Abstract
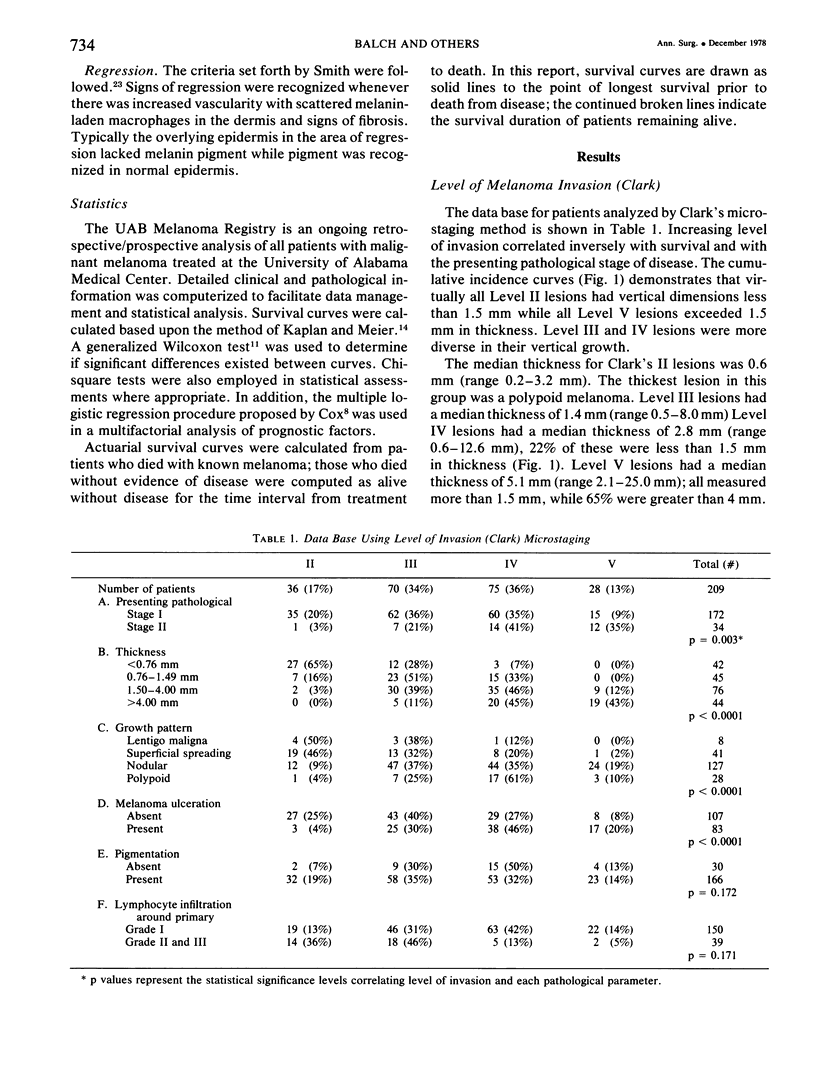
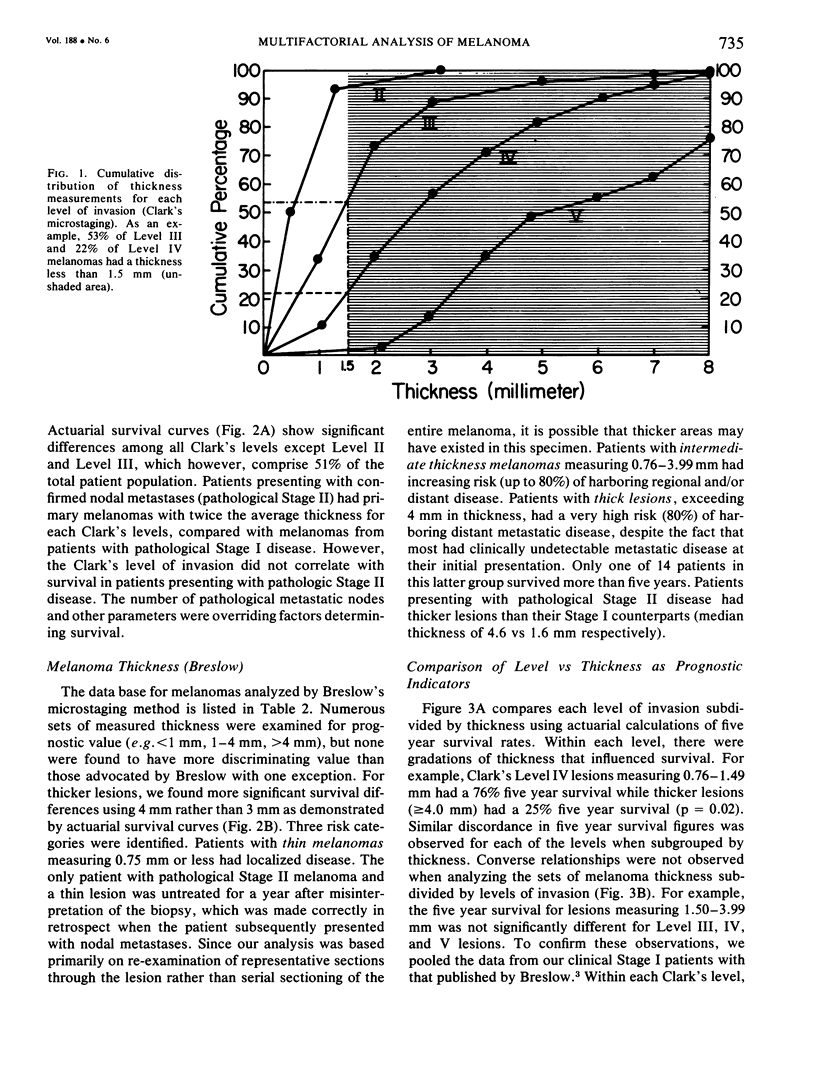
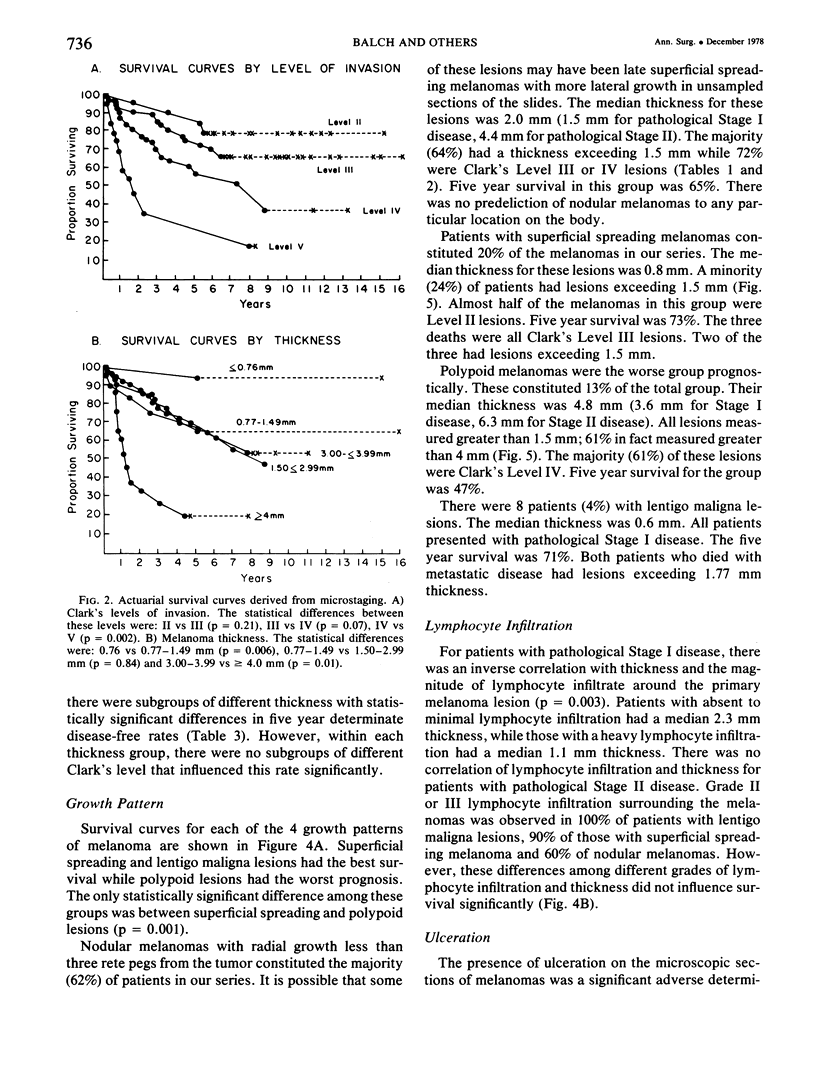
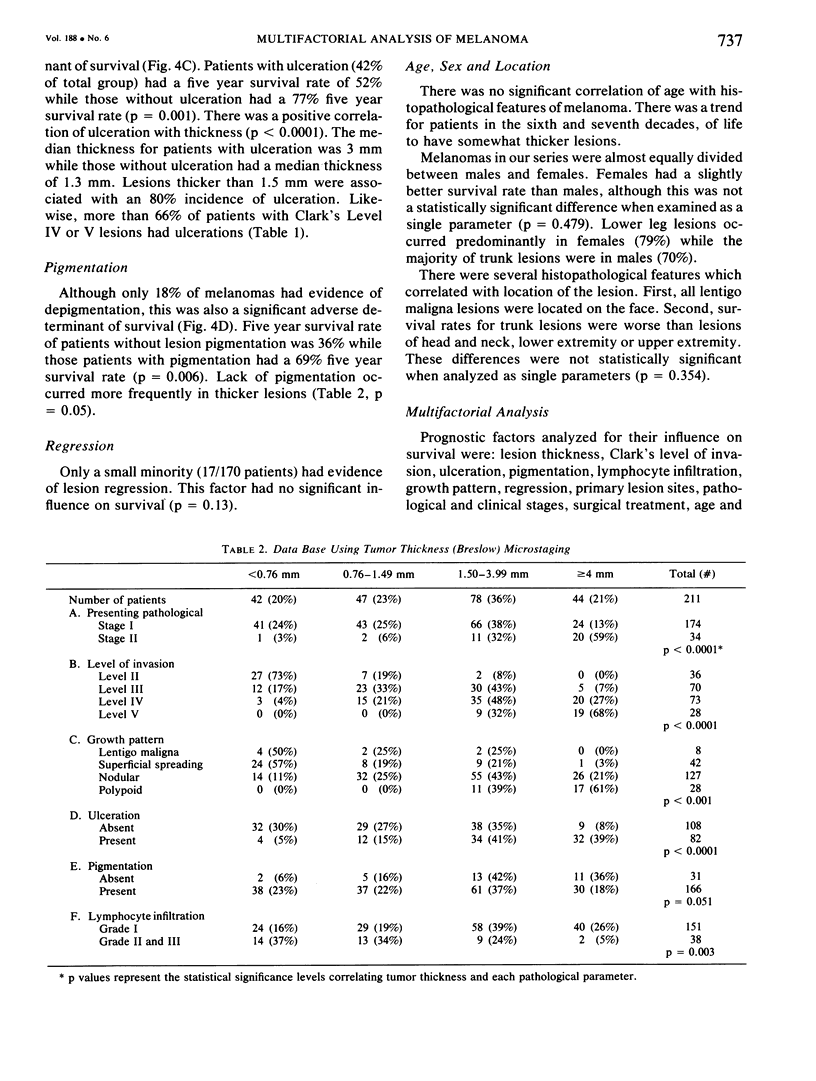
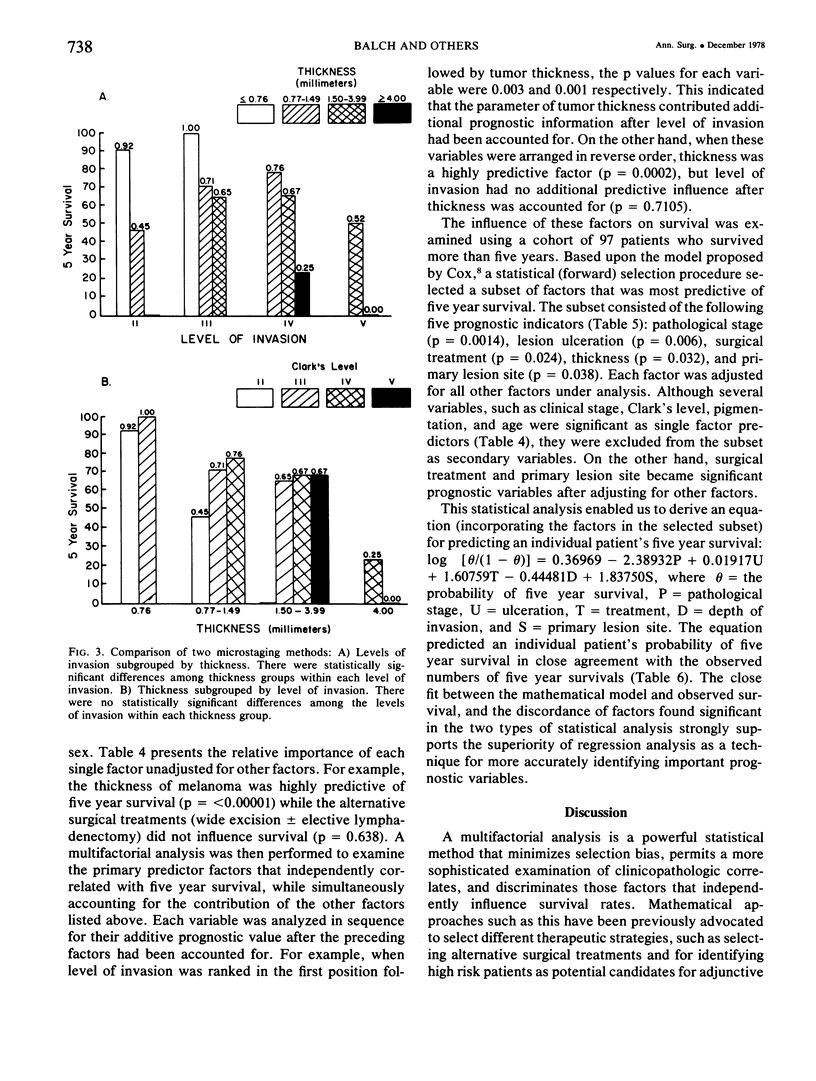
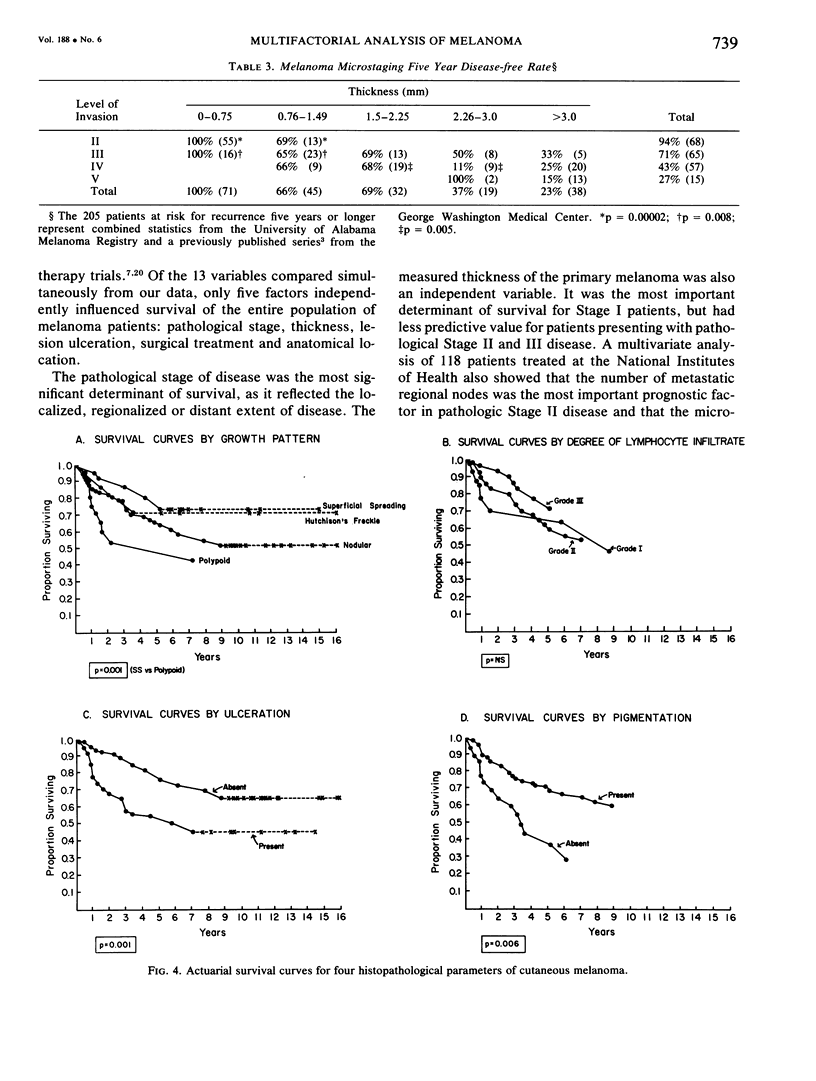
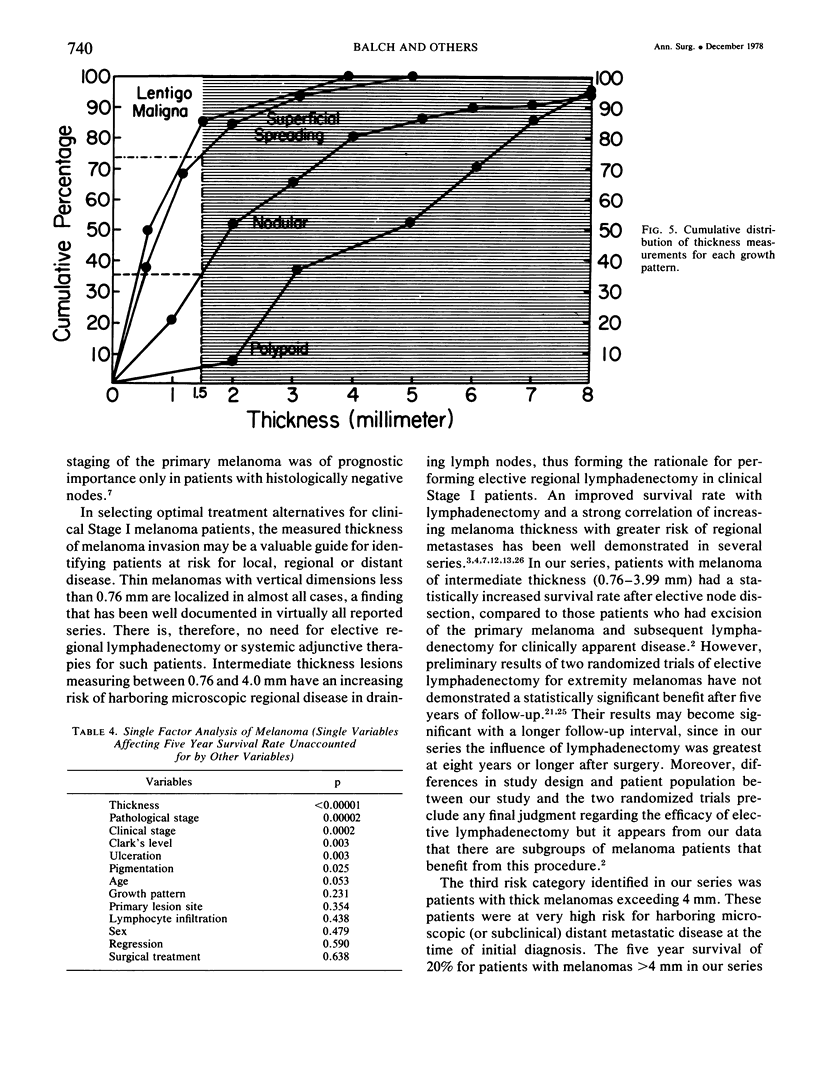
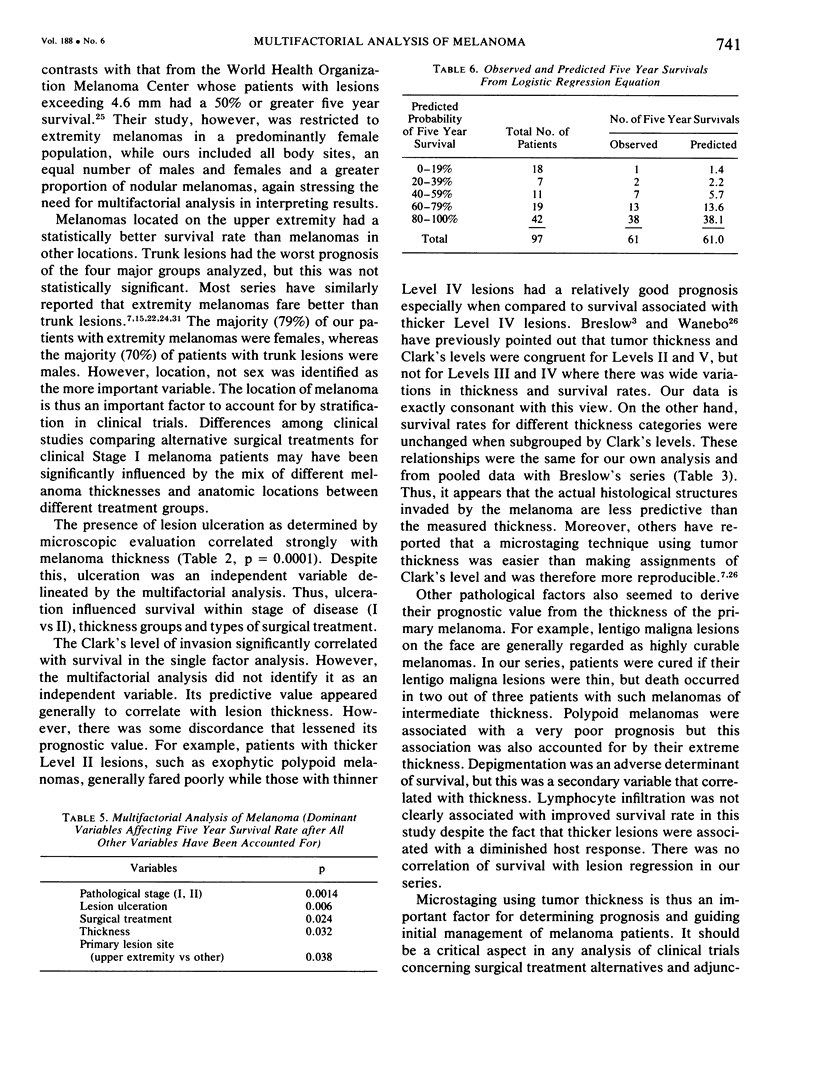
A multifactorial analysis was used to identify the dominant prognostic variables affecting survival from a computerized data base of 339 melanoma patients treated at this institution during the past 17 years. Five of the 13 parameters examined simultaneously were found to independently influence five year survival rates: 1) pathological stage (I vs II, p = 0.0014), 2) lesion ulceration (present vs absent, p = 0.006), 3) surgical treatment (wide excision vs wide excision plus lymphadenectomy, p = 0.024), 4) melanoma thickness (p = 0.032), and 5) location (upper extremity vs lower extremity vs trunk vs head and neck, p = 0.038). Additional factors considered that had either indirect or no influence on survival rates were