Abstract
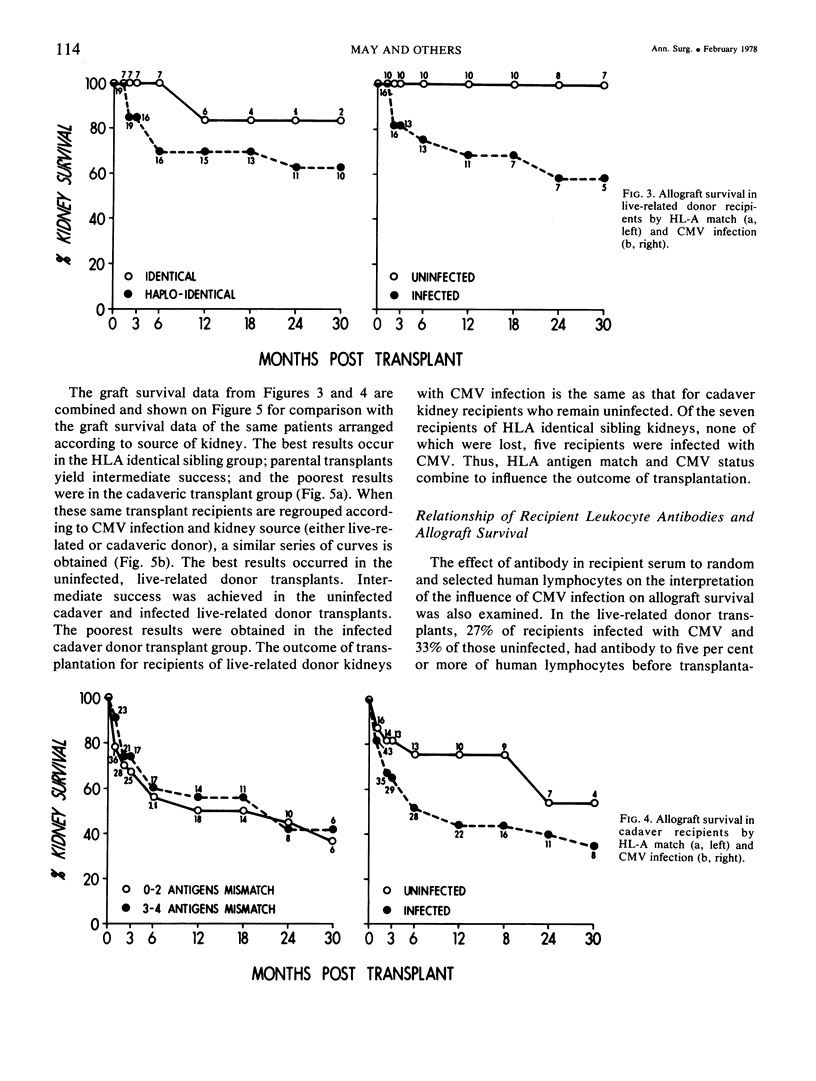
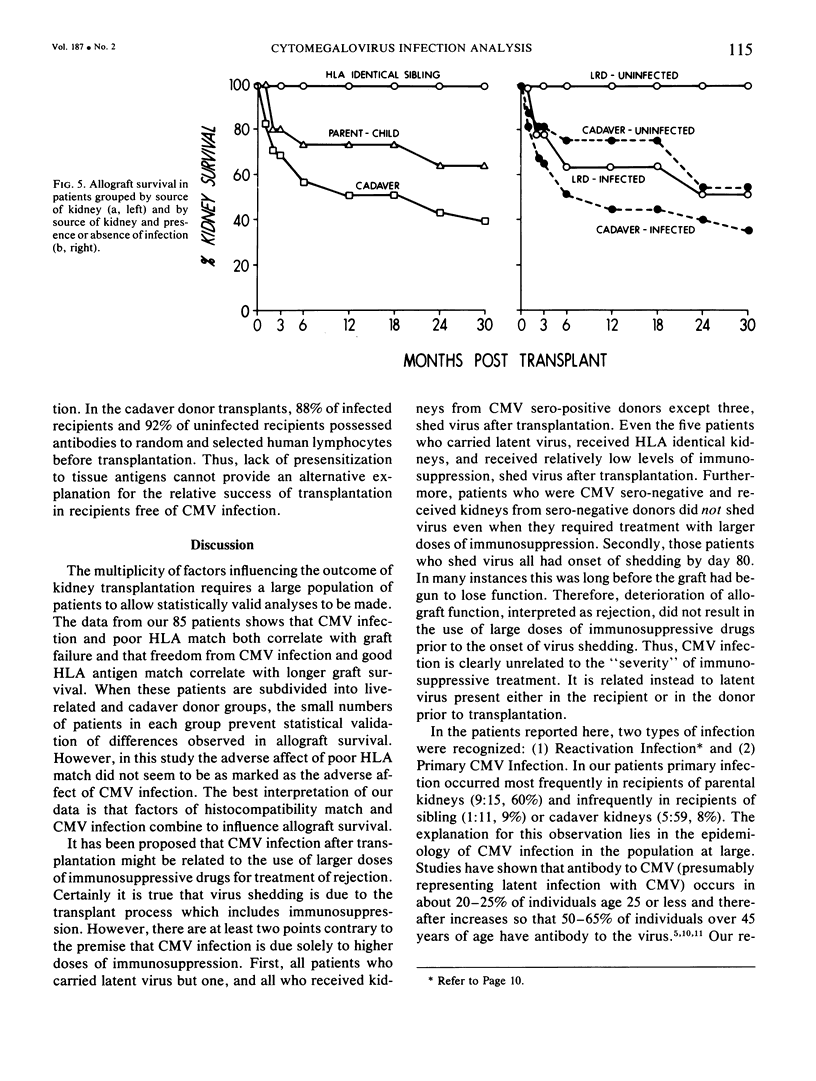
Eighty-five recipients and donors of renal allografts were examined for evidence of cytomegalovirus infection before and repeatedly after transplantation. The recipients were also divided into two group on the basis of HLA antigen matching. Better allograft survival was noted in patients well matched for HLA antigens (0-2) mismatched antigens) compared to those poorly matched (three or more antigens mismatched), and in patients free of cytomegalovirus compared to those infected. Cytomegalovirus infection had a more marked influence on allograft survival than did HLA antigen matching. The differing rates of success of transplantation, apparently dependent on blood relationship between donor and recipient, have been assumed largely to be due to inherited factors. This study, however, revealed an important factor to be the disparate incidence of cytomegalovirus infection in sibling, parental, and cadaveric categories of transplantation. The mechanism of this disparity can be explained on the basis of the incidence of latent CMV infection in the recipients and various categories of kidney donors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNES B. A. SURVIVAL DATA OF RENAL TRANSPLANTATIONS IN PATIENTS. N Engl J Med. 1965 Apr 15;272:776–779. doi: 10.1056/NEJM196504152721505. [DOI] [PubMed] [Google Scholar]
- Betts R. F., Freeman R. B., Douglas R. G., Jr, Talley T. E. Clinical manifestations of renal allograft derived primary cytomegalovirus infection. Am J Dis Child. 1977 Jul;131(7):759–763. doi: 10.1001/archpedi.1977.02120200041010. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Dunlop M. B., Parish C. R., Zinkernagel R. M. Inflammatory process in murine lymphocytic choriomeningitis is maximal in H-2K or H-2D compatible interactions. J Immunol. 1976 Jul;117(1):187–190. [PubMed] [Google Scholar]
- Ho M., Suwansirikul S., Dowling J. N., Youngblood L. A., Armstrong J. A. The transplanted kidney as a source of cytomegalovirus infection. N Engl J Med. 1975 Nov 27;293(22):1109–1112. doi: 10.1056/NEJM197511272932201. [DOI] [PubMed] [Google Scholar]
- Hors J., Feingold N., Fradelizi D., Dausset J. Critical evaluation of histocompatibility in 179 renal transplants. Lancet. 1971 Mar 27;1(7700):609–612. doi: 10.1016/s0140-6736(71)91548-0. [DOI] [PubMed] [Google Scholar]
