Abstract
The age, sex, and seasonal distributions of invasive Kingella kingae infections in southern Israel were examined and compared to the epidemiology of respiratory carriage of the organism. Medical records of all patients diagnosed between 1988 and 2002 were reviewed, and 2,044 oropharyngeal specimens were cultured on selective media during two periods (February to May and October to December) in 2001. Invasive infections significantly affected children (73 of 74 patients [98.6%] were younger than 4 years), 50 patients (67.8%) were males (P = 0.045), and 55 episodes (74.3%) occurred between July and December (P = 0.004). Carriage was higher in the 0- to 3-year-old group and decreased with increasing age (P for trend = 0.0008). Carriage rates were similar in both sexes and did not significantly differ between the February-to-May and October-to-December periods. The highest rate of carriage of K. kingae coincided with the age (less than 4 years) at which invasive infections were especially frequent. The peculiar sex and seasonal distributions of invasive disease, however, cannot be readily explained by the epidemiology of respiratory carriage. Viral infections and other yet-to-be-defined cofactors may play a role in the causation of invasive K. kingae infections.
For most of the four decades that elapsed since the first characterization of Kingella kingae, this gram-negative bacterium was considered a rare cause of human disease and therefore was mostly neglected (5, 8, 20). In recent years, as the result of improved isolation techniques, there has been an increasing number of reports on invasive K. kingae infections in the United States, Western Europe, and Israel, suggesting that the organism is an important cause of bacteremia and septic arthritis in pediatric patients (2, 4, 6, 7, 13, 14, 21, 22, 25). Moreover, studies conducted to elucidate the natural niche of K. kingae have shown that the organism is a frequent component of the oropharyngeal flora of young children and is readily transmitted from person to person among day care center (DCC) attendees (19, 24).
Despite the enlarging body of information on the clinical and diagnostic aspects of K. kingae infections, the epidemiology of the organism remains largely unknown. A study was performed to investigate the sex, age, and seasonal distributions of invasive K. kingae infections and to correlate these data with the respiratory carriage of the organism.
MATERIALS AND METHODS
Soroka University Medical Center (SUMC) is a 950-bed university hospital located in the city of Beer-Sheva and is the only medical facility providing inpatient medical services to the entire population of southern Israel (450,000 inhabitants). The Clinical Microbiology Laboratory (CML) of SUMC provides diagnostic microbiology services to hospitalized patients and to the outpatient population of 139 community clinics in the area.
Bacteriological methods.
Patients referred to the emergency department or hospitalized at SUMC with a febrile illness undergo routine blood cultures with the BACTEC system (Becton Dickinson Diagnostic Instrument Systems, Towson, Md.), which are processed according to established bacteriologic procedures (16). Bone exudates and synovial fluid specimens are routinely inoculated into blood culture bottles in addition to routine plating onto solid media, because this procedure has been shown to improve the recovery of K. kingae (22).
Throat cultures obtained from hospital and community patients for isolation of Streptococcus pyogenes are sent to the CML of SUMC in transport medium (MW 173 Amies medium; Transwab; Medical Wire and Equipment, Potley, United Kingdom) and arrive within 4 h. For isolation of K. kingae from the respiratory tract, swabs are seeded onto a selective vancomycin-containing medium especially designed to improve detection of the organism (23) and examined once a day for 2 days. K. kingae is identified by the typical morphological and biochemical characteristics of this species (short, gram-negative bacilli with tapered ends often in pairs or forming short chains; beta-hemolysis; production of acid from glucose and maltose but not from other sugars; positive oxidase reaction; and negative catalase, urease, indole, and motility tests [16]) and confirmed with the API NH kit (bioMerieux, Marcy-l'Etoile, France).
Invasive K. kingae infections.
Medical records of all patients in whom an invasive K. kingae infection, defined as isolation of the organism from normally sterile body fluids, was diagnosed from 1 January 1988 through 31 December 2001 were reviewed, and relevant demographic data were collected. The fact that all children in southern Israel are born in and receive inpatient medical services at SUMC allowed us to calculate the annual incidence of invasive K. kingae infections in this population.
Respiratory prevalence study.