clinical stage of disease, age, sex, level of invasion, pigmentation, lymphocyte infiltration, growth pattern, and regression. Most of these latter variables derived their prognostic value from correlation with melanoma thickness, except sex which correlated with location (extremity lesions were more frequent on females, trunk lesions on males). This statistical analysis enabled us to derive a mathematical equation for predicting an individual patient's probability of five year survival. Three categories of risk were delineated by measuring tumor thickness (Breslow microstaging) in Stage I patients: 1) thin melanomas (<0.76 mm) were associated with localized disease and a 100% cure rate: 2) intermediate thickness melanomas (0.76-4.00 mm) had an increasing risk (up to 80%) of harboring regional and/or distant metastases and 3) thick melanomas (≥4.00 mm) had a 80% risk of occult distant metastases at the time of initial presentation. The level of invasion (Clark's microstaging) correlated with survival, but was less predictive than measuring tumor thickness. Within each of Clark's Level II, III and IV groups, there were gradations of thickness with statistically different survival rates. Both microstaging methods (Breslow and Clark) were less predictive factors in patients with lymph node or distant metastases. Clinical trials evaluating alternative surgical treatments or adjunctive therapy modalities for melanoma patients should incorporate these parameters into their assessment, especially in Stage I (localized) disease where tumor thickness and the anatomical site of the primary melanoma are dominant prognostic factors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN A. C., SPITZ S. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer. 1953 Jan;6(1):1–45. doi: 10.1002/1097-0142(195301)6:1<1::aid-cncr2820060102>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970 Nov;172(5):902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow A. Tumor thickness, level of invasion and node dissection in stage I cutaneous melanoma. Ann Surg. 1975 Nov;182(5):572–575. doi: 10.1097/00000658-197511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. H., Jr, From L., Bernardino E. A., Mihm M. C. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969 Mar;29(3):705–727. [PubMed] [Google Scholar]
- Cohen M. H., Ketcham A. S., Felix E. L., Li S. H., Tomaszewski M. M., Costa J., Rabson A. S., Simon R. M., Rosenberg S. A. Prognostic factors in patients undergoing lymphadenectomy for malignant melanoma. Ann Surg. 1977 Nov;186(5):635–642. doi: 10.1097/00000658-197711000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias E. G., Didolkar M. S., Goel I. P., Formeister J. F., Valenzuela L. A., Pickren J. L., Moore R. H. A clinicopathologic study of prognostic factors in cutaneous malignant melanoma. Surg Gynecol Obstet. 1977 Mar;144(3):327–334. [PubMed] [Google Scholar]
- GEHAN E. A. A GENERALIZED WILCOXON TEST FOR COMPARING ARBITRARILY SINGLY-CENSORED SAMPLES. Biometrika. 1965 Jun;52:203–223. [PubMed] [Google Scholar]
- Gupta T. K. Results of treatment of 269 patients with primary cutaneous melanoma: a five-year prospective study. Ann Surg. 1977 Aug;186(2):201–209. doi: 10.1097/00000658-197708000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. G., McCarten A. B. Tumor thickness and lymphocytic infiltration in malignant melanoma of the head and neck. Am J Surg. 1974 Oct;128(4):557–561. doi: 10.1016/0002-9610(74)90275-x. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Moseley H. S., Morton D. L., Clark W., Robinson D., Urist M. M. A rational approach to the surgical management of melanoma. Ann Surg. 1977 Oct;186(4):481–490. doi: 10.1097/00000658-197710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANE N., LATTES R., MALM J. Clinicopathological correlations in a series of 117 malignant melanomas of the skin of adults. Cancer. 1958 Sep-Oct;11(5):1025–1043. doi: 10.1002/1097-0142(195809/10)11:5<1025::aid-cncr2820110525>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- LUND R. H., IHNEN M. Malignant melanoma; clinical and pathologic analysis of 93 cases. Is prophylactic lymph node dissection indicated? Surgery. 1955 Oct;38(4):652–659. [PubMed] [Google Scholar]
