false positive (i) if the DNA fingerprint of the isolate was identical to that of an isolate from another patient processed within 7 days in the same laboratory, (ii) if the isolate was taken from a patient without clinical signs of tuberculosis, and/or (iii) if the false-positive test result was confirmed by the peripheral laboratory and/or the public health tuberculosis officer. We identified 213 false-positive cultures (2.4%). The overall incidence of false-positive cultures decreased over the years, from 3.9% in 1993 to 1.1% in 2000. Laboratories with false-positive cultures more often processed less than 3,000 samples per year (P < 0.05). Among 110 patients for whom a false-positive culture was identified from 1995 to 1999, we found that for 36% of the patients an official tuberculosis notification had been provided to the appropriate public health services, 31% of the patients were treated, 14% of the patients were hospitalized, and a contact investigation had been initiated for 16% of the patients. The application of DNA fingerprinting to identify false-positive M. tuberculosis cultures and the provision of feedback to peripheral laboratories are useful instruments to improve the quality of laboratory processes and the quality of medical care.
Mycobacterium tuberculosis is one of the most harmful human pathogens worldwide, causing about 8 million new tuberculosis cases and between 2 million and 3 million deaths yearly. Although smear microscopy and clinical diagnosis play important roles in the diagnostic process, the “gold standard” for the diagnosis of tuberculosis is still a positive culture, obtained either by using traditional solid media like Löwenstein medium or by more advanced liquid culture methods, e.g., methods that use BACTEC products. During the processes of collection and processing of clinical samples in the laboratory, contamination with M. tuberculosis sometimes occurs, leading to false-positive cultures. Such misdiagnosis can result from laboratory cross-contamination or contamination of clinical devices, such as bronchoscopes (1). In addition, clerical errors, such as mislabeling of patient material, can lead to an erroneous diagnosis of tuberculosis. Risk factors known to be associated with the occurrence of laboratory cross-contamination are the viability of the organism despite difficult environmental conditions (15) and the complexity of laboratory techniques, especially batch processing (26) and the use of liquid culture media (5, 11, 22).
The consequences of false-positive M. tuberculosis cultures for patients, their social environment, and society can be serious. False-positive cultures may lead to unnecessary treatment with potentially toxic drugs (4, 8, 19) and other unnecessary medical interventions, such as diagnostic procedures and hospitalization (5). In addition, tuberculostatic drugs may interact with a wide range of other frequently used medications, like oral contraceptives (8, 12). Moreover, a false-positive culture may falsely identify patients requiring retreatment and result in the exposure of patients to retreatment regimens that include more toxic second-line drugs (7, 18, 27). Apart from these clinical consequences, the socioeconomic and emotional consequences of false-positive cultures should not be underestimated: a diagnosis of tuberculosis often leads to stigmatization, social isolation (9, 16), interruption of work, and significant public health expenditures. These consequences are not limited only to the patients but also apply to those who are identified as contacts and who are included in targeted screening procedures. It is obvious that false-positive cultures result in excess workloads for medical staff in both the public health service and the clinical sector and thus in excess expenditures for in- and outpatient care (10, 21). Finally, false-positive cultures lead to overestimation of the incidence of tuberculosis (6).
All 44 laboratories in The Netherlands send all M. tuberculosis isolates to the national reference laboratory (NRL) at the National Institute of Public Health and the Environment for species identification, drug susceptibility testing, and DNA fingerprinting. Since 1993 possible false-positive cultures in peripheral laboratories have been detected by routine DNA fingerprinting (3, 23, 25). By combining the routine DNA fingerprinting results and the responses to specific questionnaires sent to peripheral laboratories and municipal health services, this study aims to quantify the occurrence of false-positive M. tuberculosis cultures in The Netherlands, to identify risk factors for false-positive M. tuberculosis cultures, and to investigate the consequences of such false-positive cultures for the patients and the community.
MATERIALS AND METHODS
General.
This study combines the results of typing by routine DNA fingerprinting and the responses to specific questionnaires sent to peripheral laboratories and municipal health services. The data gathered in this way span different time periods and clarify different matters (Table 1).
TABLE 1.
