Abstract
This study evaluated the performance of Directigen FluA combined with a 3-day flu screening culture for the detection of influenza virus. This abbreviated protocol was a useful and effective tool and resulted in a substantial reduction in time, effort, and money spent, while not compromising sensitivity of influenza virus detection.
Rapid, accurate detection of influenza virus has important clinical and public health implications. Prompt reporting of results can play an important role in guiding both therapeutic as well as patient management decisions. These decisions can include selection of appropriate antiviral therapies and/or cohorting of infected patients in institutional settings as a means of outbreak control. Several enzyme immunoassay-based tests for the rapid detection of influenza virus antigens in clinical specimens are presently available. The performance characteristics of one of these, the Directigen FluA test, are the most thoroughly evaluated in the literature. However, the published studies examining the performance of Directigen FluA have shown that this test exhibits a wide range of sensitivities (59 to 100%) and specificities (86 to 100%) when compared to culture (3-5, 7, 8, 10-12). Furthermore, a majority of these studies have limited their evaluation of this test to well-defined conditions based on selected patient populations, presenting symptoms, and specimen collection. Prior to the 1997-to-1998 winter season, we developed a screening protocol that sought to take advantage of the rapid results available from a direct antigen test and the sensitivity of culture. This flu screening culture (FSC) combined performance of a rapid Directigen FluA test in conjunction with a 3-day viral culture using a single tube of primary rhesus monkey kidney (PMK) cells. Physicians had the option of selecting either FSC or a standard comprehensive respiratory culture (CRC) (with or without Directigen FluA) on any upper respiratory specimen submitted to the virology laboratory. The purpose of this study was to (i) evaluate the performance of Directigen FluA in a routine laboratory setting and (ii) assess the performance and cost-effectiveness of an abbreviated culture/antigen protocol (FSCs) to detect influenza virus in clinical specimens.
(This work was presented in part at the 100th Annual Meeting of the American Society for Microbiology, Chicago, Ill., May, 1999.)
During the 1997-to-1998 and 1998-to-1999 winter respiratory virus seasons, all specimens submitted to the laboratory (>1,500 total, including nasal swabs and washes, pharyngeal swabs and washes, and sputum) were tested by either CRC or FSC, depending on the physician's orders. Specimens were collected at the patient location by a member of the direct-care team, placed in viral transport medium (1.5 ml of veal infusion broth, 0.5% gelatin, and antibiotics), transported and stored at 4°C, and processed within 24 h of collection. Uninoculated residual specimens were stored at −70°C.
CRCs were incubated for 10 days using appropriate cell cultures for the detection of influenza A and B viruses, parainfluenza virus, respiratory syncytial virus, adenovirus, rhinovirus, herpes simplex virus, and varicella-zoster virus and were incubated for 14 days for the detection of cytomegalovirus. Cultures for the detection of hemadsorbing viruses consisted of three tubes of PMK cells (Bio-Whittaker, Inc., Walkersville, Md., or Viromed Laboratories, Inc., Minneapolis, Minn.) that were incubated at 33°C (two tubes) or 37°C (one tube) on a rotating drum. Cultures were examined daily for cytopathic effect (CPE) and were hemadsorbed (HAD) after 3 and 7 (33°C tubes) and 10 (37°C tube) days of incubation or if CPE was observed. Confirmation and identification of HAD+ cultures were performed by indirect immunofluorescence antibody staining of cells from culture with type-specific monoclonal antibodies (Bartels, Inc., Issaquah, Wash.). Directigen FluA rapid antigen detection of influenza A virus could be requested separately for specimens submitted for CRC.
FSCs received a Directigen FluA test and were also inoculated into a single tube of PMK cells that was incubated for 3 days at 33°C on a rotating drum. Cultures were examined daily for CPE and were HAD on day 3 or if CPE was observed prior to day 3. HAD+ cultures from specimens that were Directigen FluA+ were reported as “presumptive influenza A” and were not confirmed by indirect immunofluorescence assay. Directigen FluA (Becton Dickinson, Cockeysville, Md.) was used for direct antigen detection per the protocol of the manufacturer on the same unprocessed specimen and at the same time as culture inoculation. Assay quality control was performed as described by the manufacturer.
Influenza A virus reverse transcriptase PCR was performed on viral RNA extracted from clinical specimens using a modification of the method described by Boom et al. (2), as previously described (1). Precautions were taken to prevent carryover contamination (1, 6), and extraction controls and negative reagent controls were run in each test. Positive results were identified by hybridization using a digoxigenin-labeled, influenza A virus-specific probe (AH2).
