Abstract
Successful carbapenem-based chemotherapy for the treatment of Pseudomonas infections has been seriously hindered by the recent appearance of IMP- and VIM-type metallo-β-lactamases, which confer high-level resistance to carbapenems and most other β-lactams. Recently, multidrug-resistant Pseudomonas putida isolates for which carbapenem MICs were ≥32 μg/ml were recovered from cultures of urine from three inpatients in the general intensive care unit of the Ospedale di Circolo, Varese, Italy. Enzyme assays revealed production of a metallo-β-lactamase activity, while molecular analysis detected in each isolate a blaVIM-1 determinant carried by an apparently identical medium-sized plasmid. Conjugation experiments were unsuccessful in transferring the β-lactamase determinant to Escherichia coli or Pseudomonas aeruginosa. Macrorestriction analysis by pulsed-field gel electrophoresis demonstrated that the isolates were of clonal origin. PCR mapping and sequencing of the variable region of the plasmid-borne class 1 integron carrying the blaVIM-1 determinant (named In110) showed that the blaVIM-1-containing cassette was identical to that previously found in strains of different species from other Italian hospitals and that the cassette array of In110 was not identical but clearly related to that of In70 (a blaVIM-1-containing plasmid-borne integron from an Achromobacter xylosoxidans isolate), pointing to a common origin of this cassette and to a related evolutionary history of their cognate integrons.
Pseudomonas putida is a nonfermenting gram-negative rod belonging to rRNA group I of the genus Pseudomonas. Due to their ability to metabolize a wide range of biogenic and xenobiotic compounds, members of this species are able to colonize several niches, including soil, freshwater, and the surfaces of living organisms (22). Infections caused by P. putida are rare and are mostly reported in compromised patients, such as newborns (13) and neutropenic and cancer patients (2, 18).
P. putida is usually susceptible to carbapenems, monobactams, and extended-spectrum cephalosporins such as cefotaxime and ceftazidime (27). However, isolates of P. putida producing acquired metallo-β-lactamases that confer resistance to most β-lactams, including carbapenems, have recently been reported from the Far East (15, 29, 33).
Metallo-β-lactamases constitute molecular class B of Ambler (1) as well as group 3 in the functional classification of Bush et al. (3). The broad-spectrum activities of these enzymes are a major concern for clinicians, as they result in difficult-to-treat infections. The spread of acquired metallo-β-lactamases in nosocomial strains of nonfastidious gram-negative rods has increasingly been reported in Japan since the early 1990s (11, 28, 29), and recent reports suggest that a similar problem may also emerge in Europe (5-7, 16, 20, 21, 25, 31).
P. putida had rarely been recovered from clinical specimens at our hospital (Ospedale di Circolo), but infections caused by multidrug-resistant isolates of this species that were also resistant to carbapenems were recently observed, mostly in the general intensive care unit (ICU). The purpose of this study was to characterize the multidrug-resistant isolates recovered from the general ICU in order to investigate the resistance mechanism(s) and determine the possible clonal origins of the isolates.
MATERIALS AND METHODS
Clinical data.
The three cases included in this study were urinary tract infections caused by multidrug-resistant strains of P. putida and were observed over a 9-month period (September 1999 to May 2000). The patients had been admitted to our ICU, and their ages ranged from 63 to 75 years. Before the recovery of P. putida the patients had received multiple courses of antibiotics, including β-lactam agents, but not carbapenems. Treatment with amikacin (500 mg twice a day) was successful in eradicating infection in all three patients.
Bacterial identification and antimicrobial susceptibility testing.
Bacterial identification was achieved with the ATB system (ID32GN strips; bioMérieux, Marcy l'Étoile, France) (8). The MICs of several antimicrobial agents (piperacillin, piperacillin plus tazobactam, aztreonam, cefotaxime, ceftazidime, cefepime, imipenem, meropenem, gentamicin, tobramycin, amikacin, and ciprofloxacin) were determined with broth microdilution panels (Sceptor System custom MIC panels; Becton Dickinson Diagnostic Systems, Sparks, Md.), which were incubated at 35°C for 18 to 24 h. Multidrug-resistant strains were also evaluated by the Etest assay (AB Biodisk, Solna, Sweden). Data were interpreted according to the criteria of the National Committee for Clinical Laboratory Standards (19). In order to assess the role of the strains involved in the general ICU infections, the epidemiology and the resistance patterns of all P. putida isolates (n = 25) recovered at the Microbiology Laboratory of the Ospedale di Circolo from 1998 to 2000 were also studied. All strains were stored at −70°C in brain heart infusion broth containing 20% (vol/vol) glycerol and were analyzed after recovery from storage and subculture on appropriate media.