A fraction of all pharyngeal swabs sent to the CML of SUMC for detection of S. pyogenes during two time periods in 2001 were cultured for isolation of K. kingae on selective medium (23). The first period (February to May) was chosen to comprise the time of the year when only a small fraction of patients with invasive K. kingae infections are usually diagnosed. The second period (October to December) coincides with the season in which the vast majority of these infections are detected. Information regarding clinical symptoms or recent antibiotic exposure of the population from which these respiratory specimens derived was not available.
Throat swabs were stratified according to patients' ages before the data were examined, as follows: 0 to 3 years, 4 to 17 years, and ≥18 years. These intervals were chosen to represent children at the age at which invasive diseases are especially common, older children, and adults, respectively. For each period and age group, samples of swabs of approximately similar sizes were randomly chosen for K. kingae isolation.
Statistical methods.
The significance of the differences between two means was assayed by using Student's t test. Assuming that there is no gender predisposition or seasonal preference for infection, episodes of invasive disease were expected to be evenly distributed between the sexes and the two study periods. The comparison of observed distribution to expected distribution was assessed by using the χ2 test. A P value of <0.05 was considered statistically significant for all calculations.
RESULTS
Invasive K. kingae infections.
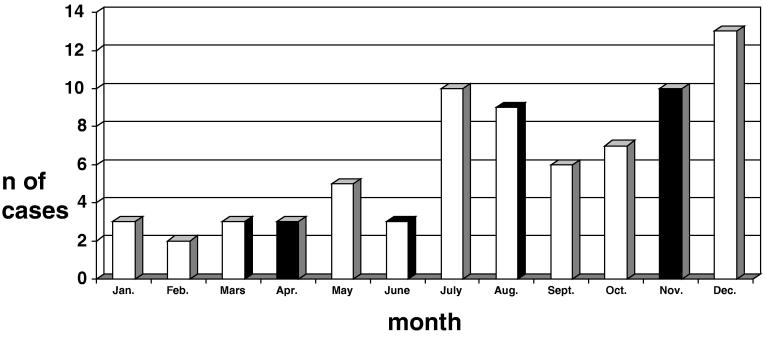
During the study period, a total of 74 patients with invasive K. kingae infections were diagnosed at SUMC, of whom 50 (67.8%) were males and 24 (32.4%) were females (P < 0.045). The annual number of cases ranged between two (in 1988) and nine (in 1994). The monthly distribution of cases is shown in Fig. 1. Nineteen patients (25.7%) were diagnosed between January and June, and 55 (74.3%) were diagnosed between July and December (P = 0.004). In 2001, three patients were diagnosed, one in May, one in August, and one in November.
FIG. 1.
Monthly distribution of cases of invasive K. kingae infections diagnosed at SUMC between 1 January 1988 and 31 December 2002.
Seventy-three patients (98.6%) were children younger than 48 months of age, and one patient was a 21-year-old adult. The median age of the children was 14 months (mean ± standard deviation, 15.0 ± 7.7 months; range, 4 to 40 months). During the 14-year study period, the average annual number of births was 10,448. The age distribution of children with invasive K kingae and the annual incidence rates by age group are shown in Table 1. At presentation, symptoms of upper respiratory tract infection were recorded in 32 children (43.8%); stomatitis was seen in 12 (16.4%), including 1 child in whom buccal lesions developed in the course of chicken pox; and diarrhea was seen in 10 (13.7%).
TABLE 1.
Prevalence of K. kingae in the respiratory tract and annual incidence of invasive disease in children younger than 48 months
| Age (mo) | Respiratory carriage
|
Invasive disease
|
|||||
|---|---|---|---|---|---|---|---|
| n | % | No. positive | % positive | n | % | Incidencea | |
| 0-11 | 29 | 4.2 | 1 | 3.4 | 31 | 42.4 | 21.2 |
| 12-23 | 173 | 24.9 | 7 | 4.0 | 33 | 45.2 | 22.6 |
| 24-35 | 222 | 32.0 | 5 | 2.3 | 7 | 9.6 | 4.8 |
| 36-47 | 270 | 38.9 | 9 | 3.3 | 2 | 2.7 | 1.4 |
| Total | 694 | 100.0 | 22 | 3.2 | 73 | 100.0 | 12.5 |
Annual incidence per 100,000 population in the period from 1988 to 2002.
