Abstract
Whole-genome fingerprinting fluorescent amplified fragment length polymorphism (FAFLP) data were compared with in silico data for the sequenced strains of Mycobacterium tuberculosis (H37Rv and CDC1551). For this G+C-rich genome, many predicted fragments were not detected experimentally. For H37Rv, only 108 (66%) of the 163 predicted EcoRI-MseI fragments between 100 and 500 bp were visualized in vitro. FAFLP was also used to identify polymorphism in 10 clinical isolates of M. tuberculosis characterized previously by IS6110 typing, examining fragments of up to 1,000 bp in size rather than up to 500 bp as was done previously. Five isolates had unique IS6110 profiles and were not known to be epidemiologically related, two isolates were the same single-band IS6110 type but were not known to be epidemiologically related, and the remaining three isolates were epidemiologically related with identical IS6110 profiles. Analysis of fragments in the 500- to 1,000-bp range using nonselective primers differentiated better between strains than analysis of fragments in the 50- to 500-bp range using a set of four selective primers. Seventeen polymorphic fragments were identified between 500 and 1,000 bp in size compared with nine polymorphic fragments between 50 and 500 bp. Using the 500- to 1,000-bp analysis, a level of discrimination similar to that of IS6110 typing was achieved which, unlike the IS6110 typing, was able to differentiate the two M. tuberculosis strains, each of which had only a single copy of IS6110.
Mycobacterium tuberculosis is a pathogenic acid-fast bacillus with a genome rich in guanine and cytosine (G+C) bases and an average G+C content of 65.6% (7). It is the causative agent of tuberculosis, a disease responsible for a total of 2 to 3 million human deaths annually. Currently, one-third of the world's population is thought to be infected, and the World Health Organization has estimated that if present trends continue, 200 million people are at risk of developing the disease in the next 20 years (22).
M. tuberculosis isolates cannot be resolved by phenotypic strain typing, and their genomes lack heterogeneity. IS6110 restriction fragment length polymorphism typing has been used to distinguish isolates of the M. tuberculosis complex (22). The method is based on the presence of different numbers of copies (varying from 0 to 25) of an insertion sequence, IS6110, in strains of M. tuberculosis and the variability of their positions in the genome. The technique is of limited value, as it cannot easily differentiate between M. tuberculosis isolates that contain only a few or no copies of the insertion sequence, as has been found in countries with a high incidence of tuberculosis, such as India (40% of isolates), and to a lesser degree (8% of isolates) in the United Kingdom (9, 15).
Amplified fragment length polymorphism (AFLP) analysis is a PCR-based technique involving the restriction of bacterial genomic DNA with two restriction enzymes, the ligation of adapters to the restriction fragments, and selective amplification of sets of the restriction fragments with adapter-specific primers. The amplified fragments are then sized by gel electrophoresis (24). The addition of a fluorescent label to one of the PCR primers allows the amplified fragments to be detected with an automated DNA sequencer.
AFLP analysis has been used in the typing of strains of different pathogens (10, 13, 19, 20) and also for plant and animal genetic mapping, medical diagnostics, and phylogenetic studies (18). The whole-genome fingerprinting technique fluorescent AFLP (FAFLP) analysis has also been used to identify polymorphisms in different strains of M. tuberculosis (12). FAFLP offers improved resolution compared with restriction fragment length polymorphism typing of isolates with a single copy of IS6110.
Using FAFLP, Goulding et al. (12) differentiated among 65 clinical M. tuberculosis isolates of known IS6110 type by analyzing DNA restriction fragments from 50 to 500 bp in size. Thirty-eight discriminatory fragments were identified, allowing differentiation of the isolates. Most of the epidemiologically related groups clustered in the same way as with IS6110 profiling, but a group of seven epidemiologically unlinked isolates with identical single-copy IS6110 profiles was split into four clusters by FAFLP analysis (12).
The present study examines whether including DNA fragments of up to 1,000 bp in the analysis increases the resolving power of FAFLP and, by comparing the results with the predicted in silico results obtained from the two sequenced strains of M. tuberculosis (8; http://www.tigr.org/CMR2), investigates whether a G+C-rich genome can be sized and identified accurately.