Data gathered for the study
| Method | Data gathered | Time period |
|---|---|---|
| DNA fingerprinting results | Incidence | 1993-2000 |
| Laboratory inventory 1 | Laboratory risk factors (isolation of strains, possible causes of false-positive cultures) | 1997 |
| Laboratory inventory 2 | Laboratory risk factors (characteristics of the safety cabinet, processing of M. tuberculosis cultures) | 1998 |
| Patient questionnaire | Clinical consequences (notification, treatment, contact investigation) | 1995-1999 |
Incidence.
NRL performs species identification, susceptibility testing, and DNA fingerprinting for all M. tuberculosis patient isolates received from 44 peripheral microbiological laboratories in The Netherlands. If more than one M. tuberculosis isolate was received from the same patient, only the first one was included in this study. Standard DNA fingerprinting consisted of IS6110-based restriction fragment length polymorphism (RFLP) analysis (24); for strains with less than five IS6110 copies, RFLP analysis was supplemented with subtyping with the polymorphic GC-rich sequence as a probe. GelCompar software (version 4.0 for Windows; Applied Maths, Kortrijk, Belgium) was used to analyze the IS6110 RFLP patterns (14). On the basis of the results of DNA fingerprinting, a false-positive M. tuberculosis culture is suspected if the DNA fingerprint of an M. tuberculosis isolate taken from a patient without clear clinical signs of tuberculosis, as indicated by the municipal health service, is identical to that of an isolate of another patient that was processed within 7 days in the same laboratory. Patients with suspected false-positive cultures were not epidemiologically linked to other patients. During the period from 1993 to 1996 these suspected false-positive cultures were regularly reported to the peripheral laboratories for confirmation of the results. In 1996 a routine feedback system was established. This system was based on a software application that generates a form that is sent to the peripheral laboratory when a false-positive culture is suspected. The respective peripheral laboratories are asked to confirm the result for the false-positive culture, after verification with the clinician involved. Measures to prevent overestimation of the number of false-positive cultures in the peripheral laboratories have been in place since 1996. Every 3 months a list of new tuberculosis patients is sent to the peripheral laboratories to verify the data registered at NRL. In addition, most peripheral laboratories use a national reference strain which is processed with the batch of patient isolates to be tested at a given time. As the DNA fingerprint of the national reference strain is very characteristic, NRL can identify if the fingerprint for the patient isolate matches the fingerprint of this strain and thus identify laboratory cross-contamination. The M. tuberculosis test result is registered as positive if the peripheral laboratory or the public health tuberculosis control staff do not confirm that it is false positive. Additional false-positive cultures are registered if the tuberculosis control staff of the municipal public health services report false-positive test results.
To summarize, a culture was defined as false positive (i) if the DNA fingerprint of the isolate was identical to that of an isolate of another patient that was processed within 7 days in the same laboratory, and (ii) if the isolate was taken from a patient without clinical signs of tuberculosis, and/or (iii) if the false-positive test result was reported by the peripheral laboratory and/or the public health tuberculosis officer. The incidence of false-positive M. tuberculosis cultures per year was calculated on the basis of this case definition.
Laboratory risk factors.
To identify the risk factors for the occurrence of false-positive M. tuberculosis cultures, questionnaires were sent to the peripheral laboratories. The first questionnaire was sent in 1997 and focused on the isolation of M. tuberculosis strains, the number of M. tuberculosis isolations per year, the kind of safety cabinet used, possible causes of false-positive cultures, and the use of reference strains. All 44 peripheral laboratories received this questionnaire by mail, followed by a telephone call 3 months later. The second questionnaire was sent in 1998 and included questions on characteristics of the safety cabinets used and the laboratory facilities, as well as questions on the actual method of processing of M. tuberculosis cultures. This questionnaire was sent to the peripheral laboratories that returned the first questionnaire.
Clinical consequences.
False-positive cultures were received from 29 peripheral microbiological laboratories. In 2000, these laboratories were sent a questionnaire for each patient with a false-positive culture, followed by a postal reminder after 1 month. Provided the laboratory did not object, this information was complemented by telephone interviews with the tuberculosis control nurses in the municipal public health services.
The information collected included the patient's sex, age, and ethnicity and whether the patient belonged to a known risk group for tuberculosis. Information was also gathered on whether official notification of the tuberculosis case had been provided to public heath authorities and, if so, if the notification had been withdrawn. Finally, it was investigated whether the patients had been treated and, if so, for how long, if they had been hospitalized, and whether a contact investigation had been started.
Statistical analysis.