Skeletal infections were the most common presentations of the disease and were diagnosed in 48 patients (64.9%), followed by occult bacteremia in 22 patients (29.7%), lower respiratory tract infection in 3 patients (4.1%), and endocarditis in the only adult patient in the series (1.4%).
Respiratory prevalence study.
A total of 2,044 of 11,202 throat specimens (18.2%) sent to the CML were cultured for K. kingae (1,020 of 6,205 [16.4%] in the February-to-May period and 1,024 of 4,997 [20.5%] in the October-to-December period). Demographic characteristics of the patients from whom the pharyngeal cultures were screened for K. kingae are shown in Table 2. The populations studied in the February-to-May and October-to-December periods were similar in terms of age and sex distribution. Thirty-seven specimens were positive for K. kingae (21 in the February-to-May period and 16 in the October-to-December period; P > 0.4). Overall, 978 specimens (47.8%) were obtained from males, of which 17 (1.7%) were positive for K. kingae, and 1,066 (52.2%) were obtained from females, of which 20 (1.9%) grew the organism (P > 0.9). The prevalence of the organism did not significantly differ between sexes in any of the age groups studied (P > 0.05 for all calculations; results not shown). The prevalence of the organism by season and age group is shown in Table 3. In the February-to-May period, the prevalence of K. kingae was higher among 0- to 3-year-old children than among the two older population groups and showed a significant decrease with increasing age. In the October-to-December period, the prevalence of K. kingae was also higher among the youngest population and gradually decreased in older individuals, but this trend did not reach statistical significance. The rate of carriage of the organism among children younger than 48 months is shown in Table 1. Because throat cultures are infrequently obtained in infants, the 0- to 11-month age group was underrepresented in the population (fewer than 5% of the children screened). The carriage rate did not differ significantly between the population age groups, and the trend towards higher prevalence of K. kingae in the February-to-May period did not reach statistical significance.
TABLE 2.
Demographic data for the population studied
| Age (yr) | Age distribution
|
Sex distribution
|
||||||
|---|---|---|---|---|---|---|---|---|
| February-May
|
October-December
|
Pb | No. (%) male
|
Pc | ||||
| n | Mean agea ± SD | n | Mean agea ± SD | February-May | October-December | |||
| 0-3 | 346 | 30.8 ± 10.8 | 348 | 30.0 ± 11.1 | 0.49 | 203 (58.7) | 194 (55.8) | 0.48 |
| 4-17 | 339 | 120.7 ± 46.1 | 340 | 112.8 ± 44.3 | 0.52 | 177 (52.2) | 179 (52.7) | 0.98 |
| ≥18 | 335 | 34.1 ± 13.0 | 336 | 35.6 ± 14.5 | 0.66 | 102 (30.5) | 123 (36.6) | 0.10 |
For the 0- to 3- and 4- to 17-year-old groups, age is expressed in months; for the ≥18-year-old group, it is expressed in years.
P values refer to the comparison of the age of the population screened in the periods from February to May and October to December, stratified by age group.
P values refer to the comparison of the fraction of males in the population screened in the periods from February to May and October to December, stratified by age group.
TABLE 3.
Prevalence of K. kingae in the respiratory tract by age group and study period
| Age (yr) | Total
|
February-May
|
October-December
|
Pb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | No. positive | % Positive | RRa | n | No. positive | % Positive | RR | n | No. positive | % Positive | RR | ||
| 0-3 | 694 | 22 | 3.2c | 1.0 | 346 | 15 | 4.3d,e | 1.0 | 348 | 7 | 2.0 | 1.0 | 0.13 |
| 4-17 | 679 | 10 | 1.5 | 0.46 | 339 | 4 | 1.2d | 0.26 | 340 | 6 | 1.8 | 0.88 | 0.76 |
| ≥18 | 671 | 5 | 0.8c | 0.23 | 335 | 2 | 0.6e | 0.13 | 336 | 3 | 0.9 | 0.44 | 0.99 |
| Total | 2,044 | 37 | 1.8 | 1,020 | 21 | 2.1 | 1,024 | 16 | 1.6 | 0.50 | |||
| P for trend | 0.0008 | 0.0006 | 0.24 | ||||||||||
RR, relative risk.
Significance of the differences in the prevalence rates found in each age group between the two study periods.