MATERIALS AND METHODS
The extracted DNA was the same as that used in the study by Goulding et al. (12). Ten of the 65 original strains with known epidemiological relationships were sampled (104, 129, 157, 202, 203, 218, 245, 251, 252, and 253). The genomes of isolates 104, 129, 203, 218, and 245 had unique IS6110 profiles and were not known to be epidemiologically related. Isolates 157 and 202 were the same single-band IS6110 type but were not known to be epidemiologically related. The epidemiologically related isolates 251, 252, and 253 had identical IS6110 profiles. In addition, the sequenced M. tuberculosis strains, H37Rv and CDC1551, were used for reference (8, 21). The DNAs from the reference strains were extracted using the cetyltrimethylammonium bromide method as described previously (23).
Experimental FAFLP.
We used the method described previously by Goulding et al. (12). Briefly, 500 ng of genomic DNA was digested in a total volume of 20 μl consisting of 5 U of MseI (New England Biolabs, Hitchin, Hertfordshire, United Kingdom), 2 μl of 10× MseI buffer, 0.2 μl of 10× bovine serum albumin, and 1.0 μl of DNase-free RNase A (10 μg/μl) for 1 h at 37°C. To this digest was added 5 U (1.0 μl) of EcoRI (Invitrogen, Paisley, United Kingdom), 1.68 μl of 0.5 M Tris HCl (pH 7.6), and 2.1 μl of 0.5 M NaCl (total volume, 25 μl), and the reaction mixtures were incubated for a further hour at 37°C. Slight variations of this method were carried out to optimize the reaction, including increasing the digest times from 1 to 2 and 3 h and adding known PCR-enhancing agents (5% [vol/vol] dimethyl sulfoxide [DMSO], 10% [vol/vol] glycerol, 60 mM tetramethylammonium chloride [TMAC], or 1% [vol/vol] deionized formamide) to the digest or to the PCR mixture. Endonucleases were inactivated at 65°C for 10 min prior to ligation. To the double-digested DNA was added 25 μl of a solution containing 40 U of T4 DNA ligase (New England Biolabs), 10 pmol of EcoRI adapter, 100 pmol of MseI adapter, and 5 μl of 10× T4 ligase buffer. This reaction mixture was incubated at 12°C for 17 h, heated at 65°C for 10 min to inactivate the ligase, and stored at −20°C.
PCRs were performed in 20-μl volumes containing 2 μl of ligated DNA, 0.1 μM 5-carboxy-fluorescein-labeled nonselective primer specific to the EcoRI adapter, 0.25 μM MseI nonselective primer specific to the MseI adapter, 2 μl of 10× Taq polymerase buffer, 200 μM each deoxynucleoside triphosphate, 1.5 mM MgCl2, and 0.5 U of Taq polymerase. Touchdown PCR cycling conditions were used for amplifying the fragments: a 2-min denaturation step at 94°C (one cycle) followed by 30 cycles of denaturation at 94°C for 20 s, a 30-s annealing step (see below), and a 2-min extension step at 72°C. The annealing temperature for the first cycle was 66°C; for the next nine cycles, the temperature was decreased by 1°C at each cycle. The annealing temperature for the remaining 20 cycles was 56°C. This was followed by a final extension at 60°C for 30 min. PCR was performed in a PE-9600 thermocycler (Perkin-Elmer Corp., Norwalk, Conn.).
The amplification products were separated on a 5% denaturing polyacrylamide gel (FMC SingGel) on an ABI 377 DNA sequencer (Perkin-Elmer Corp.). The sample (1 μl) was added to 2.5 μl of loading dye, which was a mixture containing 5 μl of formamide, 1 μl of dextran blue-50 mM EDTA loading solution, and 0.5 μl of the internal lane standard GeneScan 2500 labeled with the fluorophore ROX (PE Biosystems). The sample mixture was heated at 95°C for 2 min, cooled on ice, and immediately loaded onto the gel. The electrophoresis conditions were 2 kV at 51°C for 16 h, using 1× Tris-borate-EDTA as the buffer.