Univariate analysis was used to describe the results. Laboratory risk factors were compared between laboratories with false-positive cultures and laboratories without false-positive cultures. Stratified for the number of samples processed per year, these comparisons were repeated. Associations were tested by the χ2 test or, if the number of observations in any cell was less than five, by Fisher's exact test.
RESULTS
Incidence.
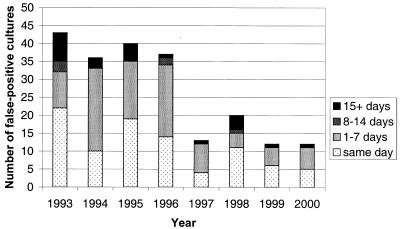
During the period from 1993 to 2000, NRL tested 8,889 M. tuberculosis patient isolates; of these, 213 were false positive by culture (2.4%). The overall incidence of false-positive M. tuberculosis cultures decreased over the years, from 43 (3.9%) in 1993 to 12 (1.1%) in 2000 (Fig. 1).
FIG. 1.
Number of false-positive cultures per year, stratified by time period since isolation of contaminating culture.
Laboratory risk factors.
A total of 34 of the 44 peripheral laboratories (77%) returned the questionnaire on false-positive cultures and related risk factors. The median number of clinical specimens tested for M. tuberculosis in these laboratories was 1,570 per year (range, 50 to 7,500 per year), and the median number of M. tuberculosis complex isolates that the laboratories submitted to NRL was 21 per year (range, 2 to 120 per year). These laboratories submitted in total 4,093 M. tuberculosis isolates to NRL; of these, 131 were false positive by culture (3.2%). In all, 22 of the 34 laboratories (65%) had encountered false-positive cultures. These laboratories had a median of two distinct false-positive episodes (range, one to nine episodes), i.e., time periods during which false-positive cultures were found but which were preceded and followed by at least a month during which a false-positive culture with a similar DNA fingerprint was not found. The median duration of these episodes was 1 day (range, 0 [false-positive cultures were found on the same day] to 63 days).
The distribution of possible risk factors in laboratories with and without false-positive M. tuberculosis cultures is presented in Table 2. Laboratories that processed less than 3,000 samples a year had encountered false-positive cultures more often (P < 0.05). Laboratories that processed more than 3,000 samples a year and that, in addition, used 70% alcohol for decontamination of laboratory equipment had encountered false-positive cultures more often than high-volume laboratories that used another decontaminant (P < 0.05) (data not shown). The 22 peripheral laboratories which encountered false-positive cultures mostly mentioned various forms of laboratory cross-contamination as possible explanations for the false positivity: contaminated buffer liquid (eight laboratories; 36%), contaminated laboratory equipment (seven laboratories; 32%), or aerosols contaminated by processing of a series of patient isolates among which one was highly positive (seven laboratories; 32%). However, preculture contamination of clinical specimens was also mentioned (contamination of a bronchoscope; two laboratories; 6%), as were clerical errors (mislabeling of patient materials; two laboratories; 6%).
TABLE 2.
Laboratory characteristics relevant for M. tuberculosis culture, isolation, and typing for laboratories without false- positive M. tuberculosis cultures and laboratories with false-positive M. tuberculosis culturesa
| Characteristic | No. (%) of laboratories:
|
|
|---|---|---|
| Without false-positive cultures | With false-positive cultures | |
| Production level per yr | ||
| <3,000 samples | 0 (0) | 9 (41)b |
| 3,000 samples or more | 12 (100) | 13 (59) |
| Safety cabinet | ||
| Safety class | ||
| Class I | 1 (8) | 1 (5) |
| Class II | 0 (0) | 5 (23) |
| Class IIA | 10 (83) | 10 (45) |
| Class IIB | 1 (8) | 6 (27) |
| Period in use | ||
| <5 yr | 4 (33) | 8 (36) |
| 5 yr or more | 6 (50) | 12 (55) |
| Unknown | 2 (17) | 2 (9) |
| Control procedures | ||
| Use of reference strain | ||
| Yes | 8 (67) | 19 (86) |
| No | 4 (33) | 3 (14) |
| Decontamination method | ||
| 70% alcohol | 3 (25) | 13 (59)c |
| Other | 9 (75) | 9 (41) |
| Culture | ||
| Method | ||
| Mbcheck | 3 (25) | 5 (23) |
| BACTEC | 0 (0) | 4 (18) |
| Other | 8 (67) | 8 (36) |
| Unknown | 1 (8) | 5 (23) |
| Medium | ||
| Solid | 9 (75) | 10 (45) |
| Solid and liquid | 2 (17) | 7 (32) |
| Unknown | 1 (8) | 5 (23) |
The data are for 1997 for 12 laboratories without false-positive cultures and 22 laboratories with false-positive cultures.
