Abstract
We have developed a rapid and easy to perform fluorescence in situ hybridization test that allows specific identification of trypanosomes from the subgenus Trypanozoon, using peptide nucleic acid probes. Probes were designed to target subgenus-specific sequences on the multiple-copy 18S rRNA, greatly facilitating the detection of a single trypanosome.
Trypanosomes belonging to the subgenus Trypanozoon are protozoan extracellular parasites that undergo continuous antigenic variation (14). Tsetse-transmitted Trypanosoma brucei gambiense and T. brucei rhodesiense cause human African trypanosomiasis (sleeping sickness) and occur in West-Central and East Africa, respectively. Animal infections with T. brucei brucei, Trypanosoma congolense, and Trypanosoma vivax appear through the whole African tsetse belt, while non-tsetse-transmitted Trypanosoma equiperdum and Trypanosoma evansi are found worldwide (5, 7, 8). Diagnosis of human and animal infections is usually based on recognition of clinical manifestations and the detection of parasites by microscopy (21), combined, in the case of T. brucei gambiense infections, with a card agglutination test for trypanosomiasis (13). Here, we present a newly developed diagnostic assay based on the use of peptide nucleic acid (PNA) probes. These molecules are pseudopeptides that hybridize to complementary nucleic acid targets (DNA and RNA). In PNA, the counterpart of the sugar phosphate backbone of DNA and RNA is a polyamide formed by repetitive units of N-2-aminoethyl glycine units to which nucleobases are covalently attached (3, 17). Previously, PNA fluorescence in situ hybridization (FISH) tests targeting rRNA have been used to differentiate and/or identify yeast and bacterium species (17-19).
For this study, trypanosomes, Leishmania donovani, and Plasmodium falciparum AS strain were obtained from the Institute of the Tropical Medicine, Antwerp, Belgium, and the Swiss Tropical Institute, Basel, Switzerland (Table 1). Other species such as Escherichia coli 25922 and Saccharomyces cerevisiae 4098 were obtained from the American Type Cell Collection, Manassas, Va. All analytical samples were fixed at 4°C for 1 h with 1 ml of 1.5% formaldehyde-PBS. After centrifugation at 10,000 × g for 10 s, pellets were resuspended and treated with 50% ethanol (Merck)-MilliQ water solution for a minimum of 1 h at −20°C. Next, 10 μl of sample suspension was applied onto a microscopy slide and dried for 10 min at 60°C. Alternatively, thin blood smears were prepared using 2 μl of human and mouse blood containing various trypanosomes. Next, smears were fixed with methanol (Merck) for 5 min at room temperature and air dried. For cytospin analysis of blood samples, erythrocytes were first lysed with erythrocyte lysing buffer from Qiagen (Westbourgh, The Netherlands). Cell suspensions (200 μl) were centrifuged and spotted onto microscopy slides using a Stlandos/Elliott cytospin. Slides were air dried and fixed with methanol as described above.
TABLE 1.
Origins of trypanosome species and subspecies used in this studya
| Subgenus | Species | Clone or strain code | Parasite form | Origin | Host |
|---|---|---|---|---|---|
| Trypanozoon | T. brucei brucei | EATRO1125 AnTat 1.1 ITMAS 121296A | Bloodstream | Uganda | Bushbuck |
| Trypanozoon | T. brucei gambiense | LiTAR1 LiTat 1.3 ITMAS 100500 | Bloodstream | Ivory Coast | Human |
| Trypanozoon | T. brucei rhodesiense | LIRI/UTRO STIB 851 ITMAS 080399C | Bloodstream | Uganda | Human |
| Trypanozoon | T. eyansi | RoTat 1.2 ITMAS 020289 | Bloodstream | Indonesia | Buffalo |
| Trypanozoon | T. equiperdum | BoTAR1 BoTat 1.1 ITMAS 240982A | Bloodstream | France | Horse |
| Nannomonas | T. congolense | TRT 17 ITMAS 020699 | Bloodstream | Zambia | Bovine |
The table indicates the strain or clone identification number, place of isolation, and type of host species. The reference trypanosomes T. brucei brucei AnTat 1.1 EATRO1125, T. brucei gambiense LiTAR1, LiTat 1.3, T. evansi RoTat 1.2, T. equiperdum BoTat 1.1, and T. congolense TRT 17 were provided by E. Magnus from the Institute for Tropical Medicine. T. brucei rhodesiense STIB 851 LIRI/UTRO was provided by R. Kaminsky from the Swiss Tropical Institute.