GeneScan and GenoTyper software (PE Biosystems) were used to ascertain the presence or absence of precisely sized fragments.
Reproducibility.
To check the reproducibility of the FAFLP analysis, at least two different sets of digestions and ligations were carried out on the DNAs of the clinical isolates. For each digestion-ligation, at least three different PCRs were carried out separately, and each was run on a different gel.
In silico FAFLP.
The complete genome sequences of M. tuberculosis strains H37Rv and CDC1551 were analyzed with the Restriction Digest Tool of The Institute for Genomic Research (http://www.tigr.org/tigr-scripts/CMR2/restrict_display.pl) using EcoRI and MseI. Data concerning the sizes and predicted number of EcoRI-MseI fragments were imported into a spreadsheet, and fragment sizes were adjusted to allow for the addition of primer sequences during PCR, enabling direct comparison between the predicted in silico and in vitro results.
RESULTS
Experimental versus in silico analysis.
For H37Rv, 66% of the 163 predicted EcoRI-MseI fragments between 100 and 500 bp, and only 8% of the 160 predicted EcoRI-MseI fragments between 501 and 1,000 bp, were detected (Table 1). For CDC1551, we found that 55% of the 160 predicted EcoRI-MseI fragments between 100 and 500 bp and 15% of the 164 predicted EcoRI-MseI fragments between 501 and 1,000 bp were detected (Table 1). When the experiments were repeated with increased magnesium in the PCR mixture (2 mM MgCl2 concentration instead of 1.5 mM) (Table 1), the number of fragments visualized increased but did not match the in silico results.
TABLE 1.
Comparison of in silico-predicted number of M. tuberculosis MseI/EcoRI fragments with actual number of fragments detected by FAFLP analysisa
| Strain | Restriction fragment size range (bp) | [MgCl2] (mM) | Predicted no. of fragments | No. of fragments detected | % Predicted fragments detected |
|---|---|---|---|---|---|
| H37Rv | 100-500 | 1.5 | 163 | 108 | 66 |
| H37Rv | 100-500 | 2.0 | 163 | 76 | 47 |
| H37Rv | 501-1,000 | 1.5 | 160 | 12 | 8 |
| H37Rv | 501-1,000 | 2.0 | 160 | 24 | 15 |
| CDC1551 | 100-500 | 1.5 | 160 | 88 | 55 |
| CDC1551 | 100-500 | 2.0 | 160 | 109 | 68 |
| CDC1551 | 501-1,000 | 1.5 | 164 | 24 | 15 |
| CDC1551 | 501-1,000 | 2.0 | 164 | 42 | 26 |
1.5 and 2 mM [MgCl2] in the PCR.
To assess how accurate FAFLP analysis could be for M. tuberculosis, the experimental analysis of H37Rv was repeated using a nonselective EcoRI-labeled primer and a selective MseI+TA primer. This primer combination was expected to result in the amplification of fewer fragments, making the comparison of fragment size data between the predicted (in silico) and the observed (i.e., experimental) data easier.
Only 5 of the 11 predicted fragments (sizes, 195, 250, 252, 434, and 821 bp) were present in the experimental results of the MseI+TA-EcoRI FAFLP analysis of H37Rv (Table 2). These five fragments were within ±2 bp of their predicted sizes. Four fragments of other sizes were detected that were not predicted in silico (Table 2).
TABLE 2.
Fragments predicted in silico and generated by FAFLP analysis of M. tuberculosis strain H37Rv using MseI + TA selective primer and EcoRI nonselective primer
| Method | Presence of fragment (size in bp)a
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 63 | 186 | 195 | 250 | 252 | 361 | 434 | 646 | 649 | 663 | 821 | 827 | 966 | 977 | |
| In silico | + | + | + | + | + | + | + | + | + | + | + | ||||
| Experimental | + | + | + | + | + | + | + | + | + | ||||||
+, present.