P = 0.003.
P = 0.022.
P = 0.004.
DISCUSSION
Invasive infections in young children are frequently caused by organisms carried asymptomatically in the respiratory tract. Streptococcus pneumoniae, Haemophilus influenzae type b, or Neisseria meningitidis residing in the mucosal surfaces is able to penetrate the bloodstream, disseminate, and invade distant organ systems (3, 9). Colonization of the respiratory tract by these organisms is, therefore, a prerequisite for later invasion, and human populations exhibiting high rates of carriage of these pathogens are at increased risk to acquire disease (3, 9, 18).
In previous studies it was demonstrated that K. kingae is frequently isolated from the upper respiratory tracts of young children. In a prospective study, the oropharynges and nasopharynges of 48 children age 6 to 42 months attending a local DCC were cultured separately (24). K. kingae was isolated from 109 of 624 oropharyngeal cultures (17.5%), and 34 children (70.8%) carried the organism at least once during an 11-month study period. The organism was not isolated from any of the nasopharyngeal cultures, suggesting that K. kingae occupies a narrow respiratory niche (24).
The results of the present investigation demonstrate that when blood cultures are routinely obtained from young febrile children and synovial fluid specimens are inoculated into blood culture bottles, K. kingae appears as a not-uncommon cause of invasive infection. Examination of our extensive experience over a 14-year period shows three remarkable epidemiological features: invasive K. kingae is a pediatric disease especially affecting populations below 4 years of age, the disease is significantly more common in males, and cases tend to cluster between July and December.
It was expected, then, that the respiratory carriage of the organism would have similar characteristics of age, sex, and seasonal predilection. The results of the carriage study confirmed that the prevalence of the organism in the pharynges of children younger than 4 years (3.2%) was significantly higher than that in older individuals (Table 3). However, this phenomenon was observed only during the early months of the year, whereas in the later cross-sectional survey, the rate of carriage of K. kingae in the segment of the population younger than 4 years decreased and was comparable to that observed in older individuals.
We could not demonstrate a correlation between the pattern of carriage of the organism and the seasonal occurrence of cases of invasive disease. In general terms, the period of the year when there was maximal prevalence of the organism in the respiratory tract did not overlap with that when the attack rate of invasive infection was highest. Whereas almost three-quarters of the patients with invasive K. kingae disease were diagnosed between July and December, the carriage rate showed an opposite, albeit not significant, trend. It might be postulated that exposure of patients with suspected streptococcal pharyngitis to antimicrobial drugs accounted for the overall low prevalence of K. kingae as well as for the unexpected low carriage rate found in the October-to-December period. Because the occurrence of respiratory infections follows a seasonal pattern, the population in general, and especially young children, is more commonly given antimicrobial drugs during the winter (1). Therefore, the prevalence of K. kingae could have been reduced by previous exposure to drugs such as β-lactams, trimethoprim-sulfamethoxazole, or macrolides, which are commonly given to patients with respiratory infections and to which the organism is exquisitely susceptible (26). Although no actual data on antibiotic exposure were collected in the present study, it should be pointed out that throat cultures are usually obtained before the onset of antimicrobial therapy. In addition, the four colder months of the year (November to March) were equally distributed between the two study periods, neutralizing the possible effect of antibiotic exposure on the rate of recovery of the organism.
The results of the present study show that there are not significant sex differences in the rate of carriage of K. kingae, whereas invasive diseases show a clear male predominance. The explanation for this observation is not obvious, although increased male-to-female ratios have been described for other infectious conditions such as those caused by S. pneumoniae and N. meningitidis (10, 12). Clearly, because most cases of K. kingae invasive infection occur among infants and toddlers, this striking feature cannot be explained by different behavioral patterns of male and female children resulting in different degrees of exposure.
The overall prevalence of K. kingae in the 0- to 4-year-old group found in this investigation is substantially lower than that detected a few years ago among children attending a DCC in the city of Beer-Sheva (24). It should be pointed out that the fraction of children attending DCCs in the present study population could not be determined. It is possible that the high rate of carriage of K. kingae found in the previous study was the result of increased transmission of the organism among susceptible young children exposed to crowded conditions prevalent in the DCC. Increased rates of carriage of other respiratory pathogens such as S. pneumoniae or H. influenzae type b have also been demonstrated among DCC attendees compared to children cared for at home (11).