P < 0.05 by Fisher's exact test.
P < 0.1 by Fisher's exact test.
Of the 34 peripheral laboratories which returned the first questionnaire, 16 (47%) also returned the second questionnaire. The use and the positioning of the safety cabinet in laboratories with false-positive cultures were not different from those in the other laboratories (Table 3). Laboratories processing more than 3,000 samples a year had encountered false-positive cultures slightly more often if the size of the room where the cultures were processed was smaller than 30 m2 (P < 0.1) (data not shown). In addition, the answers to the second questionnaire revealed that cleaning, decontamination, and waste disposal procedures were not standardized among the laboratories. Maintenance of the safety cabinet, including a regular change of filters, was carried out by the cabinet's supplier in 12 of 16 (75%) laboratories.
TABLE 3.
Use and positioning of the safety cabinet for laboratories without false-positive M. tuberculosis cultures and laboratories with false-positive M. tuberculosis culturesa
| Characteristic | No. (%) of laboratories:
|
|
|---|---|---|
| Without false-positive cultures | With false-positive cultures | |
| Use of safety cabinet | ||
| With external air outlet | ||
| Yes | 1 (17) | 5 (50) |
| No | 5 (83) | 5 (50) |
| Bunsen heater in cabinet | ||
| Yes | 4 (67) | 7 (70) |
| No | 2 (33) | 3 (30) |
| Positioning of safety cabinet | ||
| Placement | ||
| In a separate room | 1 (17) | 6 (60) |
| In a corner | 2 (33) | 0 (0) |
| At the wall | 3 (50) | 4 (40) |
| Next to a walking route | ||
| Yes | 1 (17) | 3 (30) |
| No | 5 (83) | 7 (70) |
| Draft near cabinet | ||
| Yes | 2 (33) | 3 (30) |
| No | 4 (67) | 7 (70) |
| Size of room | ||
| <30 m2 | 3 (50) | 7 (70) |
| ≥30 m2 | 3 (50) | 3 (30) |
| Air-conditioning in room | ||
| Temperature control and filtering | 5 (83) | 8 (80) |
| Filtering only | 1 (17) | 2 (20) |
The data are for 1998 for 6 laboratories without false-positive cultures and 10 laboratories with false-positive cultures.
Clinical consequences.
Nine (8%) of the 119 patients with a false-positive M. tuberculosis culture found during the period from 1995 to 1999 were excluded from the study. Two of these patients had a false-positive M. bovis BCG culture, three patients were excluded because the peripheral laboratory refused to cooperate with the study, and relevant information was no longer accessible for four patients. Of the 110 patients with a confirmed false-positive M. tuberculosis culture, 79 (72%) had been erroneously identified as having a positive culture within a week after the contaminating culture had been processed in the peripheral laboratory. In 1995, 1996, 1997, 1998, and 1999, 36, 34, 11, 18, and 11 patients, respectively, were found to have false-positive cultures. Among the 110 patients with a false-positive culture during the period from 1995 to 1999, 27 (25%) were younger than 45 years of age, whereas among the patients in the National Tuberculosis Register in 1997, 66% were younger than 45 years of age (P < 0.001). Among the patients with false-positive cultures, 9 (8%) were of non-Dutch nationality, whereas 56% of the patients in the National Tuberculosis Register in 1997 were of non-Dutch nationality (P < 0.001).
Official notification of tuberculosis cases was provided to public health services for 40 (36%) of the patients with false-positive cultures but not for 69 (63%) of the patients with false-positive cultures. The notification status was unknown for one patient. For 25 of 40 (63%) of patients for whom notification was provided, the notification was later withdrawn (Table 4). Tuberculosis treatment was started for 34 (31%) of the patients with false-positive cultures. Notification had been provided for most of the treated patients, although the notification was withdrawn for more than half of them. In total, nine patients were treated for tuberculosis for at least 6 months, 11 were treated for between 2 and 6 months, and 14 patients were treated for less than 2 months. Treatment or a lack thereof was not associated with the patient's age (data not shown).