In order to identify targets for the design of PNA probes, database-available Trypanozoon 18S ribosomal DNA sequences were aligned. BLAST searches did not detect any other protozoan ribosomal DNA sequences with a 100% identity match to the selected target sequences. Three 13- to 15-nucleotide-long PNA probes—Tbr7 (CGGAACCCAGCCA), Tbr16 (GCCCTAACAGGTGTG), and Tbr18 (GTTGCCACCAGCAGT)—were synthesized. For the analysis of PNA binding, total RNA was extracted from DE52-purified trypanosomes (12) or parasite-spiked blood, using a Qiagen extraction kit, and dot blot hybridization was performed as described before (6). Briefly, 20 ng in 10 μl of total RNA was blotted onto nylon membranes (Gibco-BRL), which were subsequently incubated in hybridization buffer with 400 nM PNA probes for 60 min at 45 or 55°C. Next, membranes were washed three times for 5 min at 50°C, and development was carried out as described before (6).
The PNA FISH itself was performed as previously described (17). Briefly, smears were treated with 20 to 40 μl of 400 nM fluorescein-labeled PNA probe in hybridization buffer and hybridized at 45 or 55°C for 90 min. The excess of PNA probe was removed by immersion of the microscope slides into in prewarmed (45°C) washing buffer for 30 min. The slides were further analyzed using fluorescence microscope (Leica) as described before (17).
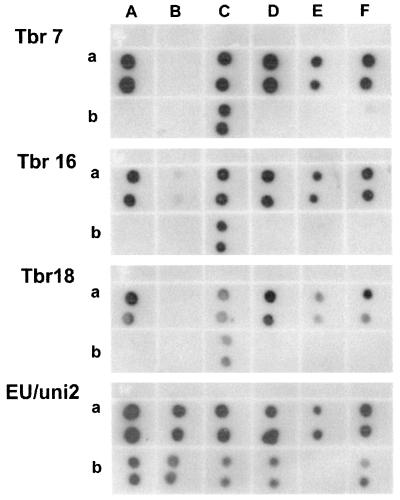
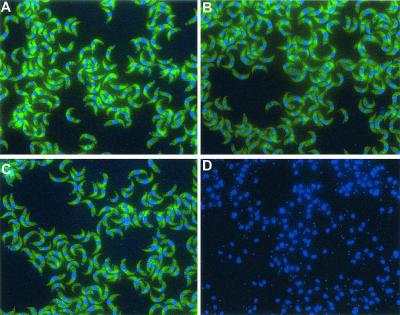
In order to validate the fluorescein-labeled PNA probes, they were first tested for their specificity in dot blot hybridization, using RNA of various reference trypanosomes as well as control pathogenic and nonpathogenic microorganisms (Table 1). As shown in Fig. 1, at 45°C, PNA probes Tbr7 and Tbr18 hybridized strongly to RNA of T. brucei gambiense, T. brucei rhodesiense, T. brucei brucei, T. evansi, and T. equiperdum (Trypanozoon subgenus) and a human blood sample spiked with T. brucei rhodesiense. PNA probes Tbr7 and Tbr18 did not hybridize to control RNA sequences of Nannomonas (T. congolense), P. falciparum, L. donovani, E. coli, or S. cerevisiae (Fig. 1). PNA probe Tbr16 gave a strong hybridization signal at 55°C, detecting RNA of all Trypanozoon species, while slightly cross hybridizing to RNA of T. congolense from the subgenus Nannomonas. The same PNA probe did not hybridize to the remaining control RNA sequences (Fig. 1). A universal PNA probe (EU/uni2) targeting many known eukaryotic sequences served as a positive control for the RNA preparations (Fig. 1). Next, PNA probes were tested in slide-based FISH for direct trypanosome identification in smears of purified parasites as well as blood and tissue samples. Concordant with the dot blot results, a clear and strong fluorescence signal was obtained with PNA probes Tbr17 and Tbr18 using 90 min of hybridization at 45°C. Probe Tbr16 strongly hybridized at 55°C. As demonstrated in Fig. 2 A to C using all probes, individual parasites were easily detectable by their bright green fluorescence, localized in the trypanosome cytoplasm. The fluorescent signal was absent in DAPI (4′,6′-diamidino-2-phenylindole)-stained organelles such as nucleus and kinetoplast. The negative control PNA probe specific for Staphylococcus aureus did not hybridize to any trypanosome 18S rRNA (Fig. 2D). Similar results were obtained when analyzing thin smears of trypanosome-containing human or mouse blood and spleen or liver cell suspension smears that had been prepared as described earlier (16). Here, a single parasite could be detected in a 2-μl blood smear. This corresponds to 500 trypanosomes/ml of blood, i.e., a detection limit 100 times more sensitive than the conventional counting chamber detection limit. This detection limit was further improved when PNA FISH was combined with a cytospin technique. Using serial dilutions of parasite-blood samples, a single parasite in a 200-μl volume could be detected, thus equaling a detection limit of five parasites/ml.
FIG. 1.