FAFLP analysis of 10 clinical isolates.
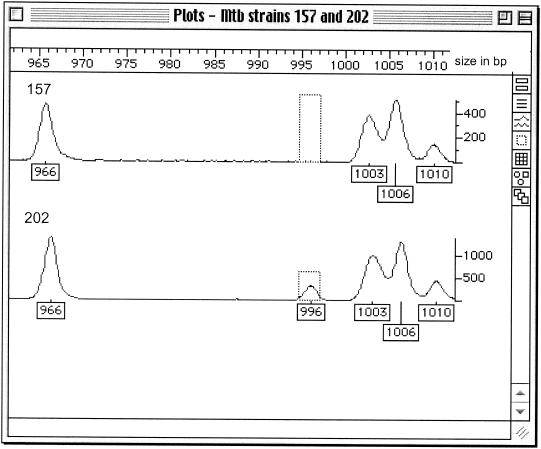
The 10 M. tuberculosis isolates showed 17 discriminatory fragments between 800 and 1,000 bp in size when nonselective EcoRI and MseI primers were used (Table 3). This discriminated between two epidemiologically unlinked strains, 157 and 202, with single copies of IS6110. Isolate 202 possessed two fragments, 898 and 996 bp in size, which were absent from isolate 157. Figure 1 shows the presence of the 996-bp fragment in isolate 202. The epidemiologically related isolates 251, 252, and 253 showed identical profiles apart from one fragment, 828 bp in size, present only in isolate 251 (Table 3).
TABLE 3.
Sizes of discriminatory fragments generated by FAFLP analysis of 10 IS6110-typed M. tuberculosis strainsa
| Strain no. | Presence of fragment (size in bp)b
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 819 | 823 | 828 | 830 | 847 | 879 | 887 | 891 | 896 | 898 | 906 | 908 | 911 | 928 | 931 | 968 | 996 | |
| 104 | + | + | + | + | |||||||||||||
| 129 | + | + | + | + | + | + | |||||||||||
| 157 | + | + | + | + | + | + | + | + | + | + | + | ||||||
| 202 | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| 203 | + | + | + | + | + | + | + | + | |||||||||
| 218 | + | + | + | + | + | ||||||||||||
| 245 | + | + | + | + | + | + | + | + | |||||||||
| 251 | + | + | + | + | + | ||||||||||||
| 252 | + | + | + | + | |||||||||||||
| 253 | + | + | + | + | |||||||||||||
Originally studied by Goulding et al. (12) using MseI and EcoRI nonselective primers. Isolates 251, 252, and 253 are known to be epidemiologically linked.
+, present.
FIG. 1.
FAFLP profiles of M. tuberculosis single-copy IS6110 isolates 157 and 202. The plot shows the fragment sizes in the range from 965 to 1,010 bp. The numbered boxes show the size in base pairs of each fragment. A fragment at 996 bp is present in isolate 202 and absent in isolate 157. The vertical scale measures the efficiency of PCR amplification of each fragment in fluorescent units.
Reproducibility.
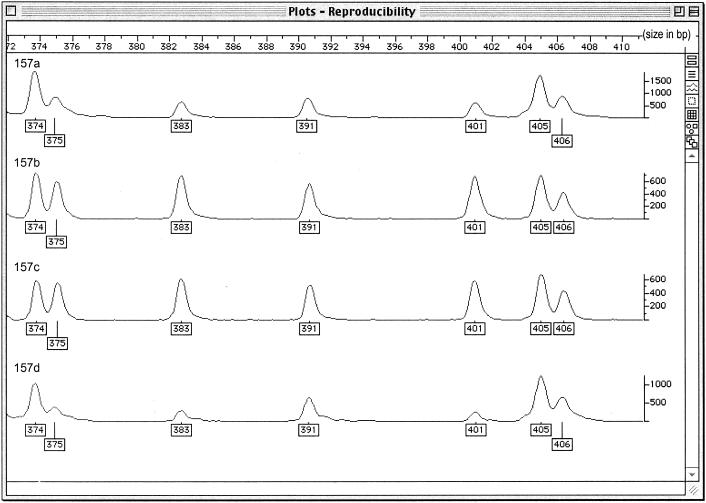
The FAFLP results were reproducible (Fig. 2); however, some background was seen in a small number of replicates, and consequently, only fragments read as above 75 fluorescent units (Fig. 2, y axis) were included. The FAFLP data showed slight variation in the relative peak heights of fragments of corresponding size (Fig. 2).