- MEHNERT J. H., HEARD J. L. STAGING OF MALIGNANT MELANOMAS BY DEPTH OF INVASION; A PROPOSED INDEX TO PROGNOSIS. Am J Surg. 1965 Aug;110:168–176. doi: 10.1016/0002-9610(65)90008-5. [DOI] [PubMed] [Google Scholar]
- PETERSEN N. C., BODENHAM D. C., LLOYD O. C. Malignant melanomas of the skin. A study of the origin, development, aetiology, spread, treatment, and prognosis. I. Br J Plast Surg. 1962 Jan;15:49–94. doi: 10.1016/s0007-1226(62)80011-3. [DOI] [PubMed] [Google Scholar]
- Polk H. C., Jr, Linn B. S. Selective regional lymphadenectomy for melanoma: a mathematical aid to clinical judgment. Ann Surg. 1971 Sep;174(3):402–413. doi: 10.1097/00000658-197109000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F. H., Taylor W. F., Ivins J. C., Pritchard D. J., Soule E. H. A prospective randomized study of the efficacy of routine elective lymphadenectomy in management of malignant melanoma. Preliminary results. Cancer. 1978 Mar;41(3):948–956. doi: 10.1002/1097-0142(197803)41:3<948::aid-cncr2820410324>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Jr, Stehlin J. S., Jr Spontaneous regression of primary malignant melanomas with regional metastases. Cancer. 1965 Nov;18(11):1399–1415. doi: 10.1002/1097-0142(196511)18:11<1399::aid-cncr2820181104>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Sugarbaker E. V., McBride C. M. Melanoma of the trunk: the results of surgical excision and anatomic guidelines for predicting nodal metastasis. Surgery. 1976 Jul;80(1):22–30. [PubMed] [Google Scholar]
- Veronesi U., Adamus J., Bandiera D. C., Brennhovd I. O., Caceres E., Cascinelli N., Claudio F., Ikonopisov R. L., Javorskj V. V., Kirov S. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977 Sep 22;297(12):627–630. doi: 10.1056/NEJM197709222971202. [DOI] [PubMed] [Google Scholar]
- Wanebo H. J., Fortner J. G., Woodruff J., MacLean B., Binkowski E. Selection of the optimum surgical treatment of stage I melanoma by depth of microinvasion: Use of the combined microstage technique (Clark-Breslow). Ann Surg. 1975 Sep;182(3):302–315. doi: 10.1097/00000658-197509000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]