β-Lactamase assays.
Metallo-β-lactamase activity in crude cell extracts was assayed spectrophotometrically, essentially as described previously (14). Reactions were performed in 10 mM HEPES buffer (HB; pH 7.5) at 30°C in a total volume of 0.5 ml. Imipenem hydrolysis was monitored at 300 nm (Δɛ = −9,000 M−1 · cm−1) by use of an initial substrate concentration of 150 μM. Inhibition of enzymatic activity by EDTA was assayed by measuring the residual carbapenemase activity after incubation of the crude extract for 20 min at 30°C in the presence of 5 mM EDTA. A control without EDTA was always run in parallel. Crude extracts were prepared from early-stationary-phase cultures grown for 16 h at 37°C in Mueller-Hinton (MH) broth. The cells were collected by centrifugation, resuspended in HB (1/10 of the original culture volume), and disrupted by sonication (three times for 15 s each time at 50 W). The supernatant obtained after centrifugation at 10,000 × g for 15 min to remove the cell debris represented the crude extract. The protein concentration in the solution was determined with a commercial kit (Bio-Rad protein assay; Bio-Rad, Richmond, Calif.) with bovine serum albumin as the standard.
Analytical isoelectric focusing of crude cell extracts for detection of β-lactamase activity was carried out in precast 5% polyacrylamide gels containing ampholytes (pH range, 3.5 to 9.5; Ampholine PAGplate; Amersham Pharmacia Biotech, Uppsala, Sweden) with a Multiphor II apparatus (Pharmacia). The gels were focused at 0.1 W/cm2 for 2 h at 10°C. β-Lactamases were detected as purple bands after the gel was overlaid with filter paper soaked with a 0.25 mM nitrocefin solution in HB supplemented with 2 mM ZnCl2.
Molecular analysis techniques.
Plasmid extraction was carried out by the alkaline lysis method (26). Whole genomic DNA was purified from P. putida as described previously (12). Southern blot hybridization was carried out directly on dried gels as described previously (32), using a 32P-labeled probe made of an amplicon containing a central 523-bp region of the blaVIM-1 gene amplified from Pseudomonas aeruginosa VR-143/97 genomic DNA (14) with primers VIM-DIA/f (5′-CAGATTGCCGATGGTGTTTGG) and VIM-DIA/r (5′-AGGTGGGCCATTCAGCCAGA) (9). PCR for the detection of VIM-type metallo-β-lactamase genes was carried out with primers VIM-DIA/f and VIM-DIA/r in a 50-μl volume by using 3.5 U of polymerase mix from the Expand High-Fidelity PCR system (Roche Biochemicals, Mannheim, Germany) in the reaction buffer provided by the manufacturer, which contained 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 50 pmol of each primer, and 5 ng of bacterial genomic DNA as the template. Reaction parameters were as follows: annealing at 55°C for 60 s, extension at 72°C for 90 s, and denaturation at 94°C for 50 s for 25 cycles.
PCR for amplification of the variable region of class 1 integrons was carried out as described previously (23) with primers INT-5′CS-f (5′-CTTCTAGAAAACCGAGGATGC) and INT-3′CS-r (5′-CTCTCTAGATTTTAATGCGGATG), designed on the basis of the 5′ conserved segment (5′-CS) and the 3′-CS of class 1 integrons, respectively. Reactions were performed in a 50-μl volume by using 3.5 U of polymerase mix from the Expand High-Fidelity PCR system (Roche Biochemicals) in the reaction buffer provided by the manufacturer, which contained 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 50 pmol of each primer, and 10 ng of bacterial genomic DNA as the template. Reaction parameters were as follows: annealing at 51°C for 60 s, extension at 70°C for 180 s (with an increment of 3 s for each cycle), and denaturation at 95°C for 40 s for 15 cycles and then annealing at 55°C for 60 s, extension at 70°C for 180 s (with an increment of 3 s for each cycle), and denaturation at 95°C for 40 s for 20 cycles, followed by a final extension step at 72°C for 20 min. PCRs were always performed in 0.2-ml tubes with a Gene Amp PCR system 2400 (Perkin-Elmer, Rahway, N.J.). Direct sequencing of PCR-generated amplicons was carried out by the dideoxy-chain termination method with an automatic DNA sequencer as described previously (23). Both strands were sequenced.
