Abstract
Colletotrichum acutatum is a cosmopolitan plant pathogen with a wide host range. While the organism's phytopathogenic potential has been well documented, it has never been reported as an etiologic agent of disease in either animals or humans. In this case, a juvenile Kemp's ridley sea turtle, Lepidochelys kempi, probably with immune compromise following cold stunning (extended hypothermia), developed a disseminated mycotic infection in the lungs and kidneys. Prophylactic treatment with oral itraconazole did not prevent or cure the infection. This report of a Colletotrichum acutatum infection in an animal extends the range of disease caused by this organism beyond that of a phytopathogen.
The genus Colletotrichum Corda (class Coelomycetes) consists of several species of fungi capable of causing anthracnosis, necrosis, leaf spot, and fruit rot in plants. Many species appear to be host specific and are primary plant pathogens. The most common species with a wide host range is Colletotrichum gloeosporioides Penzig (teleomorph Glomerella cingulata [Stonem.] Spauld. & Schrenk). Colletotrichum gloeosporioides affects tropical, subtropical, and temperate fruits, such as almond, apple, avocado, mango, pecan, nectarine, and peach (12). Colletotrichum acutatum Simmonds (teleomorph Glomerella acutata Guerber & Correll) (16), in addition to causing terminal crook disease of Pinus spp. and affecting other native plants such as lupine (8), is capable of infecting a variety of commercial crops. Some hosts with significant losses include strawberry (13), rubber (10), grape (41), almond (11), apple (3), and olive (24). Colletotrichum acutatum was considered a morphological variant of C. gloeosporioides prior to Simmonds' description in 1965 (34) during a survey of fruit rot pathogens in Queensland, Australia.
Both C. gloeosporioides and C. acutatum are cosmopolitan in distribution, and the two display considerable overlap in plant hosts affected. Although the teleomorphs of G. acutata and G. cingulata are similar, Guerber and Correll noted minor differences (16). In crosses of self-fertile strains, the brown to black perithecia of G. acutata (125 to 312 μm [mean, 183 μm] wide) were predominately ampulliform, while those of G. cingulata were more globose, ovoid, or conical. Perithecia of G. acutata also had sparse, fine setae and lacked a mycelial covering, while those of G. cingulata had a loose tuft of mycelium at the neck and were embedded in a mycelial matrix. Ascospores produced by G. acutata (8.5 to 25.1 μm by 3.1 to 8.1 μm [mean, 15.7 by 5.8 μm]) were hyaline, one-celled, usually straight, and generally smaller than those of G. cingulata, which were frequently curved.
In humans, Colletotrichum spp. are very rarely known to cause disease. Case reports include mycotic keratitis (22, 23, 26, 31, 33), usually after an eye injury. There has been a single case of human subcutaneous hyalohyphomycosis reported to have been caused by C. gloeosporioides (15) and another caused by C. crassipes (6). A number of other coelomycetes have been reported to occasionally cause similar mycoses in humans (2, 4, 7, 19, 21, 25, 38). Coelomycetes are increasing in importance, especially in the severely immunocompromised human host, and there is a growing list of implicated species (29, 37).
To date, there have been no reports in the veterinary literature of any species of Colletotrichum naturally infecting animals. One case of an experimental induction of keratitis in rabbits with C. gloeosporioides has been reported (33), but it would seem logical that this group of opportunistic fungi that affect humans would also affect other animals.
Case report.
A juvenile Kemp's ridley sea turtle (Lepidochelys kempi) was admitted to the Sea Turtle Rehabilitation Hospital, Mote Marine Laboratory, Sarasota, Fla., on 11 December 1999 along with 10 conspecifics. At admission, the turtle weighed 2.7 kg and was estimated to be 2 years of age, and the straight carapace length was measured at 27.3 cm. The turtles had been part of a mass stranding event in Cape Cod, Mass., that took place that winter and involved a total of 277 turtles of three species. The cause of the mass stranding was cold stunning (extended hypothermia), and the local rehabilitation facilities had inadequate space and personnel to deal with the large number of animals. Consequently, many of the turtles were transferred to other facilities for long-term treatment.
This particular turtle had stranded on 16 November, at which time the rectal temperature was determined to be 6.9°C and the heart rate was 7 beats/min. The turtle was maintained at the New England Aquarium in a temperature-controlled environment, and the temperature was slowly increased until the body temperature reached 18 to 20°C. The turtle was begun on prophylactic treatment with ceftazidime (SmithKline Beecham, Philadelphia, Pa.; 22 mg/kg of body weight intramuscularly every 72 h) and fluconazole (Pfizer, Inc., New York, N.Y.; 0.75 mg/kg subcutaneously every 48 h). Upon transfer and admission to the Sea Turtle Rehabilitation Hospital, the turtle was found to exhibit elevated lactate dehydrogenase, aspartate aminotransferase, creatine kinase, and white blood cell count (primarily heterophils; Table 1). For ease of administration, antifungal therapy was changed from injectable fluconazole to oral itraconazole (Ortho Biotech, Raritan, N.J.; 5 mg/kg orally once daily) and clindamycin (Pharmacia & Upjohn, Kalamazoo, Mich.; 5 mg/kg orally once daily), and Lactobacillus acidophilus (orally once daily) was added to the treatment regimen.