FIG. 2.
FAFLP reproducibility study for M. tuberculosis clinical isolate 157. The four plots show the FAFLP profiles for the same isolate across a 40-bp window (372 to 412 bp). The four reactions were carried out separately and run on different gels. The vertical scale measures the efficiency of PCR amplification of each fragment in fluorescent units.
DISCUSSION
Previous work with Escherichia coli K-12 has shown that sizing is accurate to within 1 bp and that 48 of 48 predicted fragments were visualized (2) in FAFLP analysis. This was not, however, true of M. tuberculosis H37Rv and CDC1551 (Table 1). For H37Rv, only 108 (66%) of the 163 predicted EcoRI-MseI fragment sizes between 100 and 500 bp were visualized in vitro, and when H37Rv was investigated with MseI+TA-EcoRI, only 45% of the predicted fragments were visualized. The four unexpected fragments generated by this study were thought to be caused by incomplete digestion of the M. tuberculosis genomic DNA. It is possible that, due to the rich G+C content of the genome, secondary structures form that block the restriction sites from the action of the restriction enzymes. Usually an incomplete digest would mean generating a larger number of fragments than expected because the restriction enzyme cuts the genome at that position sometimes but not always, generating two fragments instead of one, i.e., the authentic one and a larger one. This does not appear to be the case for M. tuberculosis, as fewer fragments than expected are seen, even in the larger size range, indicating that certain restriction sites are rarely (or never) cut or that some digested fragments are not amplified or are amplified poorly. Various strategies were tried (the use of DMSO, glycerol, formamide, and TMAC and increasing digestion times) to overcome these potential problems. DMSO has been shown to improve the amplification efficiency and specificity of PCR (3). It is thought to reduce secondary structure by disrupting the base pairing (11) and is therefore very useful for G+C-rich DNA templates (6). Both glycerol (16) and formamide (4, 17) improve the specificity and efficiency of PCR by changing the melting temperature of the primer-template hybridization reaction (Alkami quick guide for PCR: a laboratory reference for the polymerase chain reaction, vol. 1, Alkami Biosystems Inc., Berkeley, Calif., 1999). TMAC has been shown to improve the stringency of hybridization reactions, reduce potential DNA-RNA mismatch, and help eliminate nonspecific priming in PCR (5, 14).
Although DMSO, glycerol, and TMAC did not improve the method, preliminary results suggested that for FAFLP analysis of G+C-rich genomes, the use of deionized formamide in the digest step reduced the problem of unexpected fragments being produced and/or visualized. The identification and characterization of the four unpredicted restriction fragments generated by FAFLP analysis with the MseI+TA and EcoRI primers, and their locations in the genome, may help to elucidate the differences that occur between the in silico and experimental data.
If the failure to detect expected fragments is not due to incomplete digestion but to cut fragments not being amplified because of secondary structure, the addition of dITP in place of dGTP during the PCR step may improve the FAFLP profile. The effect of the dITP may be to increase the number of fragments seen, but it may also have an effect on their relative peak heights, as there appears to be some variability in the relative peak heights among different M. tuberculosis strains. However, this may be because amplification appears not to be uniform due to other factors, for example, if peaks with higher signal are comprised of multiple fragments of the same or similar sizes. Other potential causes for this lack of parity between in vitro and in silico data might be that there are errors in the sequences of H37Rv and CDC1551 or that passage of different stocks has led to differences between the isolates studied and the isolates sequenced. Mispriming of PCR may also have an effect on successful generation of expected fragments, but touchdown PCR is employed in FAFLP and should minimize this.