Resistance transfer experiments.
Conjugation experiments were performed on MH agar plates with Escherichia coli MKD-135 (argH rpoB18 rpoB19 recA rpsL; kindly provided by G. Kholodii, Institute for Molecular Genetics, Russian Academy of Sciences, Moscow, Russia) or P. aeruginosa 10145/3 (an rpoB and his derivative of reference strain ATCC 10145T) as the recipient. The initial donor/recipient ratio was 0.1. Mating plates were incubated at 30°C for 14 h. E. coli and P. aeruginosa transconjugants were selected on MH agar containing ceftazidime (50 mg/liter) plus rifampin (250 mg/liter). The detection sensitivity of the assay was ≥3 × 10−8 transconjugant/recipient with either recipient.
Macrorestriction analysis by PFGE.
Genomic DNA for macrorestriction analysis was prepared from cultures grown for 8 h at 37°C in Trypticase soy broth as described previously (17). Restrictions were carried out overnight at 37°C with 10 U of SpeI (Sigma, Milan, Italy). DNA fragments were resolved in a 1.3% agarose gel with a Rotaphor system R23 apparatus (Biometra, Göttingen, Germany) as described previously (17). Polymerized λ phage DNA (Sigma) served as a size standard (48.5 to 1,018 kb). The gel was stained with SYBR Gold (Molecular Probes, Eugene, Oreg.) and visualized with a Kodak CF440 camera (NEN Life Science Products, Boston, Mass.). Pulsed-field gel electrophoresis (PFGE) patterns were interpreted according to published criteria (30).
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the EMBL/GenBank/DDBJ sequence databases and assigned accession number AJ439689.
RESULTS
Detection of multidrug-resistant P. putida isolates producing the VIM-1 metallo-β-lactamase.
Over a 3-year period (1998 to 2000), 25 nonduplicate isolates of P. putida were recovered at the Microbiology Laboratory of the Ospedale di Circolo from clinical specimens including urine (n = 12), respiratory secretions (n = 8), ear swabs (n = 3), and wounds (n = 2). The infected patients were from the general ICU (n = 10), the nephrology department (n = 6), the pneumology department (n = 3), the oncology department (n = 2), the neonatal ICU (n = 2), and the department of medicine (n = 2). Most of the isolates were susceptible to aztreonam, extended-spectrum cephalosporins, carbapenems, fluoroquinolones, and aminoglycosides, whereas four of them exhibited a multidrug resistance pattern that included resistance to β-lactams and, although variably, aminoglycosides and fluoroquinolones. Three of the multidrug-resistant isolates were from the general ICU (isolates VA-304/99, VA-523/99, and VA-420/00), while the remaining one was from the nephrology department. The three isolates from the general ICU were further investigated in this work in order to assess the resistance mechanism(s) and the possible clonal origins of the isolates. The clinical data for these isolates are described in Materials and Methods section.
Antimicrobial susceptibility testing of the three multidrug-resistant P. putida isolates recovered from the ICU showed that they were characterized by the same resistance phenotype: resistance to piperacillin (MICs, ≥256 μg/ml), piperacillin plus tazobactam (MICs, ≥256 μg/ml), aztreonam (MICs, 32 μg/ml), cefotaxime (MICs, ≥256 μg/ml), ceftazidime (MICs, ≥256 μg/ml), cefepime (MICs, ≥256 μg/ml), gentamicin (MICs, 32 μg/ml), tobramycin (MICs, 16 μg/ml), ciprofloxacin (MICs, 16 μg/ml), imipenem (MICs, ≥32 μg/ml), and meropenem (MICs, ≥32 μg/ml). In contrast, amikacin remained active (MICs, 2 μg/ml).
A metallo-β-lactamase activity was detected in crude extracts of the three isolates (the specific imipenem-hydrolyzing activity ranged from 34 to 43 μmol/min/g of protein and was always inhibited >95% in the presence of 5 mM EDTA). Isoelectric focusing analysis of crude extracts revealed two β-lactamase bands of pI 5.2 and 6.4, respectively, for all three isolates (data not shown). A pI of 5.2 is in good agreement with the values reported for the VIM-type enzymes (9, 14).