TABLE 1.
Blood values determined at various stages during rehabilitation of the cold-stunned Kemp's ridley sea turtle reported in this case study
| Testa (units) | Result on:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 16 Nov 1999 | 12 Dec 1999 | 27 Dec 1999 | 24 Jan 2000 | 7 Feb 2000 | 1 Mar 2000 | 7 Mar 2000 | 13 Mar 2000 | |
| Glucose (mg/dl) | 72 | 78 | 153 | 110 | 102 | 29 | 86 | 119 |
| Sodium (mEq/liter) | 165 | 147 | 149 | 146 | 146 | 141 | 141 | 142 |
| Potassium (mEq/liter) | 4.0 | 3.0 | 2.5 | 4.1 | 3.6 | 4.2 | 4.6 | 4.4 |
| Chloride (mEq/liter) | 129 | 117 | 105 | 114 | 111 | 98 | 87 | 80 |
| CO2 (mEq/liter) | 23 | 35 | >40 | 35 | 38 | 22 | 30 | 30 |
| Blood urea nitrogen (mg/dl) | 17 | 142 | 142 | 118 | 96 | 258 | 214 | 207 |
| Creatinine (mg/dl) | 0.0 | 0.2 | 0.3 | 0.2 | 0.2 | 0.3 | 0.4 | 0.8 |
| Uric acid (mg/dl) | 4.1 | <0.5 | 0.9 | 1.7 | 3.4 | 35.5 | 35.2 | >36.0 |
| Calcium (mg/dl) | 7.8 | 6.8 | 8.4 | 8.3 | 8.7 | 12.4 | 10.7 | 10.9 |
| Phosphorus (mg/dl) | 10.1 | 7.6 | 9.7 | 8.3 | 8.2 | 10.6 | 10.4 | 14.6 |
| Total bilirubin (mg/dl) | 0.0 | 0.3 | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 |
| Alkalin phosphatase (U/liter) | 177 | 83 | 89 | 69 | 65 | 70 | 61 | 60 |
| Lactate dehydrogenase (U/liter) | 1,942 | >21,500 | 11,827 | >21,500 | ||||
| AST (U/liter) | 229 | 478 | 445 | 261 | 175 | 207 | 247 | 463 |
| ALT (U/liter) | 4 | 19 | 18 | 12 | 10 | 9 | 9 | 35 |
| Total protein (g/dl) | 2.7 | 2.9 | 4.1 | 4.7 | 4.3 | 4.7 | 4.8 | 5.0 |
| Albumin (g/dl) | 1.0 | 1.3 | 1.9 | 1.9 | 1.8 | 1.9 | 2.0 | 2.0 |
| Globulin (g/dl) | 1.7 | 1.6 | 2.2 | 2.8 | 2.5 | 2.8 | 2.8 | 3.0 |
| Albumin/globulin ratio | 0.6 | 0.8 | 0.8 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| Creatine kinase (U/liter) | 7,577 | 3,782 | ||||||
| White blood cells (103/mm3) | 7.4 | 3.6 | 2.7 | 9.1 | 22.1 | 18.2 | 23.0 | |
| Hematocrit (%) | 38 | 26 | 30 | 28 | 21 | 18 | 14 | |
| Heterophils (absolute no.) | 3,330 | 2,232 | 1,269 | 6,097 | 17,680 | 13,286 | 17,250 | |
| Progranulocytes (absolute no.) | 0 | 0 | 0 | 0 | 0 | 1,638 | 1,840 | |
| Lymphocytes (absolute no.) | 3,922 | 936 | 945 | 1,365 | 2,652 | 2,912 | 2,530 | |
| Monocytes (absolute no.) | 148 | 432 | 0 | 0 | 1,326 | 546 | 920 | |
| Eosinophils (absolute no.) | 0 | 0 | 486 | 1,456 | 442 | 0 | 230 | |
| Basophils (absolute no.) | 0 | 0 | 0 | 182 | 0 | 0 | 0 | |
| Platelets | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | |
| Red blood cell morphology | Normal | Normal | MP6 | Abnormal | Abnormal | Abnormal | Abnormal | |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
MP, moderate polychromasia.
Over the next 2 months, the turtle underwent periods of regenerative anemia and leukocytosis that were treated with vitamin K (0.5 mg/kg subcutaneously once or twice weekly), metronidazole (Sidmark Laboratories, Inc., East Hanover, N.J.; 20 mg/kg orally once daily), amikacin (Phoenix Scientific, St. Joseph, Mo.; 5 mg/kg intramuscularly initially, then 2.5 mg/kg intramuscularly every 72 h), and enrofloxacin (Bayer Corp., Shawnee Mission, Kans.; 5 mg/kg intramuscularly once daily). Itraconazole was continued throughout this period.
