Abstract
One hundred eighty-four serologically confirmed cases of hepatitis A were reported in eastern Spain in 1999. A matched case-control study implicated imported coquina clams complying with European Union shellfish standards as the source of infection; this implication was confirmed by the detection by reverse transcription-PCR of hepatitis A virus (HAV) RNA in shellfish samples. In spite of the recognized low variability of HAV, genetic characterization of the complete capsid region of virus isolates from patient serum samples revealed the existence of both synonymous and nonsynonymous variants. Two antigenic variants were detected, one in a discontinuous epitope defined by monoclonal antibody K3-4C8 and a second in a linear VP1 epitope of the virus. In spite of these antigenic variants, all isolates were assigned to genotype IB, providing further evidence that the outbreak originated from a common source, although multiple strains were likely to be involved.
Hepatitis A virus (HAV) infection is the leading cause of viral hepatitis in Spain (40) and throughout the world (9). Although transmission through the parenteral route may occur (28, 38), HAV infection is mainly propagated via the fecal-oral route (24). While in approximately 40% of the reported cases of hepatitis A the source of infection cannot be identified (9), waterborne (6) and food-borne (31, 34) outbreaks of the disease have been reported. Within the latter category, shellfish grown and harvested from waters receiving urban contaminants is a frequent cause of infectious hepatitis (17, 25).
Hepatitis A virus, the prototype of the genus Hepatovirus, belongs to the family Picornaviridae. Its 7.5-kb single-stranded RNA genome bears different regions: the 5′ and 3′ noncoding regions (NCRs); the P1 region, which encodes structural proteins VP1, VP2, VP3, and putative VP4; and the P2 and P3 regions, which encode nonstructural proteins associated with replication (18).
Immunological evidence has determined the existence of a single serotype of HAV (22), a fact which prevents the use of serological approaches to characterize different virus isolates. To overcome this difficulty, sequencing of the putative VP1/2A junction of the virus genome is used to differentiate HAV isolates into seven genotypes (32).
On 22 October 1999, the Health Department in Valencia, Spain, reported the occurrence of a hepatitis A outbreak and its possible association with imported frozen cockles. Concomitantly, a rapid alert system for food-borne outbreaks that was developed within the framework of a European Union project (QLRT-1999-0594) made us aware of the existence of the outbreak. Although the first disease reports appeared in early October, the first cases actually occurred in early September.
MATERIALS AND METHODS
Case-control study.
Cases were identified on the basis of reports from five hospitals—Hospital de La Ribera of Alzira, Hospital General of Ontinyent, Hospital Lluís Alcanyís of Xàtiva, Hospital of Requena, and Hospital La Fe of Valencia—as well as from physicians in the affected sanitary districts. A probable case was defined as a person with a symptomatology compatible with hepatitis A, together with an increase in transaminases above normal levels and the absence of other hepatic disease. A confirmed case was characterized by the detection of anti-HAV immunoglobulin M (IgM) antibody, with or without hepatitis symptoms. A secondary case was defined as a person acquiring the disease 14 to 50 days after being in contact with a person suffering from hepatitis A. All probable, confirmed, or secondary cases with an onset of symptoms between 1 September and 31 December 1999 in the affected areas were included in the study. Persons without symptoms of the disease and with similar ages, habits, and habitats were enrolled as controls. A total of 391 epidemiological surveys, corresponding to 189 cases and 202 controls, were used to elucidate whether hepatitis cases were associated with selected exposures by calculation of odds ratios (36). Studied risk factors included history of nursery, school, household, or sexual contact with a person with hepatitis A during the 50 days prior to the onset of illness; association with water distribution or sanitation network problems; and consumption of different types of water or food, including vegetables and shellfish.
Samples and sample processing.
Frozen samples of clams directly associated with the outbreak, i.e., from households and restaurants implicated in cases of hepatitis A from the towns of Alzira, Utiel, Pontón, Requena, Mislata, Castelló, and Xàtiva, were analyzed for the presence of HAV. Processing of shellfish was performed essentially by the method described by Atmar et al. (2). Briefly, the stomachs and digestive diverticula were dissected from the clams and subjected to high-speed homogenization (Sorval OCI Omni mixer; Omni International, Waterbury, Conn.). Viruses were extracted from the homogenates (corresponding to 1.5 g of shellfish tissue) by sequential extractions with chloroform-butanol and Cat-Floc T (Calgon Corp., Elwood, Pa.) and concentrated by polyethylene glycol precipitation. Nucleic acids were purified from concentrated viruses by proteinase K digestion, two sequential phenol-chloroform extractions, ethanol precipitation, cetyltrimethylammonium bromide (CTAB; Sigma) precipitation, and another ethanol precipitation (2).
Serum samples (60 μl) from patients with hepatitis A were assayed for HAV after nucleic acid extraction by guanidine thiocyanate treatment (3). Stool samples from the same patients were suspended (10% [wt/vol]) in phosphate-buffered saline containing 2 M NaNO3, 1% bovine serum albumin (fractionV), and 0.1% Triton X-100 (pH 7.2) and pelleted at 1,000 × g for 5 min; nucleic acids from 60 μl of the resulting supernatant were extracted by following the procedure described by Boom et al. (3).
Molecular procedures.