TABLE 4.
Number of patients with false-positive M. tuberculosis cultures during the period from 1995 to 1999 by notification status, treatment with tuberculostatic drugs, hospitalization, and follow-up by a contact investigationa
| Notification status | No. (%) of patients
|
||||
|---|---|---|---|---|---|
| Total | Treated | Treated >2 mo | Hospitalized | With contact investigation | |
| Notification provided | 15 (14) | 14 (41) | 10 (48) | 9 (60) | 7 (39) |
| Notification provided, notification withdrawn | 25 (23) | 17 (50) | 9 (43) | 5 (33) | 10 (56) |
| Notification not provided | 69 (63) | 3 (9) | 1 (10) | 1 (7) | 1 (6) |
| Unknown | 1 (1) | ||||
| Total | 110 (100) | 34 (100) | 20 (100) | 15 (100) | 18 (100) |
A total of 110 patients were included in the analysis.
The median time between the isolation of M. tuberculosis in the peripheral laboratory and feedback from NRL about the possibility of a false-positive culture was 86 days (range, 51 to 226 days). The median time between the time that the isolate was sent to NRL and the receipt of feedback was 33 days (range, 12 to 83 days). Treatment duration was not associated with the time between the isolation of M. tuberculosis in the peripheral laboratory and the receipt of feedback from NRL about the possibility of a false-positive culture. For five patients, NRL gave feedback to the peripheral laboratory after 8 weeks or more from the time of the initial isolation of M. tuberculosis. For 11 patients the peripheral laboratories knew why the treatment had been stopped: for 2 patients, because NRL had reported the possibility of a false-positive culture; for 5 patients, because of side effects; and for 3 patients, because of symptoms inconsistent with tuberculosis; for 1 patient, it was unclear why the treatment had stopped.
In total, 15 (12%) patients were hospitalized. However, the reason for the hospitalization was not available for this study. Notifications were officially provided for nine of these patients, and the notifications were not withdrawn (Table 4). Hospitalization was not associated with the patient's age (data not shown). Four patients were hospitalized for less than 1 week, two patients were hospitalized for 1 to 2 weeks, four patients were hospitalized for 2 to 3 weeks, and five patients were hospitalized for 3 weeks or longer. The total number of hospitalization days for all hospitalized patients was 230.
Contact investigations were carried out for 18 of 40 (45%) patients with a false-positive culture for which notification was provided (Table 4). Seven contact investigations included less than 5 people, six contact investigations included between 5 and 10 people, three contact investigations included between 10 and 15 people, and two contact investigations included more than 15 people. In total, 136 people were included in contact investigations on the basis of a false-positive culture.
DISCUSSION
We showed that the incidence of false-positive M. tuberculosis test results was 2.4% in the period from 1993 to 2000. Although false-positive test results were found to affect only a few patients, they had serious consequences for the individual patients and their environment. It should be noted that the incidence of false-positive cultures is based on the findings of NRL. In the individual peripheral laboratories, more false-positive cultures that were not sent to NRL for secondary laboratory typing may have occurred. This may explain why the incidence found in this study was lower than that reported by others (2, 3, 5, 27).
Since 1995 the National Institute of Public Health and the Environment has regularly advised peripheral laboratories on the prevention of laboratory cross-contamination and has given feedback on suspected false-positive cultures. It is probable that these actions have helped peripheral laboratories further prevent false-positive M. tuberculosis cultures, resulting in a reduction in the incidence of these errors. On the other hand, it is also conceivable that this reduction was actually the result of a registration bias. The incidence of false-positive cultures dropped sharply after 1996, suggesting that the change in the feedback reporting system in 1996 did play a role. However, the absolute number of false-positive cultures found after 7 days from the time that a contaminating culture had been processed also dropped, and for these the automated feedback system did not play a role. It can therefore be concluded that the actual incidence of false-positive M. tuberculosis cultures did decline.
As far as we know, our study is the first to investigate the relative importance of the mechanisms responsible for false-positive cultures in different laboratories. However, due to the small numbers, our findings only indicate possible mechanisms. False-positive cultures occurred more often in laboratories that processed fewer than 3,000 samples a year, suggesting that laboratories need to process a minimum volume per year to maintain optimal processing procedures. Another explanation could be that peripheral laboratories processing a larger volume of cultures a year detect relatively more false-positive cultures themselves, either because they are more aware of the problem or because they have more facilities to detect such cultures. Laboratories that had detected all false-positive cultures themselves would not have been identified as laboratories with false-positive cultures in our study.