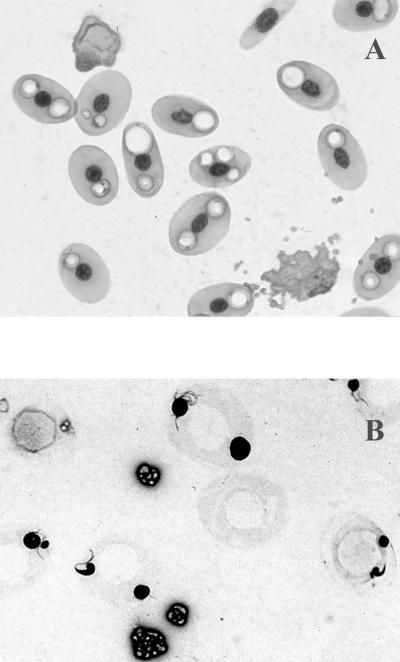
On 7 February 2000, abnormal erythrocytes began to appear in blood smears from this turtle. Affected cells appeared to have empty vacuoles in the cytoplasm when stained with RapiDiff stain (Cytocolor, Inc., Hinckley, Ohio; Fig. 1A) or Wright-Giemsa (Hemacolor; Harleco, Gibbstown, N.J.). Later, it was noted that these vacuoles stained with methylene blue (Fig. 1B). These abnormal erythrocytes increased in number over the next month until virtually 100% of the erythrocytes on a smear were affected. Blood was fixed in McDowell and Trump's solution (prepared in the laboratory [27]) overnight at 4°C to preserve it for examination by transmission electron microscopy (TEM).
FIG. 1.
Photomicrographs of blood smear from the Kemp's ridley sea turtle in this case study. (A) RapiDiff stain. (B) Methylene blue stain. Magnification, ×400.
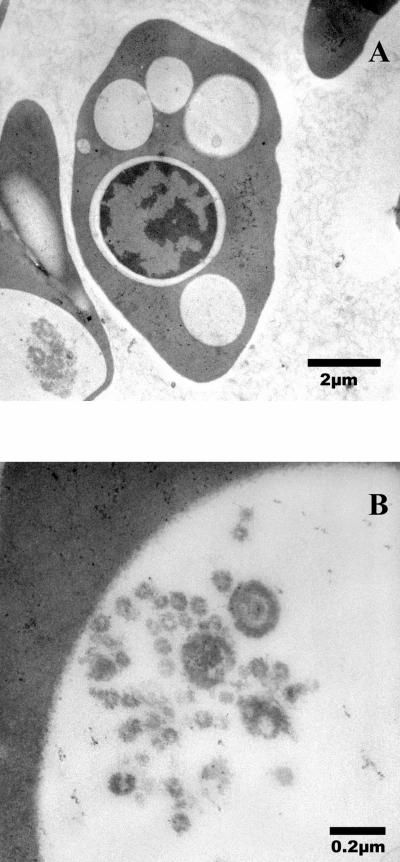
To carry out the TEM, each blood sample was washed several times in phosphate-buffered saline solution (PBS, pH 7.2) before incubating with 1% osmium tetroxide for 1 h, followed by washing in PBS solution. After several washes in distilled water, samples were dehydrated in a graded ethanol series, followed by 100% acetone. Samples were infiltrated in a graded epoxy resin series (1 h for each step) and polymerized in 100% epoxy resin for 2 days at 60°C. Ultrathin sections (70 nm) were stained with uranyl acetate and lead citrate and examined by TEM (Hitachi H-7000; Hitachi America, Ltd., Tarrytown, N.Y.). Digital micrographs were obtained (Fig. 2A and B).
FIG. 2.
TEM photomicrographs of affected erythrocyte from the Kemp's ridley sea turtle in this case study. (A) Low magnification. (B) High magnification of body within erythrocyte.
A bronchial wash was performed on 16 February. For microbiology, swabs were used to inoculate the following media: blood agar (Columbia agar base with 5% sheep blood), MacConkey agar, Hektoen enteric agar, mannitol salt agar, thiosulfate citrate bile salts agar, Sabouraud dextrose agar and broth with 150 mg of chloromycetin per ml (SAB), and tryptic soy broth (all from BD Microbiological Systems, Sparks, Md.). Bacterial cultures were incubated at 37°C (to ensure rapid growth, since virtually all bacterial pathogens grow best at the higher temperature), while fungal media were incubated at both 37°C and room temperature (ca. 22°C, as not all fungal pathogens will grow at 37°C). Media were examined daily for up to 10 days. Representative colonies from plates were transferred to tryptic soy or SAB slants, the latter for fungal isolates. Gram-negative bacteria were identified by using the API 20E system (bioMérieux, Hazelwood, Mo.). No bacterial or fungal growth was observed from the bronchial wash, even after 10 days of incubation.
At about the same time as the bronchial wash, toxic heterophils began to appear in the peripheral blood smears. Initially, these toxic cells were present in fairly small numbers, but the numbers appeared to be increasing continuously. The presence of toxic heterophils is suggestive of infectious disease, usually bacterial in nature, and an increase in the number is suggestive of a worsening of the disease process (5).