Table 1 lists the oligonucleotides and conditions used for the molecular detection and characterization of HAV in the different types of specimens by reverse transcription (RT)-PCR. Primers of the 5′ NCR were used for generic HAV detection with the Expand RT-PCR system (Roche) by following the manufacturer's instructions, except for the RT reaction, which was performed at 45°C for 1 h. For PCR amplification, an initial 4 min of denaturation at 94°C, 40 cycles of amplification (94°C for 1 min, 55°C for 1 min, and 72°C for 1.5 min), and a final extension at 72°C for 10 min were performed. PCR amplification products were confirmed by Southern blot hybridization with an internal digoxigenin-labeled probe (5′-TTAATTCCTGCAGGTTCAGG-3′) at 40°C for 2 h in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and sequential washes down to 0.5× SSC at room temperature. Additionally, sequence analyses were performed for some samples as described below.
TABLE 1.
Primers and conditions used for RT-PCR assays for the detection and characterization of HAV
| Target region | Sequence | Locationa | [Mg2+] (mM) | Primer annealing temp (°C) |
|---|---|---|---|---|
| 5′ NCR | TCACCGCCGTTTGCCTAG | 68-85 | 1.5 | 55 |
| 5′ NCR | GGAGAGCCCTGGAAGAAAGA | 241-222 | 1.5 | 55 |
| NH2-VP0 | CAGCTGGACTGTTCTTTGGG | 648-667 | 2.0 | 55 |
| NH2-VP0 | TCACCAGGAACCATAGCACAG | 1198-1178 | 2.0 | 55 |
| COOH-VP0 | TACAATGAGCAGTTTGCTGT | 1065-1084 | 2.0 | 55 |
| COOH-VP0 | GCTCTTGCATCTTCATAATTTG | 1543-1522 | 2.0 | 55 |
| NH2-VP3 | GGGACAGGAACTTCAGCTTATAC | 1380-1402 | 2.0 | 50 |
| NH2-VP3 | TCTACCTGAATGATATTTGG | 1859-1840 | 2.0 | 50 |
| Inner VP3 | GTTATTCCAGTGGACCCATATT | 1701-1722 | 2.0 | 50 |
| Inner VP3 | TCGTGTACCTATTCACTCTATA | 2031-2010 | 2.0 | 50 |
| COOH-VP3 | TGTGCAGTGATGGATATTACAG | 1938-1959 | 2.0 | 50 |
| COOH-VP3 | GTTGTTATGCCAACTTGGGGA | 2287-2267 | 2.0 | 50 |
| NH2-VP1 | AATGTTTATCTTTCAGCAAT | 2136-2155 | 2.0 | 53 |
| NH2-VP1 | ACAGCTCCAAGAGCAGTTTT | 2751-2770 | 2.0 | 53 |
| COOH-VP1 | ATGGCCTGGTTTACTCCAG | 2673-2691 | 2.5 | 45 |
| COOH-VP1 | CCCTTCATTTTCCTAGG | 3229-3213 | 2.5 | 45 |
The complete VP1/2A genome region of HAV isolates was sequenced after amplification with the Pwo RT-PCR system (Roche) by following the manufacturer's specifications and with primers corresponding to the capsid protein sequences (Table 1). Sequencing of RT-PCR products was performed in both directions with a Thermo Sequenase II dye terminator cycle sequencing premix kit (Amersham Pharmacia Biotech) by following the manufacturer's instructions and with an ABI Prism 377 automated DNA sequencer (Perkin-Elmer). Multiple sequence alignments were carried out with the ClustalW program (European Bioinformatics Institute), and neighbor-joining trees were generated with the Phylip program (Department of Genetics, University of Washington). Nucleotide diversity, i.e., the average number of nucleotide substitutions per site (21), was calculated by using the DnaSP 3.0 software package (University of Barcelona). Genetic variability was estimated by dividing the number of variant nucleotides found relative to the most frequent nucleotide sequence by the total number of nucleotides sequenced (14).
Immunological procedures.
HAV in 10% stool suspensions was titrated by a sandwich enzyme-linked immunosorbent assay (ELISA) consisting of immunocapture with convalescent-phase serum HCS-2 (generously provided by R. Lluna, Hospital Militar, Barcelona, Spain), which recognized HAV at a 1/1,000,000 dilution (5, 30), and detection with monoclonal antibody (MAb) 33Z/37/39 (generously provided by Z.-M. Yun, Institute of Virology, Beijing, China) and peroxidase-labeled goat anti-mouse IgG (Sigma) as primary and secondary antibodies, respectively. We regularly use this procedure for HAV titration by ELISA. Cytopathogenic HAV strain HM-175 (courtesy of T. Cromeans, Centers for Disease Control and Prevention, Atlanta, Ga.) (12), propagated and assayed in FRhK-4 cells (5), was used as a positive control. Mock-infected FRhK-4 cells were used as negative controls. The same procedure was used to assay virus recognition by MAbs K3-4C8 and K2-4F2 (Commonwealth Serum Laboratories, Boronia, Victoria, Australia) by replacing MAb 33Z/37/39 with these latter MAbs. All incubations were carried out at 37°C for 2 h. MAb K2-4F2 recognizes 14S-specific epitopes, present in both pentamers and capsids, while MAb K3-4C8 recognizes 70S-specific epitopes, present only in capsids (39).
All experimental procedures were performed at least in triplicate.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study for the VP1/2A region and the 5′ NCR have been deposited in GenBank and have been assigned accession numbers AF396391 to AF396408 and AY094544 to AY094575, respectively.
RESULTS
Outbreak.