It appears, then, that the striking epidemiological features of invasive K. kingae infections cannot be explained solely on the basis of the characteristics of the respiratory carriage of the organism, and, therefore, additional factors should be proposed. It is remarkable that signs of upper respiratory tract infection, stomatitis, or diarrhea were found on admission in more than half of the children who later grew K. kingae from a normally sterile body site. These findings, which were recorded in the patients' charts by physicians who were unaware of the etiology of the infection, are highly suggestive of a concomitant viral infection. The important role played by viruses in the causation of pneumococcal or meningococcal infections has been firmly established (16, 17). Respiratory viruses are frequent precipitating factors of otitis media, pneumonia, bacteremia, or meningitis among persons already colonized by these respiratory pathogens (15, 17). Recent reports have shown that specific viral infections may also predispose individuals to acquisition of invasive K. kingae infections. In a study by Amir and Yagupsky, K. kingae bacteremia was documented in 4 of 29 young children with culture-proven herpetic gingivostomatitis (2). In addition, occurrence of K. kingae endocarditis following chicken pox was also described in another report (21). These reports suggest that viral infections causing damage to the respiratory mucosa or buccal aftae facilitate local invasion by K. kingae organisms residing in the pharynx, followed by penetration of the bacterium into the bloodstream and seeding to remote sites. It is suggested that the peculiar epidemiological features of invasive K. kingae infections result from the interplay of the respiratory carriage of the organism with viral infections and possibly other, still-unidentified factors.
REFERENCES
- 1.Albanese, B. A., J. C. Roche, M. Pass, C. Whitney, M. C. McEllistrem, and L. H. Harrison. 2002. Geographic, demographic, and seasonal differences in penicillin-resistant Streptococcus pneumoniae in Baltimore. Clin. Infect. Dis. 34:15-21. [DOI] [PubMed] [Google Scholar]
- 2.Amir, J., and P. Yagupsky. 1998. Invasive Kingella kingae infection associated with stomatitis in children. Pediatr. Infect. Dis. J. 17:757-758. [DOI] [PubMed] [Google Scholar]
- 3.Aniansson, G., B. Alm, B. Andersson, P. Larsson, O. Nylen, P. Rigner, M. Svanborg, and C. Svanborg. 1992. Nasopharyngeal colonization during the first year of life. J. Infect. Dis. 165(Suppl. 1):S38-S42. [DOI] [PubMed] [Google Scholar]
- 4.Birgisson, H., O. Steingrimsson, and T. Gudnasson. 1997. Kingella kingae infections in paediatric patients; 5 cases of septic arthritis, osteomyelitis and bacteraemia. Scand. J. Infect. Dis. 29:495-498. [DOI] [PubMed] [Google Scholar]
- 5.Claesson, B., E. Falsen, and B. Kjellman. 1985. Kingella kingae infections: a review and a presentation of data from 10 Swedish cases. Scand. J. Infect. Dis. 17:233-243. [DOI] [PubMed] [Google Scholar]
- 6.deGroot, R., D. Glover, C. Clausen, A. L. Smith, and C. B. Wilson. 1988. Bone and joint infections caused by Kingella kingae: six cases and review of the literature. Rev. Infect. Dis. 10:998-1004. [DOI] [PubMed] [Google Scholar]
- 7.Goutzmanis, J. J., G. Gonis, and G. L. Gilbert. 1991. Kingella kingae infection in children: ten cases and review of the literature. Pediatr. Infect. Dis. 10:677-683. [PubMed] [Google Scholar]
- 8.Graham, D. R., J. D. Band, C. Thornsberry, D. G. Hollis, and R. E. Weaver. 1990. Infections caused by Moraxella, Moraxella urethralis, Moraxella-like groups M-5 and M-6, and Kingella kingae in the United States, 1953-1980. Rev. Infect. Dis. 12:423-431. [DOI] [PubMed] [Google Scholar]