Shortly after the bronchial wash, clear fluids were noted in the trachea, bronchi, and lungs, and culture of this fluid yielded Citrobacter freundii, several Pseudomonas spp., and a Streptococcus sp. At that time, because of antibiotic susceptibility test results, the turtle was started on rifampin (Hoechst Marion Roussel, Kansas City, Mo.; 40 to 80 mg nebulized once daily) and doxycycline (Watson Laboratories, Inc., Corona, Calif.; 10 mg/kg orally once daily) as well as furosemide (Burns Veterinary Supply, Rockville Center, N.Y.; 5 mg/kg orally as needed) and aminophylline (American Regent Laboratories, Inc., Shirley, N.Y.; 16 mg, nebulized, once daily). The turtle initially appeared to be responding to the therapy, but on 7 March a marked deterioration was evident. Blood work indicated kidney failure and possible hepatic compromise (Table 1), and about 80% of all heterophils observed on peripheral blood smears were toxic by this time. The turtle continued to decline and died on 14 March 2000.
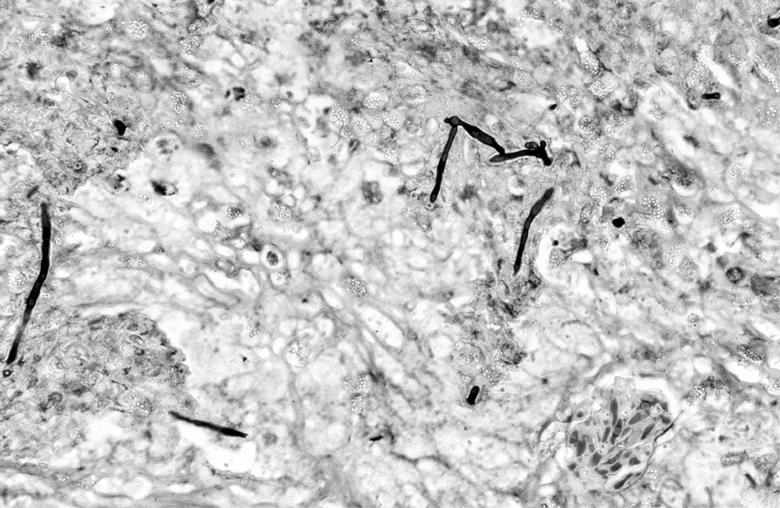
Gross necropsy findings included abnormalities in the lungs, kidneys, and liver. Histopathological findings included severe chronic granulomatous mycotic nephritis and pneumonia and a chronic moderate granulomatous hepatitis as well as a granulocytic hyperplasia of the bone marrow of the carapace. The Gomori methenamine silver stain revealed hyphae in tissue sections from the lungs and kidneys (Fig. 3). No significant lesions were detected in tissue samples examined from the pancreas, spleen, trachea, stomach, large intestine, or ovary. Lesions observed were consistent with multicentric systemic fungal disease.
FIG. 3.
Photomicrograph of Gomori methenamine silver-stained kidney from the Kemp's ridley sea turtle, showing hyphae within the granulomatous lesions. Magnification, ×100.
At the time of the necropsy, cultures were taken and performed as above. In addition to the bacteria cultured earlier, a filamentous, conidium-producing fungus was cultured from the lungs and kidneys on SAB after 5 days of incubation at room temperature. Both the aerial hyphae and agar under and surrounding colonies turned orange in 7 days after being a light rust color initially.
A subculture of the isolate was sent to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio, where it was identified as Colletotrichum acutatum and added to their stock collection as UTHSC 00-564. Identification was based on the isolate's cultural characteristics on potato flake agar (prepared in-house), temperature requirements, and microscopic morphology. Colonies exhibited a slow to moderate growth rate (11 mm in 5 days on a 60-mm petri dish) and were white initially.
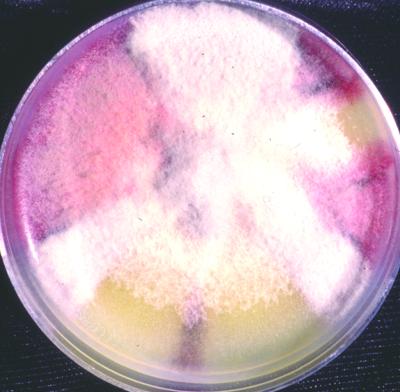
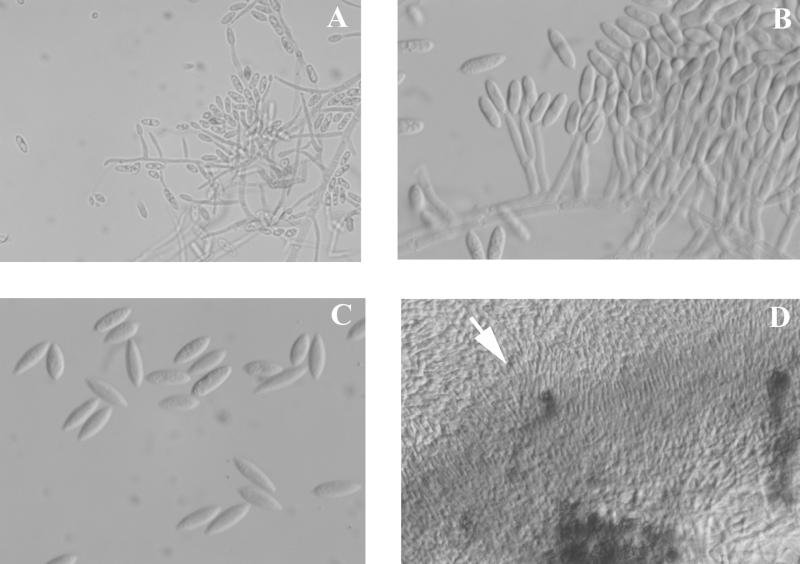
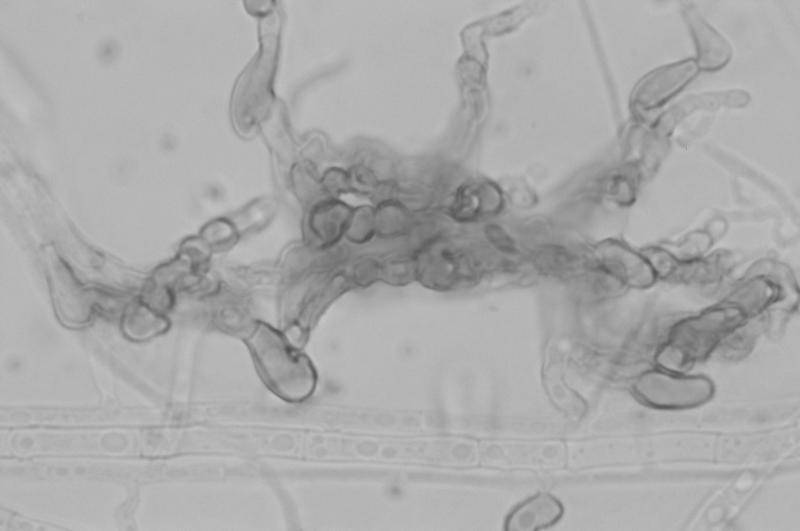