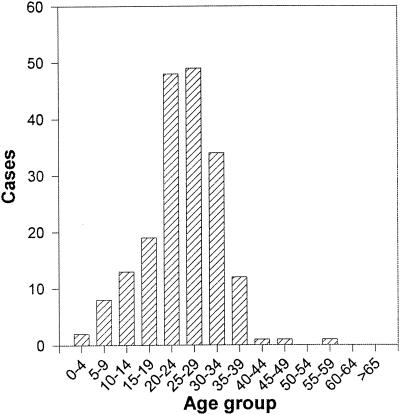
One hundred eighty-four confirmed cases of hepatitis A were reported in health areas 6, 7, 10, and 13 of the Autonomous Community of Valencia in eastern Spain. The curve of hepatitis A incidence clearly followed a holomiantic pattern, indicating a common source (data not shown). Secondary cases accounted for only 0.5% of the total cases. The affected areas comprise a total population of 593,373 inhabitants. Dates of illness onset ranged from weeks 39 to 52 of 1999. In addition to the conclusive presence of serum IgM antibody to HAV, hepatitis patients showed jaundice (88%), vomiting (44%), nausea (46%), asthenia (60%), anorexia (60%), choluria (86%), acholia (48%), and fever (57%). The median age of the patients was 24.6 years (range, 3 to 57), and 70% of the cases occurred in individuals 20 to 34 years old (Fig. 1). Sixty percent of the patients were male, and 40% were female.
FIG. 1.
Age distribution (years) of patients with hepatitis A in the Valencia outbreak.
Case-control study.
The case-control study revealed that the only significant positive association was found with the consumption of coquina clams at an odds ratio of 3.81 (95% confidence interval, 2.33 to 6.26) (Table 2). Surveys performed by the Department of Epidemiology of the Generalitat Valenciana indicated a common source of the clams implicated in the outbreak. All clams had been imported frozen from Peru and could be classified as Donax spp.
TABLE 2.
Consumption of selected food or water items by case patients and control individuals in the affected areas between 1 September and 31 December 1999
| Food | No. (%) of persons who consumed the food
|
||
|---|---|---|---|
| Cases (n = 189) | Controls (n = 202) | Odds ratio (95% confidence interval) | |
| Coquina clams | 155 (82.0) | 110 (54.4) | 3.81 (2.33-6.26) |
| Other clams | 0 (0.0) | 2 (1.0) | |
| Mussels | 37 (19.6) | 46 (22.8) | 0.83 (0.49-1.39) |
| Jackknife clams | 8 (4.2) | 4 (1.9) | 2.19 (0.58-8.89) |
| Oysters | 0 (0.0) | 0 (0.0) | |
| Crustacean shellfish | 30 (15.9) | 39 (19.3) | 0.79 (0.45-1.38) |
| Vegetables | 131 (69.3) | 137 (67.8) | 1.07 (0.68-1.69) |
| Spring water | 156 (82.5) | 178 (88.1) | 0.64 (0.35-1.17) |
| Tap water | 45 (23.8) | 54 (26.7) | 0.86 (0.53-1.39) |
| Bottled water | 54 (28.5) | 55 (27.2) | 1.07 (0.67-1.71) |
HAV detection in shellfish and clinical samples.
HAV RNA was detected by RT-PCR with primers of the 5′ NCR in 75% (15 of 20) of the frozen coquina clam samples directly related to hepatitis cases. The same molecular procedure enabled the amplification of HAV in 60% (34 of 57) of the serum samples. This method, regularly used by us for generic HAV detection, has a sensitivity inversely proportional to the time elapsed between the onset of symptoms and sample collection. For instance, 90% of the IgM-positive samples collected during the first week of the disease were found positive by RT-PCR, while only 50% of the samples collected 1 week later were found positive by RT-PCR (data not shown). This time limitation is due to the low level and transient nature of viremia. The decline in viremia over time makes the use of nested PCR a requirement for the detection of viral RNA 1 month after the alanine aminotransferase peak (8).
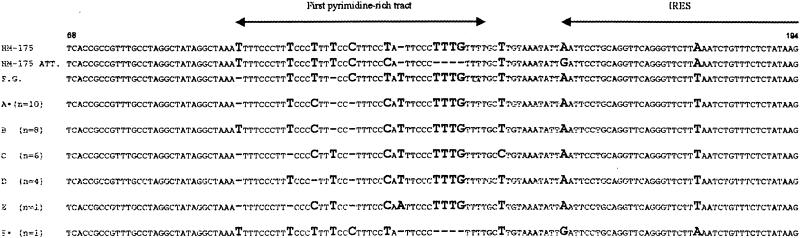
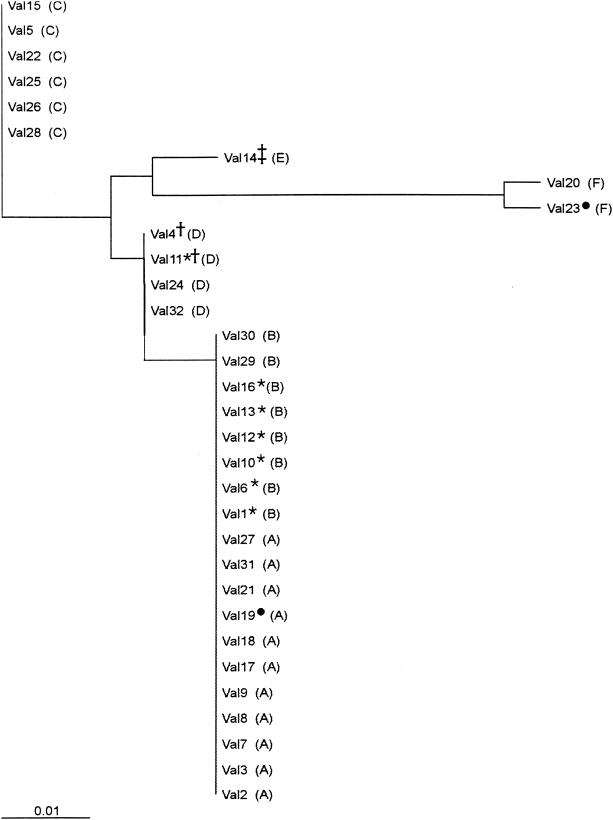
The shellfish-borne origin of the outbreak was confirmed by aligning a 127-nucleotide (nt) fragment from the 5′ NCR (nt 68 to 194) of 30 serum samples and 2 shellfish samples. Six different groups were observed after alignment of the serum sequences, as shown in Fig. 2. However, the neighbor-joining tree depicts only five groups, since groups A and B differed only in a single point mutation and were considered a single group (Fig. 3). In group F, a specific deletion of 4 nt (nt 131 to 134) was detected, indicating the potential circulation of at least two different viral strains. The highest homologies among groups A to E were observed between groups A and D (98.4%) and between groups C and E (98.4%), while the lowest homology (96%) was detected between groups B and E. When deletions were not considered for the calculations, 100% homology was observed among groups A, B, and D, and the largest distance appeared between groups C and E (98.4% homology). All but three detected mutations were contained in the first pyrimidine-rich tract region. The two shellfish sequences were 100% homologous to groups A and F.