PCR analysis with primers for blaVIM genes yielded a 0.5-kb amplicon from each of the three isolates, indicating the presence of a blaVIM allele (data not shown). Restriction of the amplicons with RsaI always yielded two 0.25-kb fragments, revealing a pattern compatible with blaVIM-1 (14).
Genetic support and transferability of blaVIM-1 determinant.
Analysis of the plasmid contents of the three isolates revealed that all of them carried an apparently identical plasmid of approximately 52 kb. Southern blot hybridization with a blaVIM probe returned a strong hybridization signal that corresponded to the plasmid DNA, indicating that the blaVIM-1 determinant was plasmid borne and that it was carried on a 7.5-kb EcoRI fragment. Figure 1 shows the results obtained with isolate VA-304/99; identical results were obtained with the other two strains, VA-523/99 and VA-420/00 (data not shown).
FIG. 1.
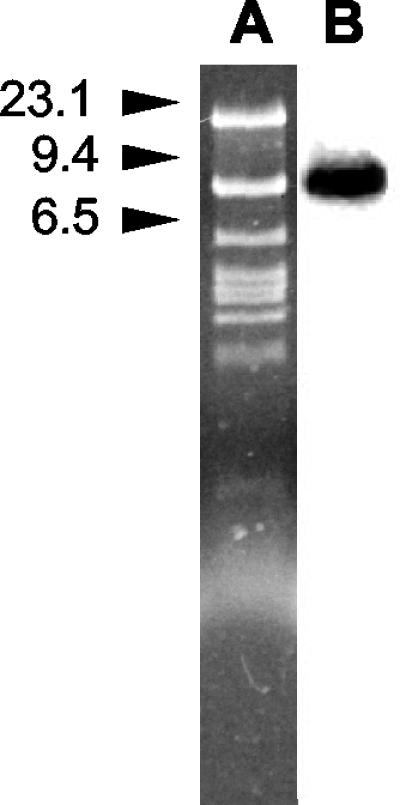
(A) Agarose gel electrophoresis of the plasmid DNA preparation from isolate VA-304/99 after digestion with EcoRI. (B) Results of Southern blot hybridization of the sample shown in panel A with a blaVIM-specific probe. Size standards (in kilobases) are indicated on the left.
Conjugation experiments failed to demonstrate the possibility that the β-lactamase determinant could be transferred from each of these isolates to either E. coli MKD-135 or P. aeruginosa 10145/3 under the experimental conditions adopted.
Structure of blaVIM-1-containing integron.
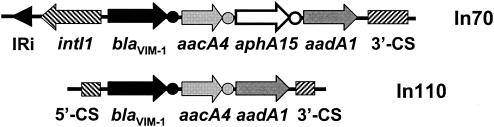
PCR amplification of the variable region of class 1 integrons (with primers INT-5′CS-f and INT-3′CS-r) with the plasmid extracted from VA-304/99 (pVA304) as the template yielded a 2.6-kb product, which was recognized by the blaVIM probe in a Southern blot experiment (data not shown). This confirmed that the blaVIM determinant is present in the amplicon. Sequencing of the amplicon showed an original array of three gene cassettes consisting of a blaVIM-1 metallo-β-lactamase determinant, an aacA4 aminoglycoside acetyltransferase determinant, and an aadA1 aminoglycoside adenylyltransferase determinant, respectively. The cassettes were inserted within the 5′-CS and the 3′-CS of a class 1 integron, termed In110 (Fig. 2).
FIG. 2.
Structure of the gene cassette array of integron In110, carried by plasmid pVA304, compared to that of In70, carried by plasmid pAX22 from A. xylosoxidans AX22 (24). Genes are indicated by arrows. The attC (also called the 59-base-element) recombination sites of gene cassettes are indicated by circles. The map is not drawn to scale.
The blaVIM-1-containing cassette was identical to those previously found in a chromosomal-borne integron of P. aeruginosa VR-143/97 (14) and in the In70 plasmid-borne integron from Achromobacter xylosoxidans AX22 (24). The aacA4-containing cassette was almost identical to that present in In70 (the only differences were a silent G-to-A transition at the third position of codon 145 and a C insertion within the attC site). The aadA1-containing cassette was identical to that of In70 and exhibited the same deletion of the attC recombination site (24). The variable region of In110, therefore, was virtually identical to that of In70 except for the lack of the aphA15-containing cassette present in the latter integron (Fig. 2). The nature of the gene cassettes present in In110 was also consistent with the aminoglycoside resistance pattern shown by these isolates (resistance to gentamicin and tobramycin, but not to amikacin).