Dot blot hybridization of membrane-blotted RNA of trypanosomes and various controls using anti-fluorescein alkaline phosphatase-labeled PNA probes Tbr7, Tbr16, and Tbr18 and positive control EU/uni2. Hybridization reaction is revealed by addition of chemiluminescent substrate. Isolates shown (indicated by lane letter and row letter) are as follows: Aa, RNA of T. evansi; Ba, T. congolense; Ca, T. brucei brucei; Da, T. brucei rhodesiense; Ea, T. brucei gambiense; Fa, T. equiperdum; Ab, P. falciparum; Bb, L. donovani; Cb, human blood sample containing trypanosomes; Db, E. coli; Eb, negative control in which RNA content was omitted; Fb, S. cerevisiae.
FIG. 2.
Fluorescence microscope detection of purified T. brucei rhodesiense parasites stained with fluorescein-labeled PNA probes in FISH. The blue DAPI staining indicates the position of trypanosome nucleus and kinetoplast. (A) Probe Tbr7; (B) probe Tbr16; (C) probe Tbr18; (D) negative control PNA probe specific for S. aureus.
To date, control of sleeping sickness heavily relies on active patient diagnosis and correct drug treatment (21). As conventional parasite detection by microscopy suffers from limited sensitivity, we present in this paper a new diagnostic test, the Trypanozoon-specific PNA FISH. This test facilitates the microscopic detection of a single trypanosome cell by targeting widely distributed and conserved cytoplasmic multicopy 18S rRNA sequences with PNA probes. Advantages of the PNA probes are as follows. (i) They are designed by BLAST search to match specifically phylogenetically highly conserved Trypanozoon 18S rRNA, avoiding loss of detection by antigenic variation and avoiding cross-reactivity with other protozoan parasites. (ii) Their high hybridization performance saves time over DNA-based hybridization (1, 2, 14). (iii) Their resistance to cellular proteases and nucleases makes them less prone to degradation during hybridization procedures (3, 17). (iv) Methanol-fixed smears prepared for Plasmodium screenings can be costained for PNA FISH analysis. (v) The use of fluorescence microscopy makes Trypanozoon PNA FISH adaptable to the majority of clinical laboratories.
In the past, various PCR tests have been developed to detect trypanosomes. Although primers were derived from the repetitive DNA sequences, the detection limit obtained with blood samples was only in the range of 40 trypanosomes/ml of blood. This is not significantly different from conventional parasitological laboratory detection techniques such as the mini-anion-exchange column technique (4, 9-11, 15, 20). In comparison, Trypanozoon PNA FISH with undiluted thin blood smears is a much-simplified rapid way of specific parasite detection, still reaching a detection limit of 500 trypanosomes/ml of blood. By adding a cytospin step, this limit can be improved to five parasites/ml, equaling an optimal PCR detection limit on blood samples. To prove the test's field reliability in detection of sleeping sickness in the different infection foci known today, future on-site studies will be needed. However, based on the blood-spiked tests provided here, PNA FISH looks to be a promising new tool in the fight against sleeping sickness.
Acknowledgments
We are particularly grateful to Eddy Magnus, Luc Verhelst, Rudi Baelmans, and Peter Ilegems for their technical assistance.
S.M. is supported by a postdoctoral fellowship of the Foundation for Scientific Research-Flanders.
REFERENCES
- 1.Bromidge, T., W. Gibson, K. Hudson, and P. Dukes. 1993. Identification of Trypanosoma brucei gambiense by PCR amplification of variant surface glycoprotein genes. Acta Trop. 53:107-119. [DOI] [PubMed] [Google Scholar]
- 2.Dukes, P., W. C. Gibson, J. K. Gashumba, K. M. Hudson, and T. J. Bromidge, A. Kaukus, T. Asonganyi, and E. Magnus. 1992. Absence of the LiTat 1.3 (CATT antigen) gene in Trypanosoma brucei gambiense stocks from Cameroon. Acta Trop. 51:123-134. [DOI] [PubMed] [Google Scholar]
- 3.Egholm, M., O. Buchard, L. Christensen, C. Behrens, S. M. Freirer, D. A. Driver, R. H. Berg, S. K. Kim, B. Norden, and P. E. Nielsen. 1993. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen bonding rules. Nature 365:556-568. [DOI] [PubMed] [Google Scholar]
- 4.Enyaru, J. C. K., E. Matovu, M. Akol, C. Sebikali, J. Kyambadde, C. Schmid, R. Brun, R. Kaminsky, L. M. Ogwal, and F. Kansiime. 1998. Parasitological detection of Trypanosoma brucei gambiense in serologically negative sleeping sickness suspects from North-west Uganda. Ann. Trop. Med. Parasitol. 92:845-850. [DOI] [PubMed] [Google Scholar]
- 5.Gibson, W. 1986. Will the real Trypanosoma brucei gambiense please stand up? Parasitol. Today 2:255-257. [DOI] [PubMed] [Google Scholar]
- 6.Gildea, B. D., C. Shelagh, J. MacNeill, H. Perry-O Keefe, D. Sorensen, and J. M. Coull. 1998. PNA solubility enhancers. Tetrahedron Lett. 39:7255-7258. [Google Scholar]
- 7.Hide, G. 1999. History of sleeping sickness. Clin. Microbiol. Rev. 12:112-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoare, C. A. 1972. The trypanosomes of mammals. Blackwell Scientific Publication, Oxford, United Kingdom.