FIG. 2.
Alignment of a 127-nt fragment from the 5′NCR of 30 serum samples from hepatitis A patients. The first three sequences were obtained from GenBank (accession no. M14707, M16632, and X83302). The number of samples included in each group of sequences is shown in parentheses. Two shellfish sample sequences showed 100% homology with groups labeled with an asterisk. Nucleotide variants are depicted in bold. ATT., attenuated; IRES, internal ribosome entry site.
FIG. 3.
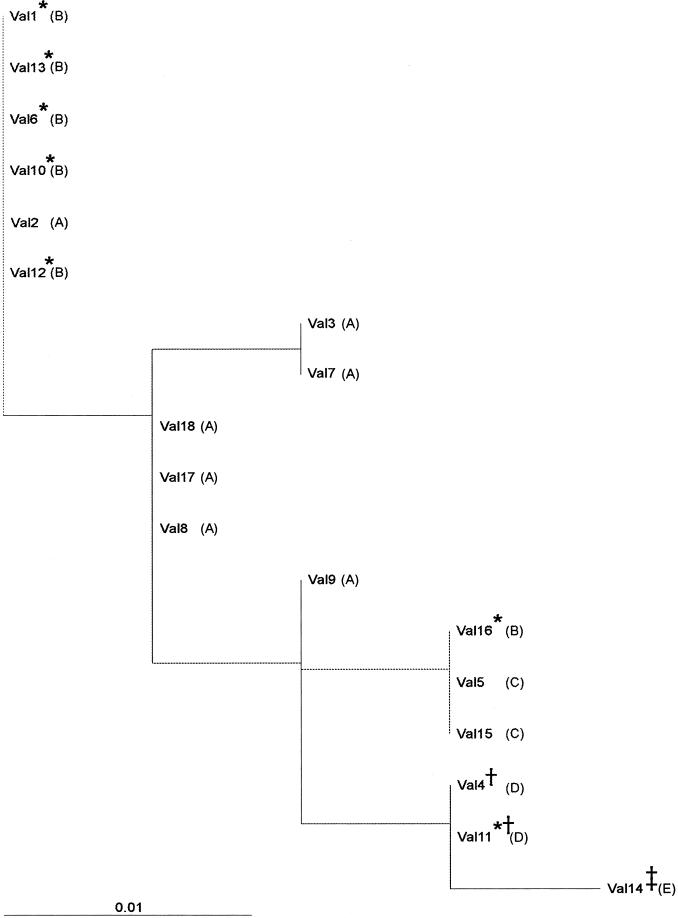
Phylogenetic tree based on the sequences of the 5′ NCR shown in Fig. 2. Letters corresponding to the same groups as those depicted in Fig. 2 are shown in parentheses. Symbols: circle, shellfish samples; asterisk, samples with a valine-to-isoleucine change at position 72 of protein VP3; dagger, samples with a valine-to-alanine change at position 63 of protein VP0; double dagger, samples with a methionine-to-valine change at position 28 of protein VP1.
Capsid variability of HAV isolates.
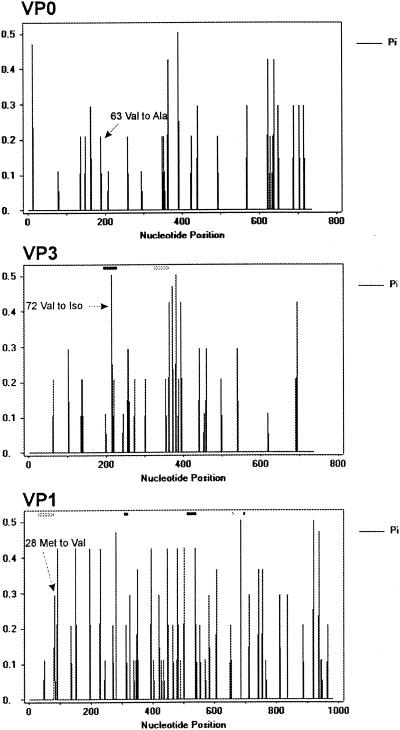
The complete VP1/2A region sequence corresponding to the HAV capsid proteins was determined for 18 of the 5′ NCR RT-PCR-positive samples. Analysis of the capsid sequences indicated that the frequency of variation was 6 × 10−3. For VP0 protein, nucleotide changes were detected all along the VP0 region, although distinct regions with higher nucleotide diversity were observed around nt 150 to 200, 350 to 400, 625 to 650, and 700 (Fig. 4). All these nucleotide changes were synonymous mutations, with the exception of one mutation at position 189, found in 2 out of 18 isolates, which induced a conservative change of Val to Ala at amino acid 63 of VP0 or 40 of VP2.