Another way of improving the FAFLP method for M. tuberculosis would be the use of different sets of enzymes, for example, XhoI and HhaI, which cut at IS6110 as well as throughout the rest of the genome. This combination should give FAFLP profiles corresponding to the IS6110 elements in a genome, as well as sampling throughout that genome.
The four-primer combination used in the study by Goulding et al. (12) of 65 IS6110-typed clinical isolates generated 38 discriminatory fragments under 500 bp in size. Of these fragments, only nine discriminated among the 10 (of 65) randomly selected isolates when studied as a subgroup. However, when nonselective primers were used, these 10 isolates gave a further 17 discriminatory fragments between 800 and 1,000 bp in size (Table 1). Two of these 17 polymorphic fragments (800 to 1,000 bp) differentiated between isolates 157 and 202, which both contain only a single copy of IS6110 (Fig. 1), making this a useful supplementary technique for M. tuberculosis isolates containing a single copy of IS6110. The profiles of the three isolates known to be epidemiologically linked (251, 252, and 253) were identical apart from one fragment observed in isolate 251 only. The reason for this is unclear, but it may be a reflection of real changes in the genome of isolate 251 compared with 252 and 253. The reason for the disproportionate number of polymorphisms in the higher size range is not understood. It is not due to the lack of reproducibility of amplification of larger-sized fragments.
In conclusion, experimental FAFLP does not exactly match in silico prediction, even when known PCR enhancers, such as TMAC (5) and formamide (17), are added to the digestion and/or PCR. The FAFLP technique is not as accurate for the G+C-rich genome of M. tuberculosis as for E. coli (1) and Campylobacter (10), as it does not strictly conform to the predicted model, but the fragments were precisely sized (±2 bp). The results were reproducible (Fig. 2) but not as clean as for genomes with low G+C content, such as E. coli, which again may be due to the G+C-rich content of the M. tuberculosis genome. Fragments below a certain signal strength were therefore ignored. FAFLP is capable of resolving strains that cannot be differentiated using IS6110 typing due to their low IS6110 copy numbers, as demonstrated by the two polymorphic fragments (898 and 996 bp) that were present in isolate 202 but not in 157 (both of which contain single IS6110 copies). This difference is significant, as up to 40% of M. tuberculosis isolates in certain countries have only one copy of IS6110 (9). Our study also shows that FAFLP has a greater resolving power when fragments between 501 and 1,000 bp are included, as well as those between 100 and 500 bp.
Acknowledgments
We thank Julie Logan and Fiona Scott for their invaluable assistance with this project.
REFERENCES
- 1.Arnold, C., L. Metherell, J. P. Clewley, and J. Stanley. 1999. Predictive modelling of fluorescent AFLP: a new approach to the molecular epidemiology of E. coli. Res. Microbiol. 150:33-44. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, C., L. Metherell, G. Willshaw, A. Maggs, and J. Stanley. 1999. Predictive fluorescent amplified-fragment length polymorphism analysis of Escherichia coli: high-resolution typing method with phylogenetic significance. J. Clin. Microbiol. 37:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bookstein, R., C.-C. Lai, H. To, and W.-H. Lee. 1990. PCR-based detection of a polymorphic BamHI site in intron 1 of the human retinoblastoma (RB) gene. Nucleic Acids Res. 18:1666.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti, R., and C. E. Schutt. 2001. The enhancement of PCR amplification by low molecular weight amides. Nucleic Acids Res. 29:2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevet, E., G. Lemaitre, and M. D. Katinka. 1995. Low concentrations of tetramethylammonium chloride increase yield and specificity of PCR. Nucleic Acids Res. 23:3343-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, J.-S., J.-S. Kim, C.-O. Joe, S. Kim, K.-S. Ha, and Y.-M. Park. 1999. Improved cycle sequencing of GC-rich DNA template. Exp. Mol. Med. 31:20-24. [DOI] [PubMed] [Google Scholar]