- 9.Gray, B. M., G. M. Converse III, and H. C. Dillon. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 10.Harrison, L. H., M. A. Pass, A. B. Mendelsohn, M. Egri, N. E. Rosenstein, A. Bustamante, J. Razeq, and J. C. Roche. 2001. Invasive meningococcal disease in adolescents and young adults. JAMA 286:694-699. [DOI] [PubMed] [Google Scholar]
- 11.Holmes, S. J., A. L. Morrow, and L. K. Pickering. 1996. Child-care practices: effect of social change on the epidemiology of infectious diseases and antibiotic resistance. Epidemiol. Rev. 18:10-28. [DOI] [PubMed] [Google Scholar]
- 12.Ip, M., D. J. Lyon, and A. F. Cheng. 2001. Pattern of antibiotic resistance, serotype distribution, patient demographics of Streptococcus pneumoniae in Hong Kong. Chemotherapy 47:110-116. [DOI] [PubMed] [Google Scholar]
- 13.Lacour, M., M. Duarte, A. Beutler, R. Auckenthaler, and S. Suter. 1991. Osteoarticular infections due to Kingella kingae in children. Eur. J. Pediatr. 150:612-618. [DOI] [PubMed] [Google Scholar]
- 14.Lundy, D. W., and D. K. Kehl. 1998. Increasing prevalence of Kingella kingae in osteoarticular infections in young children. J. Pediatr. Orthop. 18:262-267. [PubMed] [Google Scholar]
- 15.Moore, P. S., J. Hierholzer, W. DeWitt, K. Gouan, D. Djore, T. Lippeveld, B. Pikaytis, and C. V. Broome. 1990. Respiratory viruses and mycoplasma as cofactors for epidemic group A meningococcal meningitis. JAMA 264:1271-1275. [PubMed] [Google Scholar]
- 16.Murray, P. R., E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). 1999. Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 17.O'Brien, K. L., M. I. Walters, J. Sellman, P. Quinlisk, H. Regnery, B. Schwartz, and S. F. Dowell. 2000. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin. Infect. Dis. 30:784-789. [DOI] [PubMed] [Google Scholar]
- 18.Riordan, T., K. Cartwright, N. Andrews, J. Stuart, A. Burris, A. Fox, R. Borrow, T. Douglas-Riley, J. Gabb, and A. Miller. 1998. Acquisition and carriage of meningococci in marine commando recruits. Epidemiol. Infect. 121:495-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slonim, A., E. Walker, E. Mishori, N. Porat, R. Dagan, and P. Yagupsky. 1998. Person-to-person transmission of Kingella kingae among day-care-center attendees. J. Infect. Dis. 178:1843-1846. [DOI] [PubMed] [Google Scholar]
- 20.Verbruggen, A. M., D. Hauglustaine, L. van der Hauwert, J. J. Rombouts, G. W. Wauters, and J. Vanderpitte. 1986. Infections caused by Kingella kingae: report of four cases and review. J. Infect. 13:133-142. [DOI] [PubMed] [Google Scholar]
- 21.Waghorn, D. J., and C. H. Cheetham. 1997. Kingella kingae endocarditis following chickenpox in infancy. Eur. J. Clin. Microbiol. Infect. Dis. 16:944-946. [DOI] [PubMed] [Google Scholar]
- 22.Yagupsky, P., R. Dagan, C. B. Howard, M. Einhorn, I. Kassis, and A. Simu. 1992. High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J. Clin. Microbiol. 30:1278-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yagupsky, P., M. Merires, J. Bahar, and R. Dagan. 1995. Evaluation of a novel vancomycin-containing medium for primary isolation of Kingella kingae from upper respiratory tract specimens. J. Clin. Microbiol. 33:1426-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagupsky, P., R. Dagan, F. Prajgrod, and M. Merires. 1995. Respiratory carriage of Kingella kingae among healthy children. Pediatr. Infect. Dis. J. 14:673-678. [DOI] [PubMed] [Google Scholar]
- 25.Yagupsky, P., and R. Dagan. 1997. Kingella kingae: an emerging cause of invasive infections in young children. Clin. Infect. Dis. 24:860-866. [DOI] [PubMed] [Google Scholar]
- 26.Yagupsky, P., O. Katz, and N. Peled. 2001. Antibiotic susceptibility of Kingella kingae isolates from respiratory carriers and patients with invasive infections. J. Antimicrob. Chemother. 47:191-193. [DOI] [PubMed] [Google Scholar]