Abstract
A fluorescence-based real-time 5′ nuclease PCR capable of detecting all four human malaria parasites was developed to screen large numbers of samples during an outbreak to prevent further transmission of malaria. The effectiveness of antimalarial therapy for malaria patients can be monitored by determining the reduction of parasitemia by this method.
Malaria is a major tropical infection causing up to 500 million clinical cases and 2.7 million deaths per year worldwide. For proper treatment of malaria patients, accurate and rapid diagnosis of malaria is essential. The microscopic examination of a blood smear is the “gold standard” for malaria diagnosis. The method is sensitive and specific but laborious and time-consuming (6). Alternative methods, such as PCR and rapid antigen capture assays (ParaSight F test, OptiMAL rapid malaria test, and ICT Malaria P.f/P.v), have been developed. A PCR to detect Plasmodium falciparum and P. vivax was previously developed and evaluated (1, 7, 8, 9). We have developed a method for malaria diagnosis that can be adapted for high-throughput rapid screening of hundreds of samples with a sensitivity and specificity comparable to those of the microscopic method. The method is a fluorescence-based real-time 5′ nuclease PCR based on the TaqMan technology (Roche Molecular Diagnostics Systems) (3, 4), with primers and a probe from the small-subunit (SSU) rRNA gene.
Malaria samples.
Clinical blood samples were obtained from the Malaria Reference Center in the Department of Microbiology, National University of Singapore. Clinical samples of patients undergoing treatment were obtained from the National University Hospital, Singapore. The Gombak A strain of P. falciparum (5) was cultured by the method of Trager and Jensen (10). Two P. ovale (Po1 and Po2) and three P. malariae (Pm1, Pm2, and Pm3) samples were obtained.
DNA extraction.
DNA was extracted from 200 μl of EDTA-treated blood samples with a QIAamp DNA blood minikit (Qiagen GmbH, Hilden, Germany) and stored at 4°C.
Sequences of primers and probes.
Primers (Mach 60 [5′-ACATGGCTATGACGGGTAACG-3′] and Mach 61 [5′-TGCCTTCCTTAGATGTGGTAGCTA-3′]) and a probe (Mach 62 [5′-TCAGGCTCCCTCTCCGGAATCGA-3′]) were designed to detect the SSU rRNA genes of all four human malarial species (accession numbers M19172 for P. falciparum, X13926 for P. vivax, M54897 for P. malariae, and L48987 for P. ovale). The probe was labeled with a reporter dye, FAM (5-carboxyfluorescein), and a quencher dye, TAMRA (N,N,N,N-tetramethyl-6-carboxyrhodamine) (Applied Biosystems, Foster City, Calif.).
5′ nuclease PCR.
Five microliters of DNA, 900 nM Mach 60, 300 nM Mach 61, 200 nM Mach 62, and 1× TaqMan universal PCR master mix (Applied Biosystems) in a total reaction volume of 25 μl were amplified on an iCycler (Bio-Rad Laboratories, Hercules, Calif.). The following conditions were used for PCR: an initial denaturation at 50°C for 2 min, 95°C for 10 min, and 45 cycles of 95°C for 15 s and 60°C for 1 min. The cycle threshold value (CT) indicates the cycle where the fluorescence detected from the amplification of the quantity of the target gene exceeded the preset threshold.
Specificity.
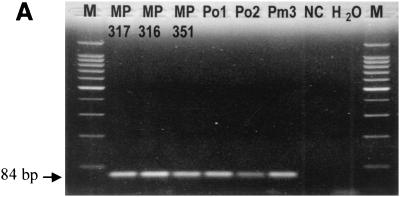
The primers amplified an 84-bp fragment from DNA prepared from P. falciparum, P. vivax, P. malariae, and P. ovale samples (Fig. 1A). The probe, designed specifically to detect the amplified PCR fragment, gave an increased fluorescent signal as the PCR progressed (Fig. 1B). The specificity of the method was evaluated with 153 blood samples, and the method was compared with blood film microscopic examination (Table 1). One hundred twenty-five samples were positive by both blood film examination and the fluorescence-based PCR method. Two other samples were positive by PCR but negative by blood film examination. These two samples were from patients who were undergoing antimalarial therapy, and the blood film method failed to detect the small amount of parasite DNA present on the last day of treatment; the PCR method, being more sensitive, could detect the parasites. All 26 samples that were negative by blood film were also negative by PCR. The positive samples had CT values of 16 to 37, while the negative samples had CT values greater than 38.
FIG. 1.
Specificity of the PCR-based method for detecting four species of human malaria parasites. (A) Agarose gel electrophoresis of PCR-amplified products with SSU rRNA primers. Lanes: MP 317 and MP316, P. falciparum samples; MP 351, P. vivax sample; Po1 and Po2, P. ovale samples; Pm3, P. malariae sample; NC, noninfected sample (negative control); H2O, no-template control; M, molecular size markers. (B) Amplification plot for the samples listed in the legend for panel A, with the threshold being set at 150 relative fluorescence units (RFU).
TABLE 1.
Comparison of fluorescence-based PCR assays with microscopic blood film examination for detection of malaria parasites in blood samples
| Species | No. of samples detected by:
|
|
|---|---|---|
| Blood film | Fluorescence-based PCR | |
| P. falciparum | 42 | 42 |
| P. vivax | 74 | 76 |
| P. ovale | 2 | 2 |
| P. malariae | 3 | 3 |
| Mixed species | 4 | 4 |
| None (negative) | 28 | 26 |
| Total | 153 | 153 |
Sensitivity.