- Lopez C., Simmons R. L., Mauer S. M., Najarian J. S., Good R. A., Gentry S. Association of renal allograft rejection with virus infections. Am J Med. 1974 Mar;56(3):280–289. doi: 10.1016/0002-9343(74)90609-3. [DOI] [PubMed] [Google Scholar]
- Lowrie E. G., Lazarus J. M., Mocelin A. J., Bailey G. L., Hampers C. L., Wilson R. E., Merrill J. P. Survival of patients undergoing chronic hemodialysis and renal transplantation. N Engl J Med. 1973 Apr 26;288(17):863–867. doi: 10.1056/NEJM197304262881701. [DOI] [PubMed] [Google Scholar]
- Luby J. P., Shasby D. M. A sex difference in the prevalence of antibodies to cytomegalovirus. JAMA. 1972 Dec 4;222(10):1290–1291. [PubMed] [Google Scholar]
- Mirkovic R., Werch J., South M. A., Benyesh-Melnick M. Incidence of cytomegaloviremia in blood-bank donors and in infants with congenital cytomegalic inclusion disease. Infect Immun. 1971 Jan;3(1):45–50. doi: 10.1128/iai.3.1.45-50.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opelz G., Mickey M. R., Terasaki P. I. HL-A and kidney transplants: reexamination. Transplantation. 1974 Apr;17(4):371–382. doi: 10.1097/00007890-197404000-00006. [DOI] [PubMed] [Google Scholar]
- Sachs J. A., Oliver R. T., Festenstein H., Blandy J. P., Salaman J. R., Balfour I. C., Pegrum G. D., Williams G. B., Hopewell J. P., Moorhead J. F. International collaboration in renal transplantation. Lancet. 1971 Jul 31;2(7718):228–230. doi: 10.1016/s0140-6736(71)92570-0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Marchioro T. L., Terasaki P. I., Porter K. A., Faris T. D., Herrmann T. J., Vredevoe D. L., Hutt M. P., Ogden D. A., Waddell W. R. Chronic survival after human renal homotransplantation. Lymphocyte-antigen matching, pathology and influence of thymectomy. Ann Surg. 1965 Oct;162(4):749–787. doi: 10.1097/00000658-196510000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki P. I., Mickey M. R., Singal D. P., Mittal K. K., Patel R. Serotyping for homotransplantation. XX. Selection of recipients for cadaver donor transplants. N Engl J Med. 1968 Nov 14;279(20):1101–1103. doi: 10.1056/NEJM196811142792007. [DOI] [PubMed] [Google Scholar]
- Wonham V. A., Winn H. J., Russel P. S. Serotyping and genetic analysis in the selection of related renal-allograft donors. N Engl J Med. 1971 Mar 11;284(10):509–513. doi: 10.1056/NEJM197103112841001. [DOI] [PubMed] [Google Scholar]