FIG. 4.
Nucleotide diversity (Pi) in the capsid genomic region, expressed as the average number of nucleotide substitutions per site (18). Rectangles represent known HAV epitope coding regions: black, immunodominant site including discontinuous epitopes in VP3 and VP1 (24, 26); white, H7c27 epitope (26); gray, linear epitopes in VP3 and VP1 (5, 13).
The distribution for VP1 protein (Fig. 4) showed a higher level of genetic variation all along the VP1 region, although nonsynonymous variants were not detected in the nucleotides coding for the immunodominant site (27, 29). The only nonsynonymous substitution identified was at position 82; it coded for a change of Met to Val. This amino acid at position 28 is located close to linear B− T− epitopes defined at positions 11 to 25 and 10 to 33, respectively (15, 20).
Genetic variability was observed all along the VP3 region, with higher nucleotide diversity around positions 135 to 140, 215 to 220, 240 to 275, 360 to 400, and 450 (Fig. 4). Interestingly, a nonsynonymous nucleotide change, implying a conservative substitution of Val by Iso, was detected in 7 out of 18 isolates at position 214, corresponding to amino acid 72, which is located in the middle of the immunodominant site of HAV (27, 29).
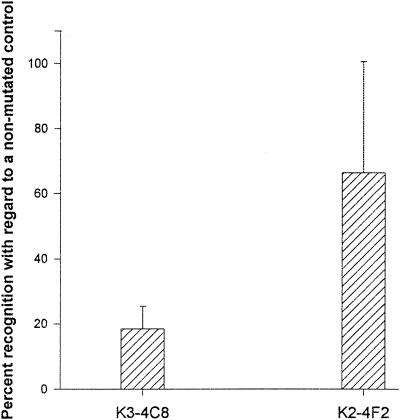
To assess whether the Iso-for-Val substitution at amino acid 72 of VP3 results in the appearance of a natural antigenic variant, a mutant virus isolated from a stool sample from a hepatitis A patient was characterized in terms of recognition by MAbs K3-4C8 and K2-4F2; for comparison, reference strain HM-175 was used as a nonmutated control. Previously, mutant and nonmutated virus suspensions had been equalized in terms of reactivity with MAb 33Z/37/39 in a sandwich ELISA and by RT-PCR amplification with primers of the 5′ NCR. Through both of these procedures it was established that 50 μl of a 10% suspension of the stool sample corresponded to approximately 50,000 50% tissue culture infective doses (TCID50) of strain HM-175. Under these conditions (50 μl of a 10% suspension of the stool sample and 50,000 TCID50 of strain HM-175), the mutant virus showed 81 and 35% reductions in recognition by MAbs K3-4C8 and K2-4F2, respectively, compared with the nonmutated HAV control (Fig. 5).
FIG. 5.
Effect of the Iso-for-Val substitution at amino acid 72 of VP3 on virus recognition by MAbs K3-4C8 and K2-4F2, as measured by an ELISA. Recognition of the virus isolate from 50 μl of a 10% stool suspension was compared with that of approximately 50,000 TCID50 of strain HM-175. Results are expressed as the mean and standard deviation.
In spite of the antigenic variants detected in both VP3 and VP1, all isolates were assigned to genotype IB, based on a sequence analysis of the VP1/2A junction region. Furthermore, samples showing 100% homology in this junction region bore amino acid changes in the capsid region (Fig. 6). The overall homology of the sequences in the VP1/2A junction region was 98%.
FIG. 6.
Phylogenetic tree based on the nucleotide sequence of the VP1/2A region (nt 3024 to 3191). Letters corresponding to the same groups as those depicted in Fig. 2 and 3 are shown in parentheses. Symbols: asterisk, samples with a valine-to-isoleucine change at position 72 of protein VP3; dagger, samples with a valine-to-alanine change at position 63 of protein VP0; double dagger, samples with a methionine-to-valine change at position 28 of protein VP1.
Control measures.
The Spanish Ministry of Health activated a national system of epidemiological surveillance to prevent the distribution of any further coquina clam shipments and informed officials at border checkpoints and the European Community Rapid Alert System for Foodstuffs. Peruvian shellfish imports are still banned by the European Union at the time of this writing.
The confirmation of the shellfish-borne nature of the outbreak led to the adoption of prophylactic measures in order to decrease the severity of the outbreak; these consisted of the immobilization and/or removal of 176,544 kg of coquina clams in the Autonomous Community of Valencia and 12,544 kg in the rest of Spain. At the same time, passive and active immunizations of the population in contact with affected individuals were undertaken. These measures led to the conclusion of the outbreak by the end of December 1999.