In order to determine the “real-life” performance of the screening culture protocol described here, all specimens received by the virology laboratory were included in this evaluation. Patient demographics varied considerably, including both adult and pediatric patients, as well as inpatient and outpatient locations. Influenza A virus was detected in approximately 30% of the total specimens from both seasons (182 of 591 in 1997 to 1998; 276 of 913 in 1998 to 1999). Interestingly, the 1998-to-1999 season exhibited a 50% increase in total number, as well as in influenza A virus-positive specimens, compared to 1997 to 1998. Influenza B virus was not detected during the 1997-to-1998 season but constituted 10% (32 of 308) of the positive specimens during the 1998-to-1999 season.
The ability of Directigen FluA to detect influenza A virus in clinical specimens was compared to that of the 10-day CRC as well as that of the 3-day FSC for both winter seasons. The calculated performance characteristics of the Directigen FluA test against each culture standard are shown in Table 1. Although the sensitivity of Directigen FluA appears slightly lower (61 to 66%) when CRC was the standard than when FSC was the standard (76 to 77%), this is not unexpected when the comparison is made to a more sensitive “gold standard” (multiple culture tubes whose contents are incubated for a longer time). Our data show, however, that Directigen FluA performed consistently from season to season compared to each culture standard, even though the sensitivity described here is lower than that stated by the manufacturer (Directigen FluA package insert; Becton Dickinson) and previous studies (3, 7, 10, 12).
TABLE 1.
Performance of Directigen compared to culture during 2 winter seasons
| Culture type | Season | No. of specimens tested | Sensitivity (%)a | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|---|
| FSC | 1997-1998 | 261 | 77 (68-86) | 91 (87-95) | 78 (69-87) | 90 (86-94) |
| 1998-1999 | 692 | 76 (70-82) | 96 (94-98) | 88 (83-93) | 92 (90-94) | |
| CRC | 1997-1998 | 137 | 61 (44-78) | 94 (89-99) | 77 (61-93) | 88 (82-94) |
| 1998-1999 | 153 | 66 (50-82) | 97 (94-100) | 88 (76-100) | 88 (82-94) |
Numbers in parentheses are 95% confidence intervals.
During the 1997-to-1998 season, 23 specimens were determined to be positive by the antigen test but negative by culture. In an attempt to resolve these discrepant results, frozen aliquots of the original specimens and controls were subjected to reverse transcriptase PCR for influenza A virus in a blinded fashion. Control specimens were matched for age, sex, source, and type of culture requested (FSC or CRC). Negative control specimens (n = 10) were off-season specimens from which no virus was isolated. Positive control specimens were culture positive and either influenza A virus antigen positive (n = 8) or antigen negative (n = 6). Since one of the 23 discrepant specimens identified was a duplicate specimen, only the 22 nonduplicate specimens were included in the analysis. We found that, in addition to the 100% accuracy in identifying influenza A virus among the control samples, 14 of the 22 antigen-positive and culture-negative patient specimens were found to contain influenza A viral RNA. This reduces the apparent false-positive rate for the 1997-to-1998 season from 21% (22 of 103 nonduplicate specimens) to 8% (8 of 103). Although 17 of 22 (77%) of these discrepant specimens were submitted for FSC, these specimens were represented in the same proportions of those that were PCR+ (11 of 14, 78%) and PCR− (six of eight, 75%). No other associations among age, sex, source, and patient location were observed in the discrepant samples.
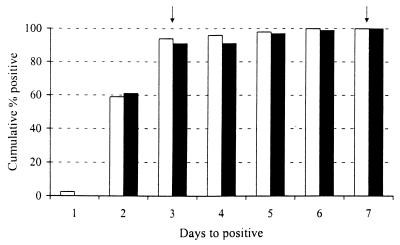
At the time that the FSC protocol was designed and implemented, the decision to limit the duration of these cultures to 3 days was based on literature reports and anecdotal evidence from our laboratory. A “validation” of this decision using our data required a retrospective assessment of the cumulative time to positivity for all CRCs received during the study period, since only those specimens for which CRCs were requested were cultured for >3 days. Figure 1 shows that, while 100% of these cultures were positive by day 7, >90% were positive by day 3 during both winter seasons. In addition, although the Directigen FluA kit used in this study is unable to detect influenza B virus, 29 of 32 influenza B virus-positive specimens were detected by culture of ≤3 days. This indicates that virtually all of our positive results have a turnaround time that can have a significant impact on the management of the patient. An approximation of the relative costs associated with performing FSCs and CRCs with or without Directigen FluA is shown in Table 2. The totals reflect our cost for performing culture and hemadsorption for an individual specimen. The tech cost is the salary plus benefits for a midlevel medical technologist at the time of the study. The antigen supply cost is the calculated price of an individual Directigen FluA antigen test based on the contract price at the time. FSCs (which include the rapid antigen test) save approximately $40.00 per specimen (>50% reduction in our cost) and markedly reduce turnaround time compared to CRCs that include a Directigen FluA test. Although an actual efficiency study to quantify the technologist time saved was not performed, the practical impact of decreased turnaround time was less “hands-on” review of tube cultures and fewer hemadsorptions performed.