Molecular characterization of VIM-1-producing P. putida isolates.
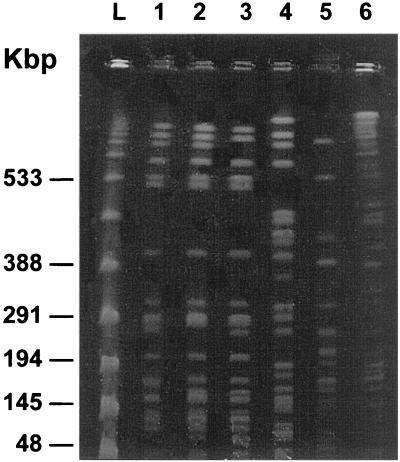
Macrorestriction analysis was conducted with the three VIM-1-producing isolates by using the SpeI restriction enzyme. For comparison, the multidrug-resistant P. putida isolate from the nephrology department (VA-758/00) and two of the β-lactam-susceptible P. putida isolates obtained from the general ICU in the year 2000 were also studied. PFGE analysis showed that the three VIM-1-producing isolates exhibited closely related restriction profiles (Fig. 3), indicating that they were of clonal origin. By contrast, comparison with the other isolates revealed patterns that differed by more than six bands.
FIG. 3.
PFGE banding patterns after SpeI digestion. Lanes 1 to 3, VIM-1-producing P. putida isolates from the general ICU (lane 1, isolate VA-304/99; lane 2, isolate VA-523/99; lane 3, isolate VA-420/00); lane 4, multidrug-resistant P. putida isolate from the nephrology department; lanes 5 and 6, two antibiotic-susceptible P. putida isolates obtained from the general ICU at the same hospital in 2000; lane L, λ phage DNA concatemers as a size marker.
DISCUSSION
Metallo-β-lactamases are emerging worldwide as acquired resistance determinants in nosocomial strains of nonfastidious gram-negative nonfermenters and members of the family Enterobacteriaceae (4). This is the first report of a P. putida isolate carrying the VIM-1 metallo-β-lactamase determinant and the first report of metallo-β-lactamase-producing isolates of this species in Europe.
The VIM-1 enzyme was first described in a multidrug-resistant P. aeruginosa strain (14) that caused a small outbreak in the general ICU of the University Hospital of Verona (Verona, Italy) in early 1997 (7). It was subsequently detected also in an A. xylosoxidans isolate from the same hospital (24) and in additional P. aeruginosa strains from different Italian hospitals (G. M. Rossolini, unpublished data), although, unlike VIM-2, it was never detected outside Italy. Studies carried out with the purified enzyme demonstrated that VIM-1 is able to hydrolyze not only carbapenems but also virtually all β-lactams with the exception of monobactams (10), thus highlighting its clinical relevance.
Consistent with this observation, in the P. putida isolates analyzed in our study, production of VIM-1 was associated with broad-spectrum β-lactam resistance including high-level resistance to carbapenems. However, the contribution of an additional enzyme(s), possibly produced in an inducible fashion, could not be ascertained at this stage. In particular, the isolates were also resistant to aztreonam (even though the MICs were lower), fluoroquinolones, and most aminoglycosides with the exception of amikacin. The aminoglycoside resistance pattern was consistent with the resistance genes carried by In110. In the patients with reported cases, treatment with amikacin was consistently effective. However, since several aminoglycoside-modifying enzymes can be encountered in nosocomial environments, a similar multidrug resistance pattern could represent a serious therapeutic problem.
Although uncommon, P. putida may be a cause of severe nosocomial infections in compromised hosts. In the Ospedale di Circolo (a 900-bed teaching hospital in northern Italy), 25 microbiologically confirmed P. putida infections were observed over a 3-year period (1998 to 2000). Of the 25 isolates, 4 showed a multidrug resistance phenotype, including high-level resistance to carbapenems. Three of them represented the VIM-1-producing isolates of clonal origin from the general ICU patients analyzed in this study, whereas the fourth one was a clonally independent isolate from a different ward. Overall, these findings demonstrate that metallo-β-lactamases can be important resistance determinants emerging in these opportunistic pathogens.