The use of 70% alcohol for decontamination of laboratory equipment seemed to be a risk factor for false-positive cultures. However, the mycobactericidal activity of 70% alcohol has been reported to be good (13). In our setting, perhaps not the product but the method was the determining factor, as suggested by the great variety of decontamination procedures reported by the laboratories. Our data do not support published findings that a liquid medium or batch processing are risk factors for false-positive M. tuberculosis cultures (5, 11, 22, 26), but this may be due to the small numbers in our study. In laboratories that process more than 3,000 samples a year, processing of the samples in a relatively small room seemed to be a risk factor for false-positive cultures. This is perhaps related to the more natural ventilation in a larger room. Although our findings regarding the circulation of air (the presence of air-conditioning systems, an external air outlet, and the placement of the safety cabinet) did not confirm this, other studies note the role of aerosols in causing false-positive M. tuberculosis cultures (4, 21).
It is likely that the chance of a false-positive culture per specimen tested is similar for different age groups and different nationalities. Our findings that patients with false-positive cultures were relatively older and more often had a Dutch nationality therefore suggest that these patients had more often been unnecessarily tested for tuberculosis than young and non-Dutch patients.
The proportion of patients with a false-positive culture who were treated (31%) was smaller than the proportion (67%) published elsewhere (4). It is possible that a diagnosis of tuberculosis was doubtful for patients with a false-positive M. tuberculosis test before the laboratory test result was known. Perhaps the feedback on suspected false-positive cultures to the peripheral laboratories has also prevented unnecessary treatment. However, our findings suggest that the feedback from NRL influenced the clinical management to only a limited extent.
We found a remarkable discrepancy between the number of official notifications (n = 40) and the number of contact investigations. Contact investigations were started for only 16% of patients with a false-positive culture. This could indicate that there were doubts about the diagnosis from the beginning or that the diagnosis was made upon the entrance of the patient into the country (and therefore the patient did not yet have contacts). Extrapulmonary specimens were tested for only three patients, and therefore, contact investigations were not indicated. It should be stressed, however, that 136 contacts were investigated, with all the accompanying socioeconomic consequences. The percentage of false notifications (15 in roughly 7,500 notifications [0.2%] from 1995 to 1999 [20]) was so low that it can be concluded that tuberculosis surveillance data are almost unbiased.
It is probable that the direct medical costs related to false-positive M. tuberculosis cultures will be determined largely by the tuberculosis treatment for 3,447 days, 230 hospital days, consultations of the 34 treated patients, investigations of 136 contacts, and extra diagnostic tests for the 110 patients with false-positive cultures. The indicative minimum costs of drug therapy for the treated patients with false-positive cultures were $7,000 (all monetary units provided here are in U.S. dollars). In this calculation a 2-month regimen of isoniazid (300 mg/day in tablets), rifampin (600 mg of rifadin tablets per day), and pyrazinamide (1,500 mg/day in tablets) was used, followed by a regimen of isoniazid (300 mg/day in tablets) and rifampin (600 mg of rifadin tablets per day). Drug costs were based on Dutch pharmaceutical outlet prices (17). The indicative costs of hospitalization in Dutch hospitals for all hospitalized patients with false-positive cultures were $66,780. The cost of a hospital stay for a tuberculosis patient, based on admission to a pulmonary ward in a university hospital and including only the costs of nursing, materials, nutrition, medication, laundry, housing and cleaning, and overhead and equipment, was assumed to be $290 per day (J. B. Oostenbrink, T. Buijs-Van der Woude, M. Van Agthoven, M. A. Koopmanschap, and F. F. H. Rutten, submitted for publication). The treated tuberculosis patients were assumed to have had, on average, five outpatient consultations with a specialized medical doctor in The Netherlands, each for about $50, amounting to $8,500. The cost required to contact 136 persons was estimated to be $5,000, and the cost required to perform extra diagnostic tests for the 110 patients with false-positive cultures was estimated to be $10,000. Therefore, the total direct medical costs in the Dutch health care system related to false-positive cultures from 1995 to 1999 were almost $100,000. Although this is not a large amount in the Dutch health care system, these costs could have been avoided. In addition, nonmedical costs (e.g., loss of working days and psychosocial consequences) and indirect medical costs may have been even more important than the calculated direct medical costs. However, these were not investigated.