FIG. 1.
Cumulative percentage of positive hemadsorbing viruses by day detected from CRCs over 2 winter seasons. Respiratory specimens submitted for CRC during the 1997-to-1998 (white bars) and 1998-to-1999 (black bars) winter seasons were cultured for hemadsorbing viruses in three tubes of primary rhesus monkey kidney cells incubated at 33°C (two tubes) or 37°C (one tube) on a rotating drum. Cultures were examined daily for CPE and were HAD after 3 and 7 (33°C tubes; indicated by arrows) and 10 (37°C tube) days of incubation or if CPE was observed. Data are the cumulative percentage positive of hemadsorbing viruses isolated by day of detection. All isolates were detected by day 7, and all were influenza A or B.
TABLE 2.
Approximate expenses associated with each culture
| Test | Cost (in U. S. dollars)
|
Tech time (h) | TATd (days) | ||||
|---|---|---|---|---|---|---|---|
| Culturec | Antigen | All supplies | Tech salary | All supplies and tech salary combined | |||
| FSC + Aga | 3.00 | 13.00 | 16.00 | 18.75 | 34.75 | 1 | 3 |
| CRCb | 15.00 | 0.00 | 15.00 | 37.50 | 52.50 | 2 | 10 |
| CRC + Ag | 15.00 | 13.00 | 28.00 | 47.00 | 75.00 | 2.5 | 10 |
One tube was HAD by day 3. Ag, Directigen FloA.
Three tubes were HAD by days 3, 7, and 10, and four additional tubes were held for up to 14 days for the isolation of other viruses.
Culture costs include cell cultures, media, and reagents for hemadsorptions.
TAT, turnaround time.
The ability of a clinical microbiology laboratory to detect influenza virus in clinical specimens efficiently and accurately has important patient care implications. The primary goals in offering FSCs as an option for clinicians were to provide a diagnostic tool that was rapid and accurate, as well as one that fit well into the routine of the laboratory. Although the data were not available when the protocol was designed, we found that >90% of positive CRCs during the 2 winter seasons were identified by day 3 of culture (Fig. 1). A retrospective analysis of our laboratory data from previous seasons also showed >90% positive by day 3 of culture (data not shown). This supported our decision to limit FSCs to 3 days and is also consistent with previously published data demonstrating a 100% recovery of influenza A virus and 94% recovery of influenza B virus by day 3 in cultures of clinical specimens that were HAD daily (9). In addition to the efficiency with which FSCs were able to detect influenza virus, an approximation of the cost showed that FSCs were 35% less expensive than CRCs and >50% less expensive than CRCs in combination with Directigen FluA (Table 2). A further reduction in expense and workload may be attained if the single-tube culture is eliminated from FSCs in the case of Directigen FluA-positive specimens. However, we feel that it is important to include culture backup for a number of reasons: (i) to have a continuing monitor of commercial test performance, (ii) to obtain viral isolates for epidemiological characterization, and (iii) to have the potential to isolate a broader range of viruses. This last point is highlighted by the fact that, during each winter season, both a large number (>100) and a wide variety of viruses (adenovirus, cytomegalovirus, herpes simplex virus, parainfluenza virus, respiratory syncytial virus, and rhinovirus) were detected in specimens cultured by FSC and CRC. This screening strategy has also had a significant impact on the daily operations of the virology laboratory during the winter season. The FSCs have allowed us to focus our efforts on answering the question highest on the physician's differential diagnosis during these months. The positive impact of reducing the hands-on time for these cultures by 50% was seen not only financially but also in lab efficiency and morale. This was especially important during the peak of the season when upwards of 30 specimens/day were respiratory specimens—a number that more than doubled the workload in the virology laboratory.