DISCUSSION
Genetic analysis may provide valuable information on the source of a virus in both sporadic and epidemic infections (40). This report describes an outbreak of hepatitis A epidemiologically linked to coquina clams. Genetic analysis of the 5′NCR demonstrated the occurrence of identical sequences in shellfish and patient serum samples, thus confirming the shellfish-borne origin of the outbreak. Although, because of technical impairments, only two sequences from shellfish samples could be analyzed, one of them contained a specific 4-nt deletion that was also present in one patient sequence. Additionally, the other shellfish sequence showed 100% homology with the sequences of 33% of the patient samples. The overall homology among the different sequences was 96%, and nucleotide changes were concentrated in the first pyrimidine-rich tract, as previously described (37).
HAV is a virus with recognized low antigenic variability, as reflected by the existence of a single serotype (22). However, antigenic variants selected for their resistance to different MAbs have been reported (27, 29). In our study, the complete capsid region sequence analysis revealed the existence of both synonymous and nonsynonymous variants among the HAV isolates, with an overall genetic variability in the range of those reported for other picornaviruses (14). Among these isolates, a natural antigenic variant inducing a loss of recognition by MAb K3-4C8 and a second variant in a linear epitope of VP1 (15, 20) were detected. However, a specific analysis of the VP1/2A junction, usually used for genotyping (32), determined that all sequences belonged to a single genotype, IB, in spite of the antigenic variants detected in both VP3 and VP1. This observation indicates that genotyping methods may not always reflect antigenic variations. On the other hand, it should be pointed out that although all sequences belonged to genotype IB, the overall homology was 98%, in contrast to other outbreaks with a common origin, where the homology among sequences of the VP1/2A junction was 100% (13, 19, 32). These data suggest that the clams were contaminated with several different strains prior to harvest; this suggestion is supported by the evidence of cocirculation of different HAV strains in a same geographic area (11) and the requirement for many HAV-infected shedding individuals for contamination of shellfish-containing waters. However, the potential capacity of RNA viruses to mutate their genomes, to exist as quasispecies (14), and thus to generate genetic variants should not be ignored.
The crystallographic structure of HAV is yet to be resolved. However, assuming that tolerance to amino acid substitutions is higher at surface protein sites free of structural constraints, it appears reasonable to presume that all observed amino acid changes are located on the capsid surface. Given that antigenic sites are frequently located at surface sites, it can be postulated that several of the detected substitutions should be involved in HAV antigenic sites, such as mutations in residue 28 of VP1 (15, 20) and residue 72 of VP3 (27, 29). Since this latter epitope site recognized by MAb K3-4C8 is conformation dependent and is detected only in 70S particles and not in 14S pentamers (39), it can be postulated that these changes constitute a natural antigenic variant for this MAb, whose site should be located near the threefold axis. The Iso-for-Val substitution is located at amino acid 72 of VP3, in the middle of the HAV immunodominant site (27, 29). No escape mutations have been described so far at this position. However, three of the amino acids surrounding this Val, Asp, Ser, and Gln, have been associated with several escape mutants (23). For other picornaviruses, such as rhinovirus 14 and several coxsackieviruses, Iso appears in this same position (35). An escape mutant associated with this Val position has been described for poliovirus 1 (26).
In regions of endemicity, the majority of HAV infections occur in childhood (16). In these areas, distinct outbreaks rarely happen and clinical disease related to HAV infections is uncommon, as children generally experience asymptomatic infections. In regions of nonendemicity, food-borne and waterborne hepatitis A requires specific prevention until high levels of immunity are achieved in all age groups as a result of routine vaccinations during childhood. Through continuous improvements in hygiene and environmental quality, the incidence of hepatitis A in Spain has dramatically decreased in recent years, with attack rates of 56.28 cases per 100,000 inhabitants in 1989 and of 2.48 cases per 100,000 inhabitants in 2000 (41). Furthermore, data from 1989 to 1991 show that the young population (<24 years old) accounted for 80% of reported hepatitis A cases (41). The age distribution of hepatitis cases in this outbreak also hints that Spain is no longer an area of endemicity for hepatitis A, since in this situation, people 20 to 35 years old should be immune, like those 40 to 60 years old.
Currently available virus diagnostic procedures enable the specific and sensitive detection of viruses in shellfish. The presence of HAV RNA in coquina clams provided definitive evidence that this molluscan shellfish was the cause of the hepatitis outbreak, as previously implied by the case-control study. Other enteric viruses, such as rotaviruses and enteroviruses, had also been found as contaminants of the same shellfish samples, while astroviruses, Norwalk-like viruses, and hepatitis E virus were not detected (7).
As a consequence of globalization, transnational outbreaks of food-borne infections are reported with increasing frequency. The reported outbreak was caused by imported frozen coquina clams complying with all European Union shellfish standards, which rely solely on bacteriological parameters (1). This outbreak reaffirms the fact that bacterial control is an insensitive proxy for viral contamination, since hepatitis A cases have been associated with the consumption of shellfish controlled through legal standards (25) and enteric viruses are often detected in shellfish with bacterial counts meeting the current criteria for public consumption (4, 33). Since bacterial indicators are unacceptable as indicators of viral pathogens, rapid and standardized assessment of virus contamination of shellfish should be implemented to prevent the occurrence and international spread of shellfish-borne viral infections. The outbreak described here could have been avoided if shellfish had been monitored for viruses.