The sensitivity of the method was determined by fivefold dilution of genomic DNA isolated from the cultured Gombak A strain of P. falciparum. Fluorescence can be detected from 90 ng (CT, 16) to 0.002 pg (CT, 35) of P. falciparum DNA. The amount of 0.002 pg of P. falciparum DNA is equivalent to 0.1 parasite, based on one genome equivalent of the parasite being 0.02 pg (2). The assays were repeated seven times and found to be reproducible, with the CT value varying from 34 to 36 for 0.002 pg of DNA.
Monitoring antimalarial treatment.
The PCR method was used to monitor the progress of the antimalarial treatment of three patients and compared with the microscopic examination of blood film. The numbers of parasites in the patients undergoing treatment were determined daily with fluorescence-based PCR and blood film methods. The parasitemia determined from the blood film for one patient fell from 0.45 to 0% from days 1 to 5 of treatment. The fluorescence PCR method indicated that the number of parasites detected fell from 2 × 104 parasites to 0.17 parasite per 5 μl of blood from days 1 to 5, with the serially diluted cultured Gombak A strain being used as the standard. Both methods could monitor the reduction of parasitemia during the course of treatment, though the fluorescence-based PCR method was more sensitive and could detect very small numbers of parasites from the samples on the day before the patients were discharged. Similar results were obtained for two other patients undergoing antimalarial therapy (data not shown).
In summary, a real-time fluorescence PCR method with specific primers and a labeled probe of the SSU rRNA gene was developed for the detection of malaria parasites. This method has a sensitivity of 0.002 pg of P. falciparum DNA, which is equivalent to 0.1 parasite. The method is also specific for all P. falciparum, P. malariae, P. ovale, and P. vivax parasites, since all the samples that tested positive by blood film were also positive by real-time PCR.
The method would be useful in monitoring the effectiveness of antimalarial therapy, especially in situations where drug-resistant strains of the parasites are prevalent. Determining parasitemia by microscopy could be subjective, depending on the technician counting the numbers of parasites and red blood cells, and could vary from person to person and from day to day. The real-time PCR will provide an objective method for determining the parasitemia in the samples.
The method developed in this study will be useful in countries where malaria is not endemic and there is a lack of skilled technicians. A large number of negative samples may need to be screened for malaria as a measure to prevent further transmission of malaria. This was demonstrated in a recent malaria outbreak in Singapore, where 242 samples from asymptomatic individuals suspected of harboring the malaria parasites were screened. One positive malaria case was detected by PCR but not from blood film examination (data not shown).
Acknowledgments
Victor Koh provided technical support for this study. For malaria samples, we are grateful to Mak Jeon Wah and Patricia Lim, Institute for Medical Research, Kuala Lumpur, Malaysia; Bill Collins, Malaria Branch, Centers for Disease Control and Prevention, Atlanta, Ga.; and M. Kimura, Institutes of Medical Sciences, University of Tokyo, Tokyo, Japan.
This work was supported by the Defence Medical Research Institute, Defence Science and Technology Agency, and the Singapore Armed Forces.
REFERENCES
- 1.Das, A., B. Holloway, W. E. Collins, V. P. Shama, S. K. Ghosh, S. Sinha, S. E. Hasnain, G. P. Talwar, and A. A. Lal. 1995. Species-specific 18S rRNA gene amplification for the detection of P. falciparum and P. vivax malaria parasites. Mol. Cell. Probes 9:161-165. [DOI] [PubMed] [Google Scholar]
- 2.Goman, M., G. Langsley, J. E. Hyde, N. K. Yankovsky, J. W. Zolg, and J. G. Scaife. 1982. The establishment of genomic DNA libraries for the human malaria parasite Plasmodium falciparum and identification of individual clones by hybridization. Mol. Biochem. Parasitol. 5:391-400. [DOI] [PubMed] [Google Scholar]
- 3.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 4.Livak, K. J., S. J. A. Flood, J. Marmaro, W. Giusti, and K. Deetz. 1995. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 4:357-362. [DOI] [PubMed] [Google Scholar]
- 5.Mak, J. W., P. K. C. Lim, M. A. J. A. Tan, P. L. W. Lam, A. Noor Rain, G. D. Selvadurai, and K. Hanjeet. 1987. Parasitological and serological surveys for malaria among the inhabitants of an aborigine village and an adjacent Malay village. Acta Trop. 44:83-89. [PubMed] [Google Scholar]
- 6.Payne, D. 1988. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull. W. H. O. 66:621-626. [PMC free article] [PubMed] [Google Scholar]
- 7.Sethabur, O., A. E. Brown, S. Panyim, K. C. Kain, H. K. Webster, and P. Echeverria. 1992. Detection of Plasmodium falciparum by polymerase chain reaction in a field study. J. Infect. Dis. 166: 145-148. [DOI] [PubMed] [Google Scholar]
- 8.Snounou, G., S. Viriyakosol, W. Jarra, S. Thaithong, and K. N. Brown. 1993. Identification of the four human malaria parasite species in field samples by polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 58:283-292. [DOI] [PubMed] [Google Scholar]
- 9.Tham, J. M., S. H. Lee, T. M. C. Tan, R. C. Y. Ting, and U. A. K. Kara. 1999. Detection and species determination of malaria parasites by PCR: comparison with microscopy and with ParaSight-F and ICT Malaria Pf tests in a clinical environment. J. Clin. Microbiol. 37:1269-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]