- 7.Cole, S. T. 1999. Learning from the genome sequence of Mycobacterium tuberculosis H37Rv. FEBS Lett. 452:7-10. [DOI] [PubMed] [Google Scholar]
- 8.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 9.Das, S., C. N. Paramasivan, D. B. Lowrie, R. Prabhakar, and P. R. Narayanan. 1995. IS6110 restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from patients with pulmonary tuberculosis in Madras, south India. Tubercle Lung Dis. 76:550-554. [DOI] [PubMed] [Google Scholar]
- 10.Desai, M., J. M. J. Logan, J. A. Frost, and J. Stanley. 2001. Genome sequence-based fluorescent amplified fragment length polymorphism of Campylobacter jejuni, its relationship to serotyping, and its implications for epidemiological analysis. J. Clin. Microbiol. 39:3823-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frackman, S., G. Kobs, D. Simpson, and D. Storts. 1998. Betaine and DMSO: enhancing agents for PCR. Promega Notes 65:27. [Google Scholar]
- 12.Goulding, J. N., J. Stanley, N. Saunders, and C. Arnold. 2000. Genome-sequence-based fluorescent amplified fragment length polymorphism analysis of Mycobacterium tuberculosis. J. Clin. Microbiol. 38:1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grady, R., G. O'Neill, B. Cookson, and J. Stanley. 2000. Fluorescent amplified-fragment length polymorphism analysis of the MSRA epidemic. FEMS Microbiol. Lett. 187:27-30. [DOI] [PubMed] [Google Scholar]
- 14.Hung, T., K. Mak, and K. Fong. 1990. A specificity enhancer for polymerase chain reaction. Nucleic Acids Res. 18:4953.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar, D., N. A. Saunders, J. M. Watson, A. M. Ridley, S. Nicholas, K. F. Barker, R. Wall, Q. N. Karim, S. Barrett, R. C. George, and A. C. McCartney. 2000. Clusters of new tuberculosis cases in north-west London: a survey from three hospitals based on IS6110 RFLP typing. J. Infect. 40:132-137. [DOI] [PubMed] [Google Scholar]
- 16.McPherson, M. J., and S. G. Moller. 2000. PCR. BIOS, Oxford, United Kingdom.
- 17.Sarkar, G., S. Kapelner, and S. S. Sommer. 1990. Formamide can dramatically improve the specificity of PCR. Nucleic Acids Res. 18:7465.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savelkoul, P. H. M., H. J. M. Aarts, J. de Haas, L. Dijkshoorn, B. Duim, M. Otsen, J. L. W. Rademaker, L. Schouls, and J. A. Lenstra. 1999. Amplified-fragment length polymorphism analysis: the state of an art. J. Clin. Microbiol. 37:3083-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott, F., J. Threfall, J. Stanley, and C. Arnold. 2001. Fluorescent amplified fragment length polymorphism genotyping of Salmonella enteritidis: a method suitable for rapid outbreak recognition. Clin. Microbiol. Infect. 7:479-485. [DOI] [PubMed] [Google Scholar]
- 20.Smith, D., G. Willshaw, J. Stanley, and C. Arnold. 2000. Genotyping of verocytotoxin-producing Escherichia coli O157: comparison of isolates of a prevalent phage type by fluorescent amplified-fragment length polymorphism and pulsed-field gel electrophoresis analyses. J. Clin. Microbiol. 38:4616-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valway, S. E., M. P. C. Sanchez, T. F. Shinnick, I. Orme, T. Agerton, D. Hoy, J. S. Jones, H. Westmoreland, and I. M. Onorato. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338:633-639. [DOI] [PubMed] [Google Scholar]
- 22.van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 249:1-26. [DOI] [PubMed] [Google Scholar]
- 23.van Soolingen, D., P. E. W. da Haas, P. W. M. Hermans, and J. D. A. van Embden. 1994. DNA fingerprinting of Mycobacterium tuberculosis. Methods Enzymol. 235:196-205. [DOI] [PubMed] [Google Scholar]
- 24.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. van de Lee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeau. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]