Acknowledgments
We thank the Department of Epidemiology of the Directorate of Public Health of the Generalitat Valenciana for cooperation in this study. We acknowledge the skillful assistance of Àngels Rabassó and the technical expertise of the Serveis Científic-Tècnics of the University of Barcelona. We thank F. Le Guyader, Ifremer, Nantes, France, for active collaboration and D. N. Lees, CEFAS, Weymouth, United Kingdom, for useful discussions.
This study was supported in part by grants ERB3514PL973098, QLRT-1999-0634, and QLRT-1999-0594 from the European Union; grant BIO99-0455 from the CICYT, Ministry of Science and Technology, Spain; and grant 1997SGR 00224 from the Generalitat de Catalunya.
REFERENCES
- 1.Anonymous. Council directive of 15th of July, 1991. 91/492/EEC. J. Eur. Commun. L268:1-14. [Google Scholar]
- 2.Atmar, R. L., F. H. Neill, J. L. Romalde, F. Le Guyader, C. M. Woodley, T. G. Metcalf, and M. K. Estes. 1995. Detection of Norwalk virus and hepatitis A virus in shellfish with the PCR. Appl. Environ. Microbiol. 61:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. Van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch, A., F. X. Abad, R. Gajardo, and R. M. Pintó. 1994. Should shellfish be purified before public consumption? Lancet 344:1024-1025. [PubMed] [Google Scholar]
- 5.Bosch, A., J. F. González-Dankaart, I. Haro, R. Gajardo, J. A. Pérez, and R. M. Pintó. 1998. A new continuous epitope of hepatitis A virus. J. Med. Virol. 54:95-102. [DOI] [PubMed] [Google Scholar]
- 6.Bosch, A., F. Lucena, J. M. Díez, R. Gajardo, M. Blasi, and J. Jofre. 1991. Human enteric viruses and indicator microorganisms in a water supply associated with an outbreak of infectious hepatitis. J. Am. Water Works Assoc. 83:80-83. [Google Scholar]
- 7.Bosch, A., G. Sánchez, F. Le Guyader, H. Vanaclocha, L. Haugarreau, and R. M. Pintó. 2001. Human enteric viruses in coquina clams associated with a large hepatitis A outbreak. Water Sci. Technol. 43:61-75. [PubMed] [Google Scholar]
- 8.Bower, W. A., O. V. Nainan, X. Han, and H. S. Margolis. 2000. Duration of viremia in hepatitis A virus infection. J. Infect. Dis. 182:12-17. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1995. Hepatitis surveillance report no. 56. Centers for Disease Control and Prevention, Atlanta, Ga.
- 10.Cohen, J. I., B. Rosenblum, J. R. Ticehurst, R. J. Daemer, S. M. Feinstone, and R. H. Purcell. 1987. Complete nucleotide sequence of an attenuated hepatitis A virus: comparison with wild-type virus. Proc. Natl. Acad. Sci. USA 84:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa-Mattioli, M., V. Ferrer, S. Monpoeho, L. Garcia, R. Colina, S. Billaudel, I. Vega, R. Perez-Bercoff, and J. Cristina. 2001. Genetic variability of hepatitis A virus in South America reveals heterogeneity and co-circulation during epidemic outbreaks. J. Gen. Virol. 82:2647-2652. [DOI] [PubMed] [Google Scholar]
- 12.Cromeans, T., M. D. Sobsey, and H. A. Fields. 1987. Development of a plaque assay for a cytopathogenic, rapidly replicating isolate of a hepatitis A virus. J. Med. Virol. 22:45-56. [DOI] [PubMed] [Google Scholar]
- 13.Dentinger, C. M., W. A. Bower, O. V. Nainan, S. M. Cotter, G. Myers, L. M. Dubusky, S. Fowler, E. D. P. Salehi, and B. P. Bell. 2001. An outbreak of hepatitis A associated with green onions. J. Infect. Dis. 183:1273-1276. [DOI] [PubMed] [Google Scholar]
- 14.Domingo, E. 1996. Biological significance of viral quasispecies. Viral Hep. Rev. 2:247-261. [Google Scholar]
- 15.Emini, E. A., J. V. Hughes, D. S. Perlow, and J. Boger. 1985. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 55:836-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadler, S. C. 1991. Global impact of hepatitis A virus infection changing patterns, p. 14-20. In F. B. Hollinger, S. M. Lemon, and H. S. Margolis (ed.), Viral hepatitis and liver disease. The Williams & Wilkins Co., Baltimore, Md.
- 17.Halliday, M. L., L.-Y. Kang, T.-Z. Zhou, M.-D. Hu, Q.-C. Pan, T.-Y. Fu, Y. S. Huang, and S. L. Hu. 1991. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J. Infect. Dis. 164:852-859. [DOI] [PubMed] [Google Scholar]
- 18.Hollinger, F. B., and S. U. Emerson. 2001. Hepatitis A virus, p. 799-840. In B. N. Fields D. M. Knipe, and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 19.Hutin, Y. J. F., V. Pool, E. H. Cramer, O. V. Nainanm, J. Weth, I. T. Williams, S. T. Goldstein, K. F. Gensheimer, B. P. Bell, C. N. Shapiro, M. J. Alter, and H. S. Margolis. 1999. A multistate, foodborne outbreak of hepatitis A. N. Engl. J. Med. 340:595-602. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov, V. S., L. N. Kulik, A. E. Gabrielian, L. D. Tehikin, A. T. Kozhich, and V. T. Ivanov. 1994. Synthetic peptides in the determination of hepatitis A T-cell epitopes. FEBS Lett. 345:159-161. [DOI] [PubMed] [Google Scholar]
- 21.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, Inc., New York, N.Y.