After 7 days of incubation they became overlaid with gray woolly growth, were sectored with areas of pale yellow (a feature noted by Dingley and Gilmour [8]), and produced a distinct carmine pigment which diffused into the medium (Fig. 4). Pigment production appeared not to be influenced by temperature but was medium dependent. Optimal growth occurred at 25°C, with somewhat reduced colony diameters at 30°C. No growth occurred at 35 or 40°C. Conidia produced in the aerial mycelium from long, slender phialides were extremely variable in both size and shape, ranging from 2.5 to 3 μm in width by 6 to 15 μm in length (Fig. 5A). While the majority of conidia were fusiform, some were medianly constricted, with obtuse apices. Conidia from salmon-colored acervulus-like masses were more consistent in size and shape (Fig. 5B and 5C). Appressoria were mostly nonlobed but did become quite complex (Fig. 6). Acervulus-like masses lacked setae, and no sclerotia were observed. See Table 2 for features differentiating C. acutatum from the C. gloeosporioides and C. coccodes groups.
FIG. 4.
Macroscopic morphology of C. acutatum after growth on potato flake agar at 25°C for 10 days.
FIG. 5.
Conidia of C. acutatum produced on potato flake agar at 25°C, lactophenol cotton blue mount. (A) Conidia from phialides in the aerial mycelium (magnification, ×460). (B) Conidia from a small acervulus-like mass of conidiophores (magnification, ×920). (C) Nonconstricted conidia with pointed ends from acervulus-like masses (magnification, ×920). (D) Edge (arrow) of an acervulus-like mass of conidiophores (magnification, ×230).
FIG. 6.
Complex appressoria of C. acutatum grown on potato flake agar at 25°C, lactophenol cotton blue mount. Magnification, ×920.
TABLE 2.
Features differentiating Colletotrichum acutatum from C. gloeosporioides and C. coccoides
| Organism | Colony morphologya | Rate of growth | Tempb (°C) | Conidial morphology | Conidial dimensions (μm) | Spore mass color | Reference(s) |
|---|---|---|---|---|---|---|---|
| C. acutatum | Superficial mycelium gray; sclerotia absent or poorly developed, rare; carmine pigment in agar | Slow | 33 max, 25-26.8 opt | On massed conidiophores in the nature of acervuli; also formed from single phialides; variably shaped, mostly short, narrow, with pointed ends | 8.5-16.5 × 2.5-4 | Salmon to dark orange | 6, 31 |
| UTHSC 00-564 | Initially white, later gray with yellow sectoring; intense carmine diffusing pigment; no sclerotia or setae | Slow | 25 optc | As above | 6.0-15.0 × 2.5-3 (aerial mycelium); 3 × 12 mean (spore masses) | Orange | |
| C. gloeosporioides | Superficial mycelium white to gray; sclerotia large and abundant; medium not pigmented | Fast | 35.5 max, 25-26.5 opt | On massed conidiophores in the nature of acervuli; broad, oblong, rounded ends | 10-16 × 4-7; 13.8 × 4.8 mean | Salmon | 6, 31 |
| C. coccoides | Scant superficial mycelium; medium pigmented pale salmon to pale brown; sclerotia large and abundant; setae present | Fast | 24-28 opt | On sclerotia, pointed ends, straight; consistently medianly constricted | 16-22 × 3-4 | Honey | 6, 33 |
On potato flakes agar.