The fact that the same VIM-1-producing strain was isolated from different patients over a 9-month period suggests the chance of a long-lasting persistence of this microorganism in the hospital environment. None of the infected patients had previously received treatment with carbapenems, indicating that other β-lactams could also select for metallo-β-lactamase-producing strains in a single patient. However, it should be noticed that from November 1998 to August 1999, a large outbreak caused by a PER-1-producing P. aeruginosa strain occurred in the general ICU of the same hospital, and imipenem was widely used to eradicate the infections (17). The extensive use of imipenem during the several months preceding the isolation of VA-304/99 (the first VIM-1-producing isolate) could have selected for the emergence of carbapenem-resistant strains.
The analysis of the genetic support of blaVIM-1 revealed that, in this strain, the gene was plasmid borne. Although the plasmid was apparently not self-transmissible, the presence of the gene on a plasmid could facilitate horizontal spread and underscores the role that P. putida could play as a long-lasting reservoir of resistance genes in the hospital environment. In plasmid pVA304, blaVIM-1 was part of an integron-borne gene cassette identical to that previously found in another Italian hospital (14, 24). Moreover, the variable region of the blaVIM-1-containing integron of pVA304 (named In110) was clearly related, although not identical, to that of In70, from which In110 could have been derived following excision of the aphA15-containing cassette. These findings therefore point to a common origin of the blaVIM-1 cassette and a common evolutionary history of the cognate integrons spreading in northern Italy.
Acknowledgments
This work was partially supported by the European research network on metallo-β-lactamases within the TMR program (contract FMRX-CT98-0232) and by grant 2001068755_003 (PRIN 2001) from the Italian Ministero dell'Istruzione, dell'Università e della Ricerca.
REFERENCES
- 1.Ambler, R. P. 1980. The structure of beta-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 289:321-331. [DOI] [PubMed] [Google Scholar]
- 2.Anaissie, E., V. Fainstein, P. Miller, H. Kassamali, S. Pitlik, G. Bodey, and K. Rolston. 1987. Pseudomonas putida. Newly recognized pathogen in patients with cancer. Am. J. Med. 82:1191-1194. [DOI] [PubMed] [Google Scholar]
- 3.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush, K. 2001. New β-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso, O., J. C. Sousa, R. Leitão, and L. Peixe. 1999. Carbapenem hydrolysing β-lactamase from clinical isolates of Pseudomonas aeruginosa in Portugal. J. Antimicrob. Chemother. 44:135.. [DOI] [PubMed] [Google Scholar]
- 6.Cornaglia, G., M. L. Riccio, A. Mazzariol, L. Lauretti, R. Fontana, and G. M. Rossolini. 1999. Appearance of IMP-1 metallo-β-lactamase in Europe. Lancet 353:899-900. [DOI] [PubMed] [Google Scholar]
- 7.Cornaglia, G., A. Mazzariol, L. Lauretti, G. M. Rossolini, and R. Fontana. 2000. Hospital outbreak of carbapenem-resistant Pseudomonas aeruginosa producing VIM-1, a novel transferable metallo-β-lactamase. Clin. Infect. Dis. 31:1119-1125. [DOI] [PubMed] [Google Scholar]
- 8.Croize, J., D. Rouhan, and P. Le Noc. 1987. Identification des bacilles à Gram négatif aérobies et aéro-anaérobies non exigeants par le Système ATB 32 GN. Ann. Biol. Clin. 45:74-77. [PubMed] [Google Scholar]
- 9.Docquier, J.-D., F. Luzzaro, G. Amicosante, A. Toniolo, and G. M. Rossolini. 2001. Multidrug-resistant Pseudomonas aeruginosa producing PER-1 extended-spectrum serine-β-lactamase and VIM-2 metallo-β-lactamase. Emerg. Infect. Dis. 7:910-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschini, N., B. Caravelli, J.-D. Docquier, M. Galleni, J.-M. Frère, G. Amicosante, and G. M. Rossolini. 2000. Purification and biochemical characterization of the VIM-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:3003-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirakata, Y., K. Izumikawa, T. Yamaguchi, H. Takemura, H. Tanaka, R. Yoshida, J. Matsuda, M. Nakano, K. Tomono, S. Maesaki, M. Kaku, Y. Yamada, S. Kamihara, and S. Kohno. 1998. Rapid detection and evaluation of clinical characteristics of emerging multiple-drug-resistant gram-negative rods carrying the metallo-β-lactamase gene blaIMP. Antimicrob. Agents Chemother. 42:2006-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, J. L. 1994. Similarity analysis of DNAs, p. 655-682. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 13.Ladhani, S., and Z. A. Bhutta. 1998. Neonatal Pseudomonas putida infection presenting as staphylococcal scalded skin syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 17:642-644. [DOI] [PubMed] [Google Scholar]