- 22.Lemon, S. M., and L. N. Binn. 1983. Antigenic relatedness of two strains of hepatitis A virus determined by cross-neutralization. Infect. Immun. 42:418-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemon, S. M., and L.-H. Ping. 1989. Antigenic structure of hepatitis A virus, p. 193-208. In B. L. Semler and E. Ehrenfeld (ed.), Molecular aspects of picornavirus infection and detection. American Society for Microbiology, Washington, D.C.
- 24.Mast, E. E., and M. J. Alter. 1993. Epidemiology of viral hepatitis: an overview. Semin. Virol. 4:273-283. [Google Scholar]
- 25.Mele, A., M. G. Rastelli, O. N. Gill, D. DiBisceglie, F. Rosmini, G. Pardelli, C. Valtriani, and P. Patriarchi. 1989. Recurrent epidemic hepatitis A associated with the consumption of raw shellfish, probably controlled through public health measures. Am. J. Epidemiol. 130:540-546. [DOI] [PubMed] [Google Scholar]
- 26.Minor, P. D., M. Ferguson, D. M. A. Evans, J. W. Almond, and J. P. Icenogle. 1986. Antigenic structure of polioviruses of serotypes 1, 2, and 3. J. Gen. Virol. 67:1283-1291. [DOI] [PubMed] [Google Scholar]
- 27.Nainan, O. V., M. A. Brinton, and H. S. Margolis. 1992. Identification of aminoacids located in the antibody binding sites of human hepatitis A virus. Virology 191:984-987. [DOI] [PubMed] [Google Scholar]
- 28.Noble, R. C., M. A. Kane, S. A. Reeves, and I. Roeckel. 1984. Posttransfusional hepatitis A in a neonatal intensive care unit. JAMA 252:2711-2715. [PubMed] [Google Scholar]
- 29.Ping, L.-H., and S. M. Lemon. 1992. Antigenic structure of human hepatitis A virus defined by analysis of escape mutants selected against murine monoclonal antibodies. J. Virol. 66:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pintó, R. M., J. F. González-Dankaart, G. Sánchez, S. Guix, M. J. Gómara, M. García, I. Haro, and A. Bosch. 1998. Enhancement of the immunogenicity of a synthetic peptide bearing a new epitope of hepatitis A virus. FEBS Lett. 438:106-110. [DOI] [PubMed] [Google Scholar]
- 31.Reid, T. M. S., and H. G. Robinson. 1987. Frozen raspberries and hepatitis A. Epidemiol. Infect. 98:109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson, B. H., R. W. Jansen, B. Khanna, A. Totsuka, O. V. Nainan, G. Siegl, A. Widell, H. S. Margolis, S. Isomura, K. Ito, T. Ishizu, Y. Moritsugu, and S. M. Lemon. 1992. Genetic relatedness of hepatitis A virus strains recovered from different geographical regions. J. Gen. Virol. 73:1356-1377. [DOI] [PubMed] [Google Scholar]
- 33.Romalde, J. L., E. Area, G. Sánchez, C. Ribao, I. Torrado, F. X. Abad, R. M. Pintó, J. L. Barja, and A. Bosch. 2002. Prevalence of enterovirus and hepatitis A virus in bivalve mollusks from Galicia (NW Spain): inadequacy of the EU standards of microbiological quality. Int. J. Food Microbiol. 74:119-130. [DOI] [PubMed] [Google Scholar]
- 34.Rosemblum, L. S., I. R. Mirkin, D. T. Allen, S. Safford, and S. C. Hadler. 1990. A multistate outbreak of hepatitis A traced to commercially distributed lettuce. Am. J. Public Health 80:1075-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossmann, M. G., E. Arnold, J. W. Erickson, E. A. Frankenberger, J. P. Griffitth, H.-J. Hecht, J. E. Johnson, G. Kamer, M. Luo, A. G. Mosser, R. R. Rueckert, B. Sherry, and G. Vriend. 1985. Structure of a human cold virus and functional relationship to other picornaviruses. Nature 317:145-153. [DOI] [PubMed] [Google Scholar]
- 36.Schlesselman, J. J. 1982. Case-control studies: design, conduct, analysis. Oxford University Press, Oxford, United Kingdom.
- 37.Shaffer, D. R., E. A. Brown, and S. M. Lemon. 1994. Large deletion mutations involving the first pyrimidine-rich tract of the 5′ nontranslated RNA of human hepatitis A virus define two adjacent domains associated with distinct replication phenotypes. J. Virol. 68:5568-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheretz, R. J., B. A. Russell, and P. D. Reuman. 1984. Transmission of hepatitis A by transfusion of blood products. Arch. Intern. Med. 144:1579-1580. [PubMed] [Google Scholar]
- 39.Stapleton, J. T., V. Raina, P. L. Winokur, K. Walters, D. Klinzman, E. Rosen, and J. H. McLinden. 1993. Antigenic and immunogenic properties of recombinant hepatitis A virus 14S and 70S subviral particles. J. Virol. 67:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor, M. B. 1997. Molecular epidemiology of South African strains of hepatitis A virus: 1982-1996. J. Med. Virol. 51:273-279. [DOI] [PubMed] [Google Scholar]
- 41.Velasco, M. L., and R. Cano. 2001. Vigilancia de hepatitis A en España. Años 1997-2000. Bol. Epidemiol. Nacl. 9:139. [Google Scholar]