Maximum (max) and optimum (opt) temperatures.
Optimum temperature 25°C; reduced colonial diameter at 30°C; no growth at 35 or 40°C.
In vitro antifungal susceptibility testing of the isolate performed according to the National Committee for Clinical Laboratory Standards standard M38-P (28) broth macrodilution method for filamentous fungi revealed 72- and 96-h MICs of fluconazole and itraconazole of 16 and 64 μg/ml and 0.125 and 0.5 μg/ml, respectively.
Cold stunning of sea turtles is a fairly common occurrence during the fall and winter in temperate climates in the United States but also occurs in subtropical climates during unusually cold events (40). Initial treatment of the hypothermia involves slowly increasing the body temperature to about 20°C (32). For the animals that survive to this point, the extent of immune compromise and the treatment thereof appear to determine the long-term survival of the animal. During rehabilitation of cold-stunned turtles, bacterial, fungal, and viral infections will often develop over the following 3 to 10 months (C. A. Manire, personal observation). These infections do not appear to be related to the stress of captivity. Broad-spectrum systemic antibiotics and antifungal agents are routinely used prophylactically during this extended period. Occasionally, in a very small percentage of the patients, despite the prophylactic treatment, overwhelming infections develop that may lead to the demise of the animal.
Although it is almost impossible to determine in sea turtles at present, it is suspected that the turtle in this case was immunocompromised. It is unlikely that a plant pathogen would cause primary disease in an immunocompetent animal, and one can only speculate where the turtle acquired the fungus. Coelomycetes are known to survive in salt water (36) and may have been acquired there, but it is also possible that it was acquired as an airborne inhalant during rehabilitation. None of the other turtles housed with this one developed the same infection. It is important to note that the temperature at which turtles undergoing rehabilitation are maintained at the Sea Turtle Rehabilitation Hospital (27°C) is within the range of the optimal growth temperature for the fungus (26 to 28°C). Unfortunately, there is usually little or no hematologic evidence when mycotic infections develop in sea turtles. The heterophilia that occurred several times during the course of this disease as well as the toxic heterophils were identical to those caused by bacterial infections and may well have been due to the presence of concurrent bacterial infections. In other animal groups, fungal infections are often noted to result in monocytosis or eosinophilia, but neither was present to any degree during the disease in this case (Table 1). It is not currently known whether the inclusions observed in the erythrocytes of this animal were caused by the mycotic infection or possibly some other agent. It is presented here for comparison with any other cases that may occur.
In vitro antifungal susceptibility testing on the isolate indicated that the infection should have been susceptible to the itraconazole treatment that the animal was receiving. A similar situation has been termed “breakthrough” fungal infections in human medicine, and it is being reported more commonly in immunocompromised individuals (14, 20, 30). Other conspecifics receiving the same dose of itraconazole were later determined to exhibit itraconazole concentrations in plasma of about 2 to 3 μg/ml, and kidney and lung tissue values were 8.6 and 4.7 times the plasma concentrations, respectively (C. A. Manire, H. L. Rhinehart, G. J. Pennick, D. Sutton, and M. G. Rinaldi, submitted for publication). Rifampin is known to decrease itraconazole concentrations when used concurrently in both humans (1, 9, 17, 35, 39) and animals (18). It is possible that concurrent use of rifampin and itraconazole may have contributed to the fungal infection, but a much higher dosage or a combination of two different systemic antifungal drugs for treatment may be necessary to affect a cure in immunocompromised sea turtles.
This report extends the potential pathogenicity of Colletotrichum acutatum and documents a disseminated mycosis in the Kemp's ridley sea turtle (Lepidochelys kempi).
Acknowledgments
We thank the many individuals whose donations make the work of the Sea Turtle Rehabilitation Hospital possible, and we thank David Smith, Petra Cunningham-Smith, Lisa Duffy, Eric Anderson, Jeannie Senn, and the many volunteers and interns who assisted with the rehabilitation of these turtles. We thank Karen Kelly, ICBR, Electron Microscopy Core Laboratory, University of Florida, for technical assistance, and Robert Reddick for review of the SEM erythrocyte photomicrographs. Andy Stamper and the staff of the New England Aquarium for initial treatment of the turtles and the Massachusetts Audubon Society Wellfleet Bay Wildlife Sanctuary for rescuing the turtles in extremely adverse conditions are all to be recognized for their heroic efforts to save these endangered turtles.