- 9.Kabiri, M., J. R. Franco, P. P. Simarro, J. A. Ruiz, M. Sarsa, and D. Steverding. 1999. Detection of Trypanosoma brucei gambiense in sleeping sickness suspects by PCR amplification of expression-site-associated genes 6 and 7. Trop. Med. Int. Health 4:658-661. [DOI] [PubMed] [Google Scholar]
- 10.Kanmogne, G. D., T. Asonganyi, and W. C. Gibson. 1996. Detection of Trypanosoma brucei gambiense, in serologically positive but aparasitaemic sleeping sickness suspects in Cameroon, by PCR. Ann. Trop. Med. Parasitol. 90:475-483. [DOI] [PubMed] [Google Scholar]
- 11.Kyambadde, J. W., J. C. K. Enyaru, E. Matovu, M. Odiit, and J. F. Carasco. 2000. Detection of trypanosomes in suspected sleeping sickness patients in Uganda using the polymerase chain reaction. Bull. W. H. O. 78:119-124. [PMC free article] [PubMed] [Google Scholar]
- 12.Lanham, S. M., and D. G. Godfrey. 1970. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp. Parasitol. 28:521-534. [DOI] [PubMed] [Google Scholar]
- 13.Magnus, E., T. Vervoort, and N. Van Miervenne. 1978. A card agglutination test with stained trypanosomes (CATT) for the serological diagnosis of T. b. gambiense trypanosomiasis. Ann. Soc. Belg. Med. Trop. 58:169-176. [PubMed] [Google Scholar]
- 14.Pays, E., L. Vanhamme, and M. Berberof. 1994. Genetic controls for the expression of surface proteins in African trypanosomes. Annu. Rev. Microbiol. 48:25-52. [DOI] [PubMed] [Google Scholar]
- 15.Penchenier, L., G. Simo, P. Grebaut, S. Nkinin, C. Laveissiere, and S. Herder. 2000. Diagnosis of human trypanosomiasis, due to Trypanosoma brucei gambiense in central Africa, by the polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 94:392-394. [DOI] [PubMed] [Google Scholar]
- 16.Radwanska, M., S. Magez, A. Michel, B. Stijlemans, M. Geuskens, and E. Pays. 2000. Comparative analysis of antibody responses against HSP, invariant surface glycoprotein 70, and variant surface glycoprotein reveals a complex antigen-specific pattern of immunoglobulin isotype switching during infection by Trypanosoma brucei. Infect. Immun. 68:848-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stender, H., K. Lund, K. H. Petersen, O. F. Rasmussen, P. Hongmanee, H. Miorner, and S. E. Godtfredsen. 1999. Fluorescence in situ hybridization assay using peptide nucleic acid probes for differentiation between tuberculous and nontuberculous species in smears of Mycobacterium cultures. J. Clin. Microbiol. 37:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stender, H., C. Kurtzman, J. J. Hyldig-Nielsen, D. Sorensen, D., A. Broomer, K. Oliveira, H. Perry O'Keefe, A. Sage, B. Young, and J. Coull. 2001. Identification of Dekkera bruxellensis (Brettanomyces) from wine by fluorescence in situ hybridization using peptide nucleic acid probes. Appl. Environ. Microbiol. 67:938-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stender, H., K. Oliveira, S. Rigby, F. Bargoot, and J. Coull. 2001. Rapid detection, identification, and enumeration of Escherichia coli by fluorescence in situ hybridization using an array scanner. J. Microbiol. Methods 45:31-39. [DOI] [PubMed] [Google Scholar]
- 20.Truc, P., V. Jamonneau, G. Cuny, G., and J. L. Frezil. 1999. Use of polymerase chain reaction in human African trypanosomosis stage determination and follow-up. Bull. W. H. O. 77:745-748. [PMC free article] [PubMed] [Google Scholar]
- 21.Van Meirvenne, N. 1999. Biological diagnosis of human African trypanosomiasis, p. 235-252. In M. Dumas, B. Bouteille, and A. Buguet (ed.), Progress in human trypanosomiasis, sleeping sickness. Springer, Paris, France.