Although each laboratory must determine its own testing scheme individually, we feel that FSCs are a valuable tool in our laboratory with our population of patients. In spite of some of the limitations of laboratory testing in the diagnosis of influenza, our laboratory will continue to offer FSCs as an option as long as the consistency of the Directigen FluA test is maintained and pending further technological advances in the area of rapid testing. The recently released Directigen FluA+B kit, which both detects and differentiates between influenza A and B antigens in clinical specimens, may serve as tool that provides a more complete complement to culture for the detection of influenza virus. A preliminary study from our laboratory demonstrated similar performance characteristics between the FluA+B kit and the FluA kit in detecting influenza A virus (D. Newton, W. Kuhnert, C. Mayer, and M. Menegus, Abstr. 16th Annu. Clin. Virol. Symp., abstr. S17, 2000). While the Directigen FluA kit performed consistently between seasons, its performance compared to culture in our hands (61 to 77% sensitive) was on the lower end of the range of sensitivities (59 to 100%) seen in a variety of previously published reports (3-5, 7, 8, 10-12). In our study, there were no exclusion criteria for the rejection of specimens based on quality; nor were there in-house monitors for the reliability of specimen collection or transport. Since this reflects a realistic situation for many clinical laboratories, we would expect that the overall performance of this antigen detection test would be lower than previously published or advertised. In conclusion, we have found that 3-day FSCs were a useful and effective tool during these influenza seasons and in combination with Directigen FluA resulted in a substantial reduction in time, effort, and money spent, while not compromising sensitivity of influenza virus detection.
Acknowledgments
We thank the members of the clinical and research virology laboratories of the Strong Memorial Hospital at the University of Rochester Medical Center, without whose support and expert technical assistance this study would not have been possible.
REFERENCES
- 1.Atmar, R. L., B. D. Baxter, E. A. Dominguez, and L. H. Taber. 1996. Comparison of reverse transcription-PCR with tissue culture and other rapid diagnostic assays for detection of type A influenza virus. J. Clin. Microbiol. 34:2604-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomel, J. J., M. F. Remilleux, P. Marchand, and M. Aymard. 1992. Rapid diagnosis of influenza A. Comparison with ELISA immunocapture and culture. J. Virol. Methods 37:337-343. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez, E. A., L. H. Taber, and R. B. Couch. 1993. Comparison of rapid diagnostic techniques for respiratory syncytial and influenza A virus respiratory infections in young children. J. Clin. Microbiol. 31:2286-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston, S. L., and H. Bloy. 1993. Evaluation of a rapid enzyme immunoassay for detection of influenza A virus. J. Clin. Microbiol. 31:142-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwok, S., and R. Higuchi. 1989. Avoiding false positives with PCR. Nature (London) 339:237-238. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi, G. P., H. Leib, G. S. Birkhead, C. Smith, P. Costello, and W. Conron. 1994. Comparison of rapid detection methods for influenza A virus and their value in health-care management of institutionalized geriatric patients. J. Clin. Microbiol. 32:70-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcante, R., F. Chiumento, G. Palu, and G. Cavedon. 1996. Rapid diagnosis of influenza type A infection: comparison of shell-vial culture, directigen flu-A and enzyme-linked immunosorbent assay. New Microbiol. 19:141-147. [PubMed] [Google Scholar]
- 9.Minnich, L. L., and C. G. Ray. 1987. Early testing of cell cultures for detection of hemadsorbing viruses. J. Clin. Microbiol. 25:421-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reina, J., M. Munar, and I. Blanco. 1996. Evaluation of a direct immunofluorescence assay, dot-blot enzyme immunoassay, and shell vial culture in the diagnosis of lower respiratory tract infections caused by influenza A virus. Diagn. Microbiol. Infect. Dis. 25:143-145. [DOI] [PubMed] [Google Scholar]
- 11.Ryan-Poirier, K. A., J. M. Katz, R. G. Webster, and Y. Kawaoka. 1992. Application of Directigen FLU-A for the detection of influenza A virus in human and nonhuman specimens. J. Clin. Microbiol. 30:1072-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waner, J. L., S. J. Todd, H. Shalaby, P. Murphy, and L. V. Wall. 1991. Comparison of Directigen FLU-A with viral isolation and direct immunofluorescence for the rapid detection and identification of influenza A virus. J. Clin. Microbiol. 29:479-482. [DOI] [PMC free article] [PubMed] [Google Scholar]