REFERENCES
- 1.Albengres, E., H. Le Louet, and J. P. Tillement. 1998. Systemic antifungal agents. Drug interactions of clinical significance. Drug Saf. 18:83-97. [DOI] [PubMed] [Google Scholar]
- 2.Benne, G. S., C. Neeleman, M. Bruin, G. S. de Hoog, and A. Gleer. 1993. Disseminating infection with Scytalidium dimidiatum in a granulocytopenic child. Eur. J. Clin. Microbiol. Infect. Dis. 12:118-121. [DOI] [PubMed] [Google Scholar]
- 3.Biggs, A. R., and S. S. Miller. 2001. Relative susceptibility of selected apple cultivars to Colletotrichum acutatum. Plant Dis. 85:657-660. [DOI] [PubMed] [Google Scholar]
- 4.Borderie, V. M., T. M. Bourcier, J. L. Poirot, M. Baudrimont, P. Prudhomme de Saint-Maur, and L. Laroche. 1997. Endophthalmitis after Lasiodiplodia theobromae corneal abscess. Graefe's Arch. Clin. Exp. Ophthalmol. 235:259-261. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, T. W. 1996. Clinical pathology, p. 248-257. In D. R. Mader (ed.), Reptile medicine and surgery. W. B. Saunders Co., Philadelphia, Pa.
- 6.Castro, L. G. M., C. daSilva Lacaz, J. Guarro, J. Gené, E. M. Heins-Vaccari, R. Santos de F. Leite, G. L. H. Arriagada, M. M. O. Reguera, E. M. Ito, N. Y. S. Valente, and R. S. Nunes. 2001. Phaeohyphomycotic cyst caused by Colletotrichum crassipes. J. Clin. Microbiol. 39:2321-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chabasse, D., C. de Bievre, E. Legrand, J. P. Saint-Andre, L. de Gentile, B. Cimon, and J. P. Bouchara. 1995. Subcutaneous abscess caused by Pleurophomopsis lignicola Petr: first case. J. Med. Vet. Mycol. 33:415-417. [PubMed] [Google Scholar]
- 8.Dingley, J. M., and J. W. Gilmour. 1972. Colletotrichum acutatum: Simmds. f. sp. Pinea associated with “terminal crook” disease of Pinus spp. N. Z. J. For. Sci. 2:192-201. [Google Scholar]
- 9.Drayton, J., G. Dickinson, and M. G. Rinaldi. 1994. Coadministration of rifampin and itraconazole leads to undetectable levels of serum itraconazole. Clin. Infect. Dis. 18:266.. [DOI] [PubMed] [Google Scholar]
- 10.Fernando, T. H. P. S., C. K. Jayasinghe, and R. L. C. Wijesundera. 2000. Factors affecting spore production, germination and viability of Colletotrichum acutatum isolates from Hevea brasiliensis. Mycol. Res. 104:681-685. [Google Scholar]
- 11.Forster, H., and J. E. Adaskaveg. 1999. Identification of subpopulations of Colletotrichum acutatum and epidemiology of almond anthracnose in California. Phytopathology 89:1056-1065. [DOI] [PubMed] [Google Scholar]
- 12.Freeman, S., and E. Shabi. 1996. Cross-infection of subtropical and temperate fruits by Colletotrichum species from various hosts. Physiol. Mol. Plant Pathol. 49:395-404. [Google Scholar]
- 13.Freeman, S., S. Horowitz, and A. Sharon. 2001. Pathogenic and nonpathogenic lifestyles in Colletotrichum acutatum from strawberry and other plants. Phytopathology 91:986-992. [DOI] [PubMed] [Google Scholar]
- 14.Glasmacher, A., C. Hahn, C. Leutner, E. Molitor, E. Wardelmann, C. Losem, T. Sauerbruch, G. Marklein, and I. G. H. Schmidt-Wolf. 1999. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses 42:443-451. [DOI] [PubMed] [Google Scholar]
- 15.Guarro, J., T. E. Svidzinski, L. Zaror, M. H. Fortaz, J. Gené, and O. Fischman. 1998. Subcutaneous hyalohyphomycosis caused by Colletotrichum gloeosporioides. J. Clin. Microbiol. 36:3060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerber, J. C., and J. C. Correll. 2001. Characterization of Glomerella acutata, the teleomorph of Colletotrichum acutatum. Mycologia 93:216-219. [Google Scholar]
- 17.Jaruratanasirikul, S., and S. Sriwiriyajan. 1998. Effect of rifampicin on the pharmacokinetics of itraconazole in normal volunteers and AIDS patients. Eur. J. Clin. Pharmacol. 54:155-158. [DOI] [PubMed] [Google Scholar]
- 18.Kaltenbach, G., D. Levêque, J.-D. Peter, J. Salmon, H. Elkhaili, A. Cavalier, Y. Salmon, H. Monteil, and F. Jehl. 1996. Pharmacokinetic interaction between itraconazole and rifampin in Yucatan miniature pigs. Antimicrob. Agents Chemother. 40:2043-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkness, C. M., D. V. Seal, Y. M. Clayton, and E. Punithalingam. 1991. Sphaeropsis subglobosa keratomycosis-first reported case. Cornea 10:85-89. [PubMed] [Google Scholar]
- 20.Krcmery, V., Jr., F. Mateika, A. Kunová, S. Äpánik, J. Gyarfáš, Z. Sycová, and J. Trupl. 1999. Hematogenous trichosporonosis in cancer patients: report of 12 cases including 5 during prophylaxis with itraconazol [sic]. Support Care Cancer 7:39-43. [DOI] [PubMed] [Google Scholar]