- 14.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, K., J. B. Lim, J. H. Yum, D. Yong, Y. Chong, J. M. Kim, and D. M. Livermore. 2002. blaVIM-2 cassette-containing novel integrons in metallo-β-lactamase-producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob. Agents Chemother. 46:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livermore, D. M., and N. Woodford. 2000. Carbapenemases: a problem in waiting? Curr. Opin. Microbiol. 3:489-495. [DOI] [PubMed] [Google Scholar]
- 17.Luzzaro, F., E. Mantengoli, M. Perilli, G. Lombardi, V. Orlandi, A. Orsatti, G. Amicosante, G. M. Rossolini, and A. Toniolo. 2001. Dynamics of a nosocomial outbreak of multidrug-resistant Pseudomonas aeruginosa producing the PER-1 extended-spectrum β-lactamase. J. Clin. Microbiol. 39:1865-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martino, R., C. Martinez, R. Pericas, R. Salazar, C. Sola, S. Brunet, A. Sureda, and A. Domingo-Albos. 1996. Bacteremia due to glucose non-fermenting gram-negative bacilli in patients with hematological neoplasias and solid tumors. Eur. J. Clin. Microbiol. Infect. Dis. 15:610-615. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Poirel, L., T. Naas, D. Nicolas, L. Collet, S. Bellais, J.-D. Cavallo, and P. Nordmann. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 44:891-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prats, G., E. Miro, B. Mirelis, L. Poirel, S. Bellais, and P. Nordmann. 2002. First isolation of a carbapenem-hydrolyzing β-lactamase in Pseudomonas aeruginosa in Spain. Antimicrob. Agents Chemother. 46:932-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos-Diaz, M. A., and J. L. Ramos. 1998. Combined physical and genetic map of the Pseudomonas putida KT2440 chromosome. J. Bacteriol. 180:6352-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riccio, M. L., N. Franceschini, L. Boschi, B. Caravelli, G. Cornaglia, R. Fontana, G. Amicosante, and G. M. Rossolini. 2000. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob. Agents Chemother. 44:1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riccio, M. L., L. Pallecchi, R. Fontana, and G. M. Rossolini. 2001. In70 of plasmid pAX22, a blaVIM-1-containing integron carrying a new aminoglycoside phosphotransferase gene cassette. Antimicrob. Agents Chemother. 45:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossolini, G. M., M. L. Riccio, G. Cornaglia, L. Pagani, C. Lagatolla, L. Selan, and R. Fontana. 2000. Carbapenem-resistant Pseudomonas aeruginosa with acquired blaVIM metallo-β-lactamase determinants, Italy. Emerg. Infect. Dis. 6:312-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Sanford, J. P. 1995. Pseudomonas species (including melioidosis and glanders), p. 2003-2009. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 28.Senda, K., Y. Arakawa, K. Nakashima, H. Ito, S. Ichiyama, K. Shimokata, N. Kato, and M. Ohta. 1996. Multifocal outbreaks of metallo-β-lactamase-producing Pseudomonas aeruginosa resistant to broad-spectrum β-lactams, including carbapenems. Antimicrob. Agents Chemother. 40:349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senda, K., Y. Arakawa, S. Ichiyama, K. Nakashima, H. Ito, S. Ohsuka, K. Shimokata, N. Kato, and M. Ohta. 1996. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum β-lactams. J. Clin. Microbiol. 34:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsakris, A., S. Pournaras, N. Woodford, M.-F. I. Palepou, G. S. Babini, J. Douboyas, and D. M. Livermore. 2000. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J. Clin. Microbiol. 38:1290-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsao, S. G., C. F. Brunk, and R. E. Pearlman. 1983. Hybridization of nucleic acids directly in agarose gels. Anal. Biochem. 131:365-372. [DOI] [PubMed] [Google Scholar]
- 33.Yan, J.-J., P.-R. Hsueh, W.-C. Ko, K.-T. Luh, S.-H. Tsai, H.-M. Wu, and J.-J. Wu. 2001. Metallo-β-lactamases in clinical Pseudomonas isolates in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob. Agents Chemother. 45:2224-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]