This study showed that the identification of suspected false-positive M. tuberculosis cultures by DNA fingerprinting and the provision of feedback to peripheral laboratories are useful instruments in improving the quality of laboratory processes and the quality of medical care. To further reduce the adverse consequences of false-positive M. tuberculosis cultures, it is important that (i) peripheral laboratories not delay the submission of positive M. tuberculosis isolates to NRL, (ii) NRL perform DNA fingerprinting of all positive M. tuberculosis isolates and not delay the reporting of possible false-positive test results to peripheral laboratories, (iii) confirmed false-positive test results be reported back to the clinicians as soon as possible, and (iv) clinicians balance laboratory test results with their clinical judgment on whether or not a patient has tuberculosis.
Acknowledgments
We are indebted to the staff of the peripheral laboratories who provided information on laboratory procedures and to the staff of the municipal public health services who provided information on patients with false-positive cultures. In addition, we thank Martien Borgdorff and Paul van Gerven for reviewing a draft of this paper.
This work was supported by the Ministry of Health, Welfare and Sports of The Netherlands and the European Union (New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis, project no. QLK2-CT-2000-00630).
REFERENCES
- 1.Agerton, T., S. Valway, B. Gore, C. Pozsik, B. Plikaytis, C. Woodley, and I. Onorato. 1997. Transmission of a highly drug-resistant strain (strain W1) of Mycobacterium tuberculosis. Community outbreak and nosocomial transmission via a contaminated bronchoscope. JAMA 278:1073-1077. [PubMed] [Google Scholar]
- 2.Bauer, J., V. O. Thomsen, S. Poulsen, and A. B. Andersen. 1997. False-positive results from cultures of Mycobacterium tuberculosis due to laboratory cross-contamination confirmed by restriction fragment length polymorphism. J. Clin. Microbiol. 35:988-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braden, C. R., G. L. Templeton, W. W. Stead, J. H. Bates, M. D. Cave, and S. E. Valway. 1997. Retrospective detection of laboratory cross-contamination of Mycobacterium tuberculosis cultures with use of DNA fingerprint analysis. Clin. Infect. Dis. 24:35-40. [DOI] [PubMed] [Google Scholar]
- 4.Burman, W. J., and R. R. Reves. 2000. Review of false-positive cultures for Mycobacterium tuberculosis and recommendations for avoiding unnecessary treatment. Clin. Infect. Dis. 31:1390-1395. [DOI] [PubMed] [Google Scholar]
- 5.Burman, W. J., B. L. Stone, R. R. Reves, M. L. Wilson, Z. Yang, H. El-Hajj, J. H. Bates, and M. D. Cave. 1997. The incidence of false-positive cultures for Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 155:321-326. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2000. Misdiagnoses of tuberculosis resulting from laboratory cross-contamination of Mycobacterium tuberculosis cultures—New Jersey, 1998. Morb. Mortal. Wkly. Rep. 49:413-416. [PubMed] [Google Scholar]
- 7.Das, S., S. L. Chan, B. W. Allen, D. A. Mitchison, and D. B. Lowrie. 1993. Application of DNA fingerprinting with IS986 to sequential mycobacterial isolates obtained from pulmonary tuberculosis patients in Hong Kong before, during and after short-course chemotherapy. Tuber. Lung Dis. 74:47-51. [DOI] [PubMed] [Google Scholar]
- 8.Douglas, J. G., and M. J. McLeod. 1999. Pharmacokinetic factors in the modern drug treatment of tuberculosis. Clin. Pharmacokinet. 37:127-146. [DOI] [PubMed] [Google Scholar]
- 9.Dunlap, N. E., R. H. Harris, W. H. Benjamin, Jr., J. W. Harden, and D. Hafner. 1995. Laboratory contamination of Mycobacterium tuberculosis cultures. Am. J. Respir. Crit. Care Med. 152:1702-1704. [DOI] [PubMed] [Google Scholar]
- 10.Fryatt, R. J. 1997. Review of published cost-effectiveness studies on tuberculosis treatment programmes. Int. J. Tuberc. Lung Dis. 1:101-109. [PubMed] [Google Scholar]
- 11.Gascoyne-Binzi, D. M., R. E. Barlow, R. Frothingham, G. Robinson, T. A. Collyns, R. Gelletlie, and P. M. Hawkey. 2001. Rapid identification of laboratory contamination with Mycobacterium tuberculosis using variable number tandem repeat analysis. J. Clin. Microbiol. 39:69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grange, J. M., P. A. Winstanley, and P. D. Davies. 1994. Clinically significant drug interactions with antituberculosis agents. Drug Safety 11:242-251. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths, P. A., J. R. Babb, and A. P. Fraise. 1999. Mycobactericidal activity of selected disinfectants using a quantitative suspension test. J. Hosp. Infect. 41:111-121. [DOI] [PubMed] [Google Scholar]
- 14.Heersma, H. F., K. Kremer, and J. D. van Embden. 1998. Computer analysis of IS6110 RFLP patterns of Mycobacterium tuberculosis. Methods Mol. Biol. 101:395-422. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, K. R., C. R. Braden, K. L. Cairns, K. W. Field, A. C. Colombel, Z. Yang, C. L. Woodley, G. P. Morlock, A. M. Weber, A. Y. Boudreau, T. A. Bell, I. M. Onorato, S. E. Valway, and P. A. Stehr-Green. 2000. Transmission of Mycobacterium tuberculosis from medical waste. JAMA 284:1683-1688. [DOI] [PubMed] [Google Scholar]
- 16.Kelly, P. 1999. Isolation and stigma: the experience of patients with active tuberculosis. J. Community Health Nurs. 16:233-241. [DOI] [PubMed] [Google Scholar]
- 17.Loenen, A. C. 2001. Farmacotherapeutisch kompas 2002. College voor Zorgverzekeringen, Amstelveen, The Netherlands.
- 18.Nitta, A. T., P. T. Davidson, M. L. de Koning, and R. J. Kilman. 1996. Misdiagnosis of multidrug-resistant tuberculosis possibly due to laboratory-related errors. JAMA 276:1980-1983. [PubMed] [Google Scholar]
- 19.Ormerod, L. P., and N. Horsfield. 1996. Frequency and type of reactions to antituberculosis drugs: observations in routine treatment. Tuber. Lung Dis. 77:37-42. [DOI] [PubMed] [Google Scholar]
- 20.Royal Netherlands Tuberculosis Association. 1999. Index tuberculosis 1997. Royal Netherlands Tuberculosis Association, The Hague, The Netherlands.
- 21.Segal-Maurer, S., B. N. Kreiswirth, J. M. Burns, S. Lavie, M. Lim, C. Urban, and J. J. Rahal, Jr. 1998. Mycobacterium tuberculosis specimen contamination revisited: the role of laboratory environmental control in a pseudo-outbreak. Infect. Control Hosp. Epidemiol. 19:101-105. [DOI] [PubMed] [Google Scholar]
- 22.Small, P. M., N. B. McClenny, S. P. Singh, G. K. Schoolnik, L. S. Tompkins, and P. A. Mickelsen. 1993. Molecular strain typing of Mycobacterium tuberculosis to confirm cross-contamination in the mycobacteriology laboratory and modification of procedures to minimize occurrence of false-positive cultures. J. Clin. Microbiol. 31:1677-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Duin, J. M., J. E. Pijnenburg, C. M. van Rijswoud, P. E. de Haas, W. D. Hendriks, and D. van Soolingen. 1998. Investigation of cross contamination in a Mycobacterium tuberculosis laboratory using IS6110 DNA fingerprinting. Int. J. Tuberc. Lung Dis. 2:425-429. [PubMed] [Google Scholar]
- 24.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Soolingen, D. 1998. Utility of molecular epidemiology of tuberculosis. Eur. Respir. J. 11:795-797. [DOI] [PubMed] [Google Scholar]
- 26.Wurtz, R., P. Demarais, W. Trainor, J. McAuley, F. Kocka, L. Mosher, and S. Dietrich. 1996. Specimen contamination in mycobacteriology laboratory detected by pseudo-outbreak of multidrug-resistant tuberculosis: analysis by routine epidemiology and confirmation by molecular technique. J. Clin. Microbiol. 34:1017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wurtz, R., J. Fernandez, and B. Jovanovic. 1994. Real and apparent tuberculin skin test conversions in a group of medical students. Infect. Control Hosp. Epidemiol. 15:516-519. [DOI] [PubMed] [Google Scholar]