- 21.Lacaz, C. S., A. D. Pereira, E. M. Heins-Vaccari, L. C. Cuce, C. Benatti, R. S. Nunes, N. T. de Melo, R. S. de Freitas-Leite, and G. L. Hernandez-Arriagada. 1999. Onychomycosis caused by Scytalidium dimidiatum. Report of two cases. Review of the taxonomy of the synanamorph and anamorph forms of this coelomycete. Rev. Inst. Med. Trop. Sao Paulo 41:319-323. [PubMed] [Google Scholar]
- 22.Liao, W. Q., J. Z. Shao, S. Q. Li, T. Z. Li, S. X. Wu, J. Z. Zhang, and Q. T. Chen. 1983. Colletotrichum dematium caused keratitis. Chin. Med. J. 96:391-394. [PubMed] [Google Scholar]
- 23.Liesegang, T. J., and R. K. Forster. 1980. Spectrum of microbial keratitis in South Africa. Am. J. Ophthalmol. 90:38-47. [DOI] [PubMed] [Google Scholar]
- 24.Martin, M. P., and F. Garcia-Figueres. 1999. Colletotrichum acutatum and C. gloeosporioides cause anthracnose on olives. Eur. J. Plant Pathol. 105:733-741. [Google Scholar]
- 25.Maslen, M. M., T. Collis, and R. Stuart. 1996. Lasiodiplodia theobromae isolated from a subcutaneous abscess in a Cambodian immigrant to Australia. J. Med. Vet. Mycol. 34:279-283. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki, O., M. Yasuda, and M. Ichinohe. 1988. Keratomycosis due to Glomerella cingulata. Rev. Iberam. Micol. 5(Suppl. 1):329-349. [Google Scholar]
- 27.McDowell, E. M., and B. F. Trump. 1976. Historical fixatives suitable for diagnostic light and electron microscopy. Arch. Pathol. Lab. Med. 100:405-414. [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 2000. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 29.O'Quinn, R. P., J. L. Hoffmann, and A. S. Boyd. 2001. Colletotrichum species as emerging opportunistic fungal pathogens: a report of 3 cases of phaeohyphomycosis and review. J. Am. Acad. Dermatol. 45:56-60. [DOI] [PubMed] [Google Scholar]
- 30.Plum, G., C. Scheid, C. Franzen, H. Schütt-Gerowitt, H. Seifert, and P. D. Wickramanayake. 1996. Empirical liposomal amphotericin-B therapy in a neutropenic patient: breakthrough of disseminated Blastoschizomyces capitatus infection. Zentralbl. Bakteriol. 284:361-366. [DOI] [PubMed] [Google Scholar]
- 31.Ritterband, D. C., M. Shah, and J. A. Seedor. 1997. Colletotrichum graminicola: a new corneal pathogen. Cornea 16:362-364. [PubMed] [Google Scholar]
- 32.Sadove, S. S., R. Pisciotta, and R. DiGiovanni. 1998. Assessment and initial treatment of cold-stunned sea turtles. Chelonian Conserv. Biol. 3:84-87. [Google Scholar]
- 33.Shukla, P. K., Z. A. Khan, B. Lal, P. K. Agrawal, and O. P. Srivastava. 1983. Clinical and experimental keratitis caused by Colletotrichum state of Glomerella cingulata and Acrophialophora fusispora. Sabouraudia 21:137-147. [PubMed] [Google Scholar]
- 34.Simmonds, J. H. 1965. A study of the species of Colletotrichum causing ripe fruit rots in Queensland. Qd. J. Agric. Anim. Sci. 22:437-459. [Google Scholar]
- 35.Strayhorn, V. A., A. M. Baciewicz, and T. H. Self. 1997. Update on rifampin drug interactions, III. Arch. Intern. Med. 157:2453-2458. [PubMed] [Google Scholar]
- 36.Sutton, B. C. 1980. The coelomycetes: fungi with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew, Surrey, England.
- 37.Sutton, D. A. 1999. Coelomycetous fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Rev. Iberoam. Micol. 16:171-179. [PubMed] [Google Scholar]
- 38.Sutton, D. A., W. D. Timm, G. Morgan-Jones, and M. G. Rinaldi. 1999. Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phomopsis Saccardo 1905: criteria for identification, case history, and therapy. J. Clin. Microbiol. 37:807-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucker, R. M., D. W. Denning, L. H. Hanson, M. G. Rinaldi, J. R. Graybill, P. K. Sharkey, D. Pappagianis, and D. A. Stevens. 1992. Interaction of azoles with rifampin, phenytoin, and carbamazepine: in vitro and clinical observations. Clin. Infect. Dis. 14:165-174. [DOI] [PubMed] [Google Scholar]
- 40.Witherington, B. E., and L. M. Ehrhart. 1989. Hypothermic stunning and mortality of marine turtles in the Indian River lagoon system, Florida. Copeia 1989:696-703. [Google Scholar]
- 41.Yamamoto, J., S. Toyozo, and K. Tomioka. 1999. Occurrence of ripe rot of grape (Vitis vinifera L.) caused by Colletotrichum acutatum Simmonds ex Simmonds. Ann. Phytopathol. Soc. Jpn. 65:83-86. [